Abstract
Interactions between niche cells and stem cells are vital for proper control over stem cell self-renewal and differentiation. However, there are few tissues where the initial establishment of a niche has been studied. The Drosophila testis houses two stem cell populations, which each lie adjacent to somatic niche cells. Although these niche cells sustain spermatogenesis throughout life, it is not understood how their fate is established. Here, we show that Notch signaling is necessary to specify niche cell fate in the developing gonad. Surprisingly, our results indicate that adjacent endoderm is the source of the Notch-activating ligand Delta. We also find that niche cell specification occurs earlier than anticipated, well before the expression of extant markers for niche cell fate. This work further suggests that endoderm plays a dual role in germline development. The endoderm assists both in delivering germ cells to the somatic gonadal mesoderm, and in specifying the niche where these cells will subsequently develop as stem cells. Because in mammals primordial germ cells also track through endoderm on their way to the genital ridge, our work raises the possibility that conserved mechanisms are employed to regulate germline niche formation.
Keywords: Drosophila, Gonadogenesis, Notch, Endoderm, Niche
INTRODUCTION
Interactions of tissue-specific stem cells with their local micro-environment, or niche, are vital for proper stem cell self-renewal and differentiation (for a review, see Morrison and Spradling, 2008). Although rough locations for numerous stem cell niches have been elucidated in mammals and invertebrates, in many cases we do not understand how the niche is specified, nor can we molecularly identify niche cells in vivo (Morrison and Spradling, 2008). An understanding of the principles of niche cell development will be key in order to use stem cells effectively in therapeutics, as niche cells regulate important aspects of stem cell behavior. For example, in the absence of a self-renewal signal from niche cells, Drosophila germline cells differentiate, preventing stem cell maintenance and proper tissue homeostasis (Tulina and Matunis, 2001; Kiger et al., 2001; Ward et al., 2006; Song et al., 2007). Similarly, when ectopic or excess niche cells are induced, extra cells adopt stem cell characteristics, leading to the proliferation of stem-like cells, and potentially tumors (Tulina and Matunis, 2001; Kiger et al., 2001; Ward et al., 2006; Song et al., 2007; Kitadate et al., 2007). Therefore, it is important to understand fully which signaling pathways are necessary to establish a niche.
We have a partial understanding of niche cell development in two tissues maintained by germline stem cells; however, unanswered questions remain. Studies from the Drosophila ovary have shown that Notch signaling is required during development to specify cap cells properly, which function as the niche (Song et al., 2007; Ward et al., 2006). However, it remains ambiguous how the cap cells become activated for Notch and which neighboring cells present the signaling ligand. In the development of the C. elegans germline, the distal tip cell (DTC) functions as the niche (Kimble and White, 1981; Berry et al., 1997). Although it appears that Wnt signaling and the coordinate expression of the transcription factor Nkx2.2 is essential for DTC specification, the source of the Wnt ligand remains unknown (Lam et al., 2006).
As the Drosophila testis stem cell niche is amenable to the study of signaling pathways (Tulina and Matunis, 2001; Kiger et al., 2001; Kitadate et al., 2007), we have chosen to investigate how the niche is specified in this classical model system. The adult testis is a stem cell-based tissue, operating at steady state to sustain spermatogenesis. This system comprises two distinct populations of stem cells, germline stem cells (GSCs) and cyst stem cells (CySCs), which cluster around a group of somatic cells that serve as the niche, called the hub (Hardy et al., 1979). Several signals implicated in stem cell maintenance and self-renewal emanate from hub cells, including Unpaired (Upd) and BMP ligands (Kiger et al., 2001; Tulina and Matunis, 2001; Shivdasani and Ingham, 2003; Kawase et al., 2004; Leatherman and DiNardo, 2008; Leatherman and DiNardo, 2010; Issigonis et al., 2009).
Hub cells have been thought to be specified late in embryogenesis, as they are not visible until near hatching of the first larval instar (Le Bras and Van Doren, 2006). Hub cells can then be visualized as a tight cluster of somatic cells at the anterior end of each gonad, by using either cell surface or gene expression markers (Gönczy and DiNardo, 1996; Le Bras and Van Doren, 2006; Wawersik and Van Doren, 2005; DeFalco et al., 2005; Tanentzapf et al., 2007). Until recently, no pathway necessary to promote hub cell fate had been identified (Kitadate and Kobayashi, 2010). Given the importance of hub cells to stem cell survival, it is important to know how they become specified during embryogenesis.
Bilaterally symmetric gonads are formed during mid-embryogenesis from two distinct lineages: primordial germ cells (PGCs) and mesodermally derived somatic gonadal precursor cells (SGPs) (Aboim, 1945). Germ cells develop at the posterior pole of the embryo and are internalized in the posterior midgut (PMG) during gastrulation (Campos-Ortega and Hartenstein, 1985). They then migrate through the endoderm to reach the mesoderm. While germ cells are migrating, the SGPs are specified from the lateral mesoderm in parasegments 10-12, and begin associating with germ cells at stage 11 (Sonnenblick, 1941; Brookman et al., 1992; Boyle and DiNardo, 1995; Boyle et al., 1997). The SGPs and the germ cells then migrate together anteriorly and finally coalesce at stage 14 within parasegment 10 (Boyle and DiNardo, 1995; Boyle et al., 1997; Clark et al., 2007). SGPs then extend cellular processes to ensheath the germ cells, resulting in a spherical, compacted gonad (Jenkins et al., 2003). Lineage-tracing experiments have demonstrated that hub cells derive from the anterior two-thirds of SGPs, definitively from parasegment 11, with the remaining hub cells probably derived from parasegment 10 (Le Bras and Van Doren, 2006). Only some of these parasegment 10 and 11 SGPs become hub cells; the remainder likely adopt cyst cell fate. This suggests that SGPs may give rise to both hub and cyst cells, although it is not known which signaling pathways are responsible for inducing these differential cell fates. It has been shown, however, that receptor tyrosine kinase (RTK) signaling mediated by the Boss/Sevenless and epidermal growth factor (EGFR) pathways inhibits hub cell formation among posterior SGPs, while permitting formation in the anterior (Kitadate et al., 2007; Kitadate and Kobayashi, 2010).
Here, we focus on the role of Notch signaling in niche cell specification, a pathway implicated by expression profiling of testes enriched for the niche and it stem cells (Terry et al., 2006). We uncover a key role for Notch signaling in the initial allocation of SGPs to hub cell fate (see also Kitadate and Kobayashi, 2010). Additionally, our results suggest that the posterior midgut cells are the source of the ligand Delta, which induces hub cell fate. Finally, we show that a subset of SGPs is activated to take on hub cell fate shortly after initial SGP specification and before gonad coalescence, much earlier than previously thought.
MATERIALS AND METHODS
Fly stocks
Heterozygous siblings or w1118 were used as controls as appropriate. We analyzed gonads from the following mutants, or involving these transgenic lines: N264.39 (FBal0029934), Nts1 (FBal0012887), UasNACT (Go et al., 1998), paired-Gal4 (FBal0048793), DlRF (Parody and Muskavitch, 1993), SerRX82 (FBal0030223), SerRX106 (FBal0030221), nanos-Gal4-vp16 (from Erica Selva, University of Delaware, Newark, DE, USA), DlRev10SerRX82 (FBal0029366/FBal0030223), trachealess10512 (FBal0009624), trachealess2 (FBal0017037), fogS4 (Tepass and Hartenstein, 1994), hsp70-Notch-Gal4-VP16 (Struhl and Adachi, 1998), hsp70-Dl (Gary Struhl), Uas-lacZ-nls (Bloomington Stock Center), esg-lacZ (Gönczy et al., 1992), Uas-Dl-dsRNA (FBgn0000463), drm-Gal4 (Green et al., 2002), P{GawB}48Y-Gal4 for endoderm expression (FBti0004594) and Twist-Gal4 (FBal0040491). Stocks were balanced over CyO P{w+ Ubi-gfp} or TM6 Hu P{w+ Ubi-Gfp}.
Immunostaining
Embryos were collected on apple agar plates and aged 22-24 hours in a humidified chamber to 1st instar larvae. Hatched larvae were dissected in half with tungsten needles in Ringers solution and the internal organs were gently massaged out. Unhatched larvae were dechorionated, hand-devitellinized and dissected as above. Tissue was fixed in 4% formaldehyde, Ringers and 0.1% Triton-X-100 for 15 minutes, washed in PBTX and blocked for 1 hour at room temperature in 2% normal donkey serum/normal goat serum. Primary antibodies were used overnight at 4°C. Secondary antibodies were used at 1:400 (Alexa488, Cy3 or Cy5; Molecular Probes; Jackson ImmunoResearch) or 1:1000 (biotinylated; Invitrogen) for 1 hour at room temperature. DNA was stained with Hoechst 33342 (Sigma) at 0.2 μg/ml for 2 minutes.
Immunostaining for testes was performed as previously described except 1× PBS was substituted for Buffer B (Terry et al., 2006). For embryo studies, embryos were collected, aged for the appropriate time in a humidified chamber, fixed in 4% paraformaldehyde and heptane for 15 minutes and devitellinized with methanol.
The following primary antibodies and concentrations were used: rabbit anti-Vasa 1:5000 (a gift from R. Lehmann, Skirball Institute, New York, USA), goat anti-Vasa 1:400 (Santa Cruz), chick anti-Vasa 1:5000-10,000 (K. Howard, University College London, UK), guinea pig anti-Traffic Jam 1:10,000 (Dorothea Godt, University of Toronto, Canada), mouse anti-βgal 1:10,000 (Promega), rabbit anti-STAT 1:1000 (Erika Bach, NYU Langone Medicine Centre, New York, USA), rat anti-Filamin-N terminal 1:1000 (Lynn Cooley; recognizes full length isoforms), rat anti-Filamin-C terminal 1:1000 (Lynn Cooley, Yale University, New Haven, CT, USA; recognizes C-terminal isoform), rat anti-Serrate 1:1000 (K. Irvine, Rutgers, NJ, USA), mouse anti-Delta C594.9B (Developmental Studies Hybridoma Bank), Streptavidin-HRP 1:400 (Chemicon), mouse-anti Biotin 1:1000, rabbit anti-Sox100B 1:1000 (S. Russell, University of Cambridge, UK), mouse anti-1B1 1:20 (DSHB); mouse anti-Sxl 1:25 (DSHB).
Tyramide amplification was used to increase the anti-lacZ staining. Samples were incubated with a biotinylated secondary antibody for 1 hour, washed and followed by a 25-minute incubation in SA-HRP. After a final washing, a 15-minute incubation in tyramide-Fluorescein was employed (PerkinElmer).
Sex identification, genotyping and staging of embryos
Male embryos and larvae without a gonad coalescence defect were unambiguous owing to the larger size of the gonad. For other cases, embryo or larvae sex was determined by immunostaining with Sex lethal. Balancer chromosomes containing a GFP-transgene P{w+ TM6 Hu ubi-GFP} or P{w+ ubi-GFP} were used to distinguish between heterozygous and homozygous mutant larvae. Larvae and embryos mutant for Notch or Delta were identified by their obvious neurogenic phenotype. Embryos were staged according to Campos-Ortega and Hartenstein (Campos-Ortega and Hartenstein, 1985).
In situ hybridization
Biotin-labeled probes (not size-reduced) were synthesized from cDNA plasmids obtained from the BDGP collection or the DGRC. In situ hybridization was performed as described previously (Terry et al., 2006). Hybridization signal was revealed by immunofluoescent detection using anti-Biotin (1 hour), washed four times (20 minutes each) in PBS containing 0.1% Tween-20, and incubated in a Cy3 secondary antibody (1 hour). Embryos were then blocked for at least 30 minutes and then immunostained for various antigens.
Counting the number of hub cells and germline stem cells
To count hub cell number, larval gonads were stained as needed, and also with anti-Filamin and Hoescht, and z-stacks were obtained through the depth of the gonad using a Zeiss Axioplan with an ApoTome attachment. Nuclei that were surrounded by a Filamin signal were counted as hub cells.
To count germline stem cells, larval gonads were double stained with anti-Vasa and anti-STAT or anti-1B1 antibodies. Germ cells that were directly adjacent to the hub and that accumulated STAT protein or had a dot spectrosome were scored as stem cells.
Notch rescue
We noticed that in the absence of a heat shock, hub cells were specified at a low frequency, indicating that there is leaky expression of the hsp70-Notch-Gal4-VP16 transgene. We therefore delivered a set of three heat shocks to induce robust expression of the receptor. Embryos were collected for 1 hour and aged at 25°C until the heat shock. Heat shocks at 37°C were delivered to embryos beginning at either 5-6 hours after egg lay (AEL) or 8-9 hours AEL. A recovery period of 45 minutes followed each 40-minute heat shock. Embryos were processed after aging at 25°C until they reached hatching stage.
Measuring cell size and distance
SGP cell nuclei and cell distances between SGPs and PMG cells were measured by using the Length tool in AxioVision. During stages 11-12, the diameter of the SGP nucleus is ~5-6 μm.
RESULTS
Notch signaling specifies hub cell fate
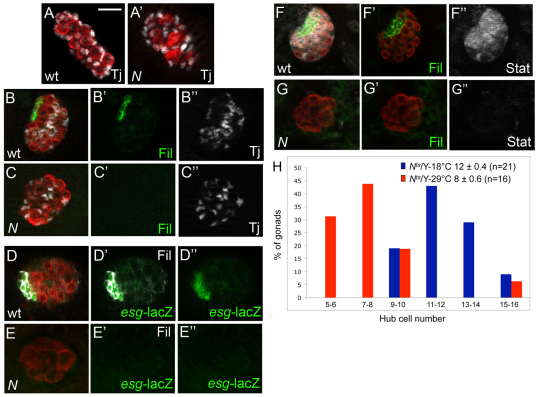
To test whether the Notch pathway was necessary to specify hub cell fate, we examined Notch mutants. We scored hub cell number shortly after larvae hatch, in animals aged 22-25 hours after egg lay (AEL) (Le Bras and Van Doren, 2006). Gonads were stained for germ cells (Vasa), for somatic cells (Traffic jam) and for hub cells, using either a cytoskeletal or gene expression marker. For example, in wild-type gonads, hub cells accumulate high levels of the F-actin-binding protein Filamin (Tanentzapf et al., 2007) and are circumscribed by a rosette of germ cells (Fig. 1B, green). We quantitated total hub cell number by stepping through z-slices in the image stack. In controls, we observed an average of 11 hub cells per gonad [11±0.3 (s.e.m.); n=12; Fig. 1B]. However, Filamin-positive hub cells were not detected in gonads from N264.39 mutant larvae (n=35; Fig. 1C). In addition, larvae carrying a hypomorphic mutation of Notch, Nts1, exhibited reduced hub cell number when grown at non-permissive temperature compared with controls (Fig. 1H; 8±0.6 versus 12±0.4, respectively; P<0.0001; we consistently found slight differences in the average hub cell number among various control genotypes, and attribute this variation to differences in genetic background. Consequently, we always report the data compared with sibling controls.). Importantly, in Notch mutants the proper number of somatic gonadal precursor cells (SGPs) were specified as stage-matched N264.39 mutants and wild-type embryos had comparable numbers of Tj-positive cells (Fig. 1A; averaging 39±2.3 versus 42±0.9, respectively; P=0.3). This indicates that although the precursor population is properly specified, SGPs cannot adopt hub cell fate in the absence of Notch. Additionally, Notch mutations did not affect the specification of posterior male-specific SGPs (data not shown). This reveals that SGPs can properly differentiate into other specialized somatic cell types within the gonad. Thus, Notch signaling appears to be specifically required for hub cell specification.
Fig. 1.
Notch signaling specifies hub cell fate. Anterior is towards the left in each panel. Gonads were stained with Vasa (red, germ cells). (A,A′) Stage 13 male embryos showing Traffic jam (white, SGPs) at the onset of coalescence. Controls [A; +/Y (n=18)] and Notch mutants [A′; N264.39/Y (n=14)] have a similar number of SGPs specified (41.5 and 39.3, respectively; P=0.30). (B-C″) First instar larval gonads showing Filamin (green, hub) and Traffic jam (white, somatic cells). (B-B″,D-D″,F-F″) In controls (+/Y) the hub is outlined by Filamin. (C-C″) In N264.39/Y gonads, the hub is absent (C'); however, somatic cells are still present (C,C″). (D-E″) +/Y and N264.39/Y larval gonads expressing an esgG66B enhancer trap. Gonads were stained with Filamin (white) and anti-β-gal (green). In control gonads (D-D″), both Filamin and esg detect hub cells. However, in N264.39/Y gonads (E-E″), most esg-positive cells are lost and Filamin staining is rarely observed. (F-G″) Gonads were stained with Filamin (green) and Stat (white). (F-F″) In +/Y gonads, Stat protein accumulates in neighboring somatic and germline cells and in the hub. (G,G″) In N264.39/Y gonads, Stat accumulation decreases drastically, indicating the lack of productive upd signaling. Scale bar: 10 μm. (H) The distribution of the number of Filamin-positive hub cells per gonad. There is a significant shift to lower hub cell numbers under non-permissive conditions for Nts (red) compared with control gonads (blue) raised at the permissive temperature (P<0.0001). The average number of hub cells per gonad (±s.e.m.) and the number of gonads (n) observed is also shown.
As an additional test for a role of Notch in hub cell specification, we assayed larval gonads using an enhancer trap at escargot (esg), a gene expression marker of hub cell fate (Le Bras and Van Doren, 2006). In control gonads, all Filamin-enriched cells were esg positive (Fig. 1D). By contrast, we observed a drastic reduction in the number of esg-expressing cells specified in N264.39 mutant gonads. Approximately 50% of gonads exhibited no esg-lacZ expression (8/17), whereas the remainder had two or fewer esg-lacZ-expressing cells (Fig. 1E). It is known that esg is detected in a number of anterior SGPs before its expression becomes restricted down to the hub during late embryogenesis (Gönczy et al., 1992; Le Bras and Van Doren, 2006). Given this, it is possible that the absence of Notch activity results in the loss of some early expressing esg-positive cell types, but there exist no specific markers for such cells to definitively establish this.
Finally, hub cells express Unpaired (Kiger et al., 2001; Tulina and Matunis, 2001), which activates the Jak-STAT pathway in adjacent somatic and germline cells (Sheng et al., 2009). One readout of pathway activation is the stabilization and accumulation of STAT protein (Chen et al., 2002). In controls, STAT protein accumulated at high levels in somatic and germline cells adjacent to the hub, as well as in hub cells themselves (Fig. 1F) (Sheng et al., 2009). By contrast, in N264.39 mutant gonads, STAT accumulation was undetectable (Fig. 1G). Taken together, we conclude that Notch signaling is necessary for proper hub cell specification.
Notch is activated within the SGP population
To determine whether SGPs within the developing embryonic gonad were activated for the Notch pathway, and whether such cells eventually contribute to the hub, we employed a Notch reporter. We used a reporter construct encoding a chimeric Notch-GAL4-VP16 receptor (under control of a hsp70 promoter). Upon heat shock, the chimera will be expressed on all cells. Subsequently, in any cells activated for Notch, processing of its intracellular domain will also release Gal4-VP16, which can induce expression of a UAS-lacZ transgene. By the time of gonad formation during embryonic stage 13, we were able to detect reporter activation in a subset of SGPs (Fig. 2A). Indeed, if such embryos were aged until the hub formed, and stained for β-galactosidase protein, we found that Notch-activated SGPs could become hub cells (Fig. 2B, arrows; 50% of hub cells were lacZ-positive; n=16). These data showed that Notch is activated in a subset of SGPs, and that such cells can contribute to the hub. Interestingly, we also noted that Notch-activated cells were not restricted to the anterior of the developing gonad, but were also found in the middle and posterior (Fig. 2A). However, receptor tyrosine kinase (RTK) pathways active in the posterior of the gonad antagonize Notch, probably preventing these middle and posterior activated cells from adopting hub cell fate (Kitadate and Kobayashi, 2010) (see Discussion).
Fig. 2.
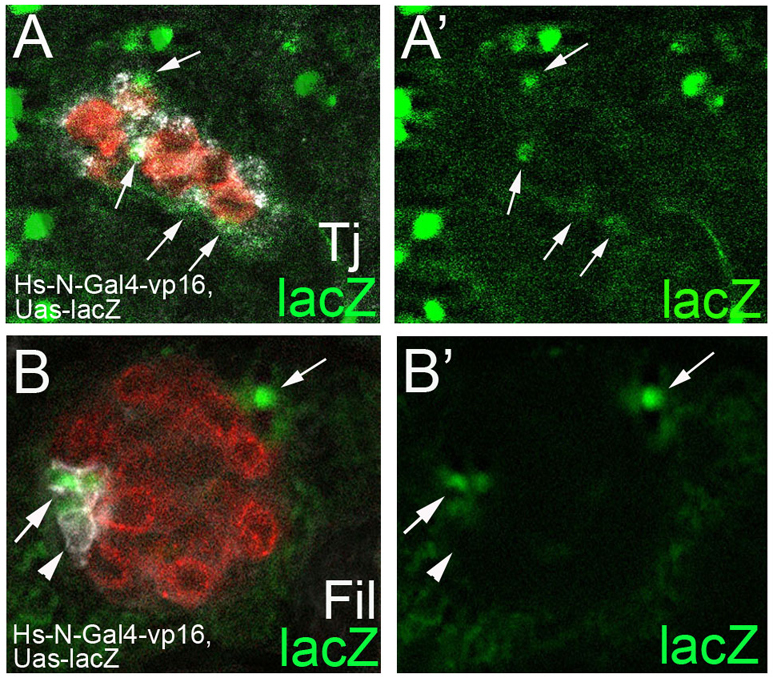
Notch activated SGPs contribute to the hub. (A,A′) Stage 13 male embryonic gonad. Notch reporter activation was assessed using the hsp70-Notch–Gal4-vp16; Uas-lacZ-nls reporter construct. Gonad showing Notch-activated lacZ-positive cells (green) that co-stain with Traffic jam (white) (arrows) and Vasa (red, germ cells). lacZ-positive cells are dispersed throughout the gonad. (B,B′) Cells activated for Notch during embryogenesis (green) contribute to the hub (Filamin, white) in the 1st larval instar gonad. Arrows indicate lacZ-positive cells. Arrowhead indicates lacZ-negative hub cells. Note that a lacZ-positive cell is also found at the posterior of the gonad. Thus, it is possible that Notch signaling also contributes to some gonadal sheath cells.
Hub cells are specified before gonad coalescence
We next wanted to identify the stage of gonadagenesis at which Notch is required to specify hub cell fate. It was previously thought that hub cell specification occurred after gonad coalescence, once germ cells and SGPs had formed a contiguous tissue (Le Bras and Van Doren, 2006). To perform our experiments, we again took advantage of the hsp70-Notch-GAL4-VP16 chimera, which functions as a wild-type receptor. In fact, delivering three heat shocks was sufficient to rescue formation of the ventral epidermis in Notch mutant embryos (Struhl and Adachi, 1998). We expressed the transgene in a Notch mutant background and assayed for the rescue of hub cell specification in larval gonads. To activate the receptor globally, we delivered three 40-minute heat shocks, each followed by a 45-minute recovery period at 25°C. Embryos that received the first heat pulse at 8-9 hours AEL (mid-stage 12) appeared similar to non-heat shocked controls. In both cases, more than two-thirds of the gonads analyzed lacked any hub cells (Fig. 3, compare yellow with blue bars). Note that a few hub cells were observed among non-heat shocked Notch-null embryos that carried the hsp70-Notch transgene (never more than 7 specified per gonad). As this is the same Notch null allele as in Fig. 1, the occasional hub cell was probably due to leaky expression of the hsp70-Notch transgene. The slightly different distribution we observed comparing non-heat shocked and late heat shocked embryos (8-9 hours AEL) is probably attributable to subtle variation in the leaky transgene expression. By contrast, we found that embryos that received the first heat pulse at 5-6 hours AEL (early-mid stage 11) exhibited significant rescue of hub cells (Fig. 3, red). In fact, 65% of gonads had five or more hub cells specified (19/29 gonads), and almost half reached our observed wild-type range of hub cells (9-14 hub cells, 13/29 gonads; Fig. 3, red). The fact that significant rescue occurred only upon early expression of Notch, suggested that hub cell specification occurred much earlier than previously appreciated, probably late-stage 11 and 12.
Fig. 3.
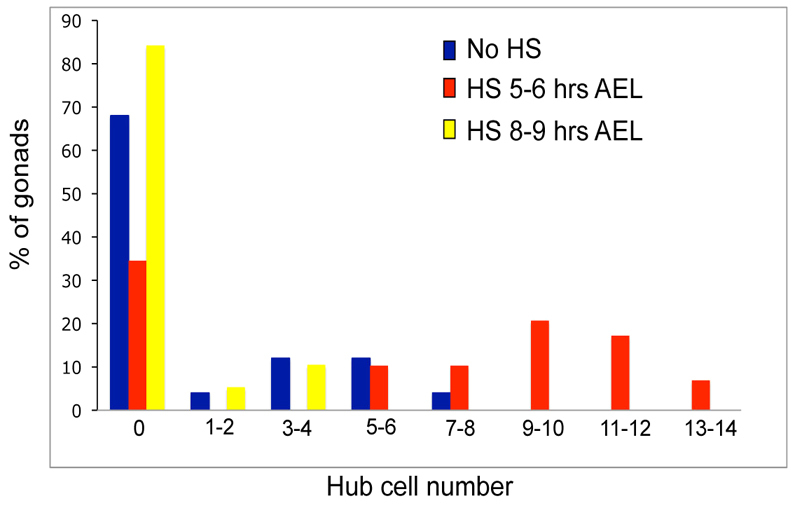
Notch activity is required before gonad coalescence to specify hub cell fate. A graph of the number of N264.39/Y; hsp70-Notch-Gal4-VP16 gonads with Filamin-positive hub cells. In this background, control gonads receiving no heat shock (blue, n=25) still have a small number of hub cells specified, indicating leaky transgene expression. The rescuing heat shock began at 5-6 hours (red, n=29) or 8-9 hours (yellow, n=19) after egg lay (AEL). There is a significant rescue of hub cells when the heat shock occurs at 5-6 hours AEL (red).
Serrate and Delta both contribute to hub cell fate
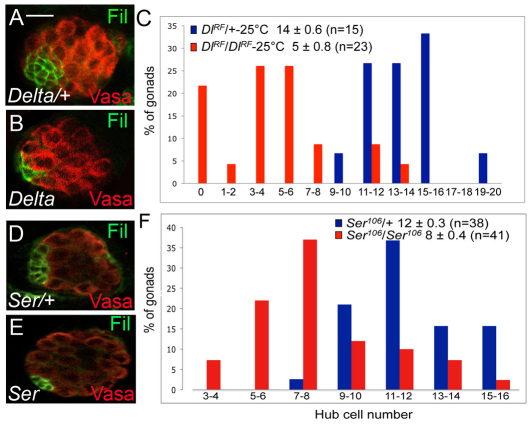
In Drosophila, there are two ligands that can activate the Notch receptor, Delta and Serrate. To determine their respective contribution to hub specification, we assayed larval gonads singly mutant for either ligand. We could not confidently score hub cell number in doubly mutant embryos owing to a severe germ cell migration defect. Germ cell migration was also severely disrupted in Delta-null mutant embryos, preventing the confidant analysis of hub cell phenotypes (Vässin and Campos-Ortega, 1987; Moore et al., 1998). We therefore assayed larval gonads that were homozygous for a hypomorphic mutation in Delta: DlRF. Delta-deficient larvae had a 70% reduction in hub cell number compared with control gonads (Fig. 4A-C; averaging 5±0.8 versus 14±0.6, respectively; P<0.0001). The effects of Serrate mutations were more modest in our hands, exhibiting a 30% decrease in hub cell number (Fig. 4D-F; averaging 8±0.4 for SerRX106 versus 12±0.3, respectively, P<0.0001; data not shown for SerRX82). This suggests that although both ligands contribute to hub cell specification, Delta has a more prominent role in this process.
Fig. 4.
Serrate and Delta both contribute to hub cell fate. (A,B,D,E) First larval instar gonads from (A) Dlts/+, (B) Dlts/Dlts, (D) Ser106/+ and (E) Ser106/Ser106 raised at 25°C. Filamin (green, hub) and Vasa (red, germ cells). Scale bar: 10 μm. (C,F) Distribution of the number of hub cells in Dlts/+ (blue) and Dlts/Dlts (red) gonads (C, P<0.0001 by Student's t-test) and Ser106/+ (blue) and Ser106/Ser106 (red) gonads (F, P<0.0001) is shown. The average number of hub cells per gonad (±s.e.m.) and the number of gonads (n) observed is also shown.
The posterior midgut activates Notch in developing SGPs
We next attempted to identify the source of the Notch ligand(s). We observed that forced expression of Delta using a mesodermal driver, Twist-Gal4, led to a 14% increase in hub cell number compared with controls (averaging 14±2.2, n=30 versus 12±2.5, n=18; P=0.027). Similarly, misexpressing Serrate from germ cells using the Nanos-Gal4 driver led to an increase in hub cell number compared with controls (averaging 14±1.5 versus 11±1.3, respectively; P=0.01). Although these gain-of-function experiments supported the notion that activation of the Notch pathway among SGPs could direct them to select hub cell fate, they do not establish which cells normally express the ligand(s). In fact, in our hands, neither Serrate nor Delta expression was detectable within the gonad [Fig. 5 (but see Kitadate and Kobayashi, 2010)]. We, thus, turned our attention to adjacent tissues as potential sources.
Fig. 5.
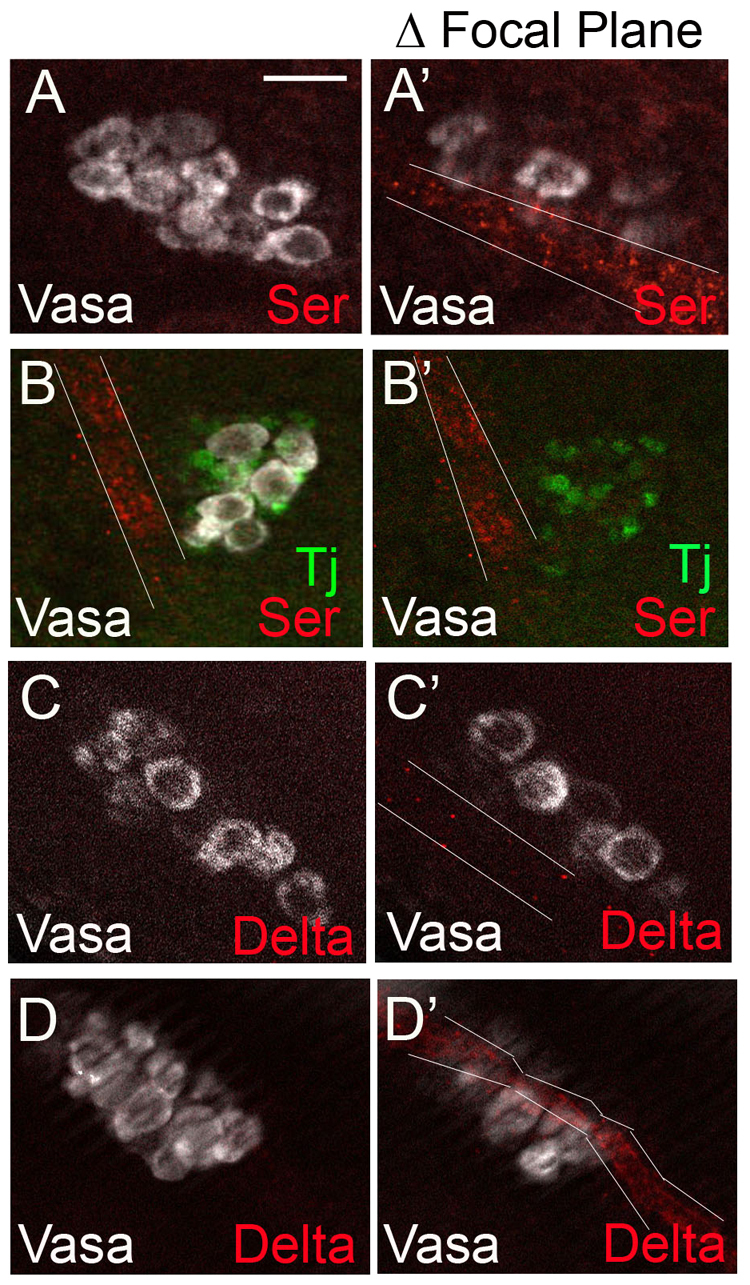
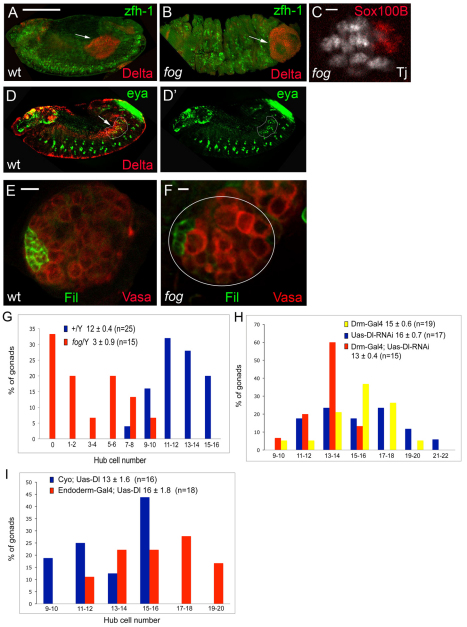
Notch ligands are expressed on neighboring tracheal cells. (A-D) Wild-type male gonads showing Vasa (white) to reveal germ cells. Ligand-expressing tracheal cells are highlighted with white lines. (A,A′) A stage 14 male gonad showing Vasa and fluorescent in situ hybridization to Serrate mRNA (red). (B,B′) A stage 15 male gonad showing Vasa, Traffic Jam (green, somatic cells) and Serrate (red). (C,C′) A stage 14 male gonad showing Vasa and fluorescent in situ hybridization to Delta mRNA (red). (D,D′) A stage 14 male gonad showing Vasa and Delta (red). Serrate mRNA (A), Serrate protein (B), Delta mRNA (C) and Delta protein (D) are not detected within the gonad proper, but are expressed from an adjacent stripe of tracheal cells in a different focal plane (A′,B′,C′,D′). Scale bar: 10 μm.
Beginning at stage 13, the gonad coalesces in very close proximity to the developing trachea, which expresses a high level of both Delta and Serrate mRNA and protein (Fig. 5). We found, however, that the loss of the trachea in trachealess or breathless mutants did not appear to affect hub cell number (data not shown). This suggests that signaling from the trachea is not necessary to specify hub cell fate.
It is known that Delta is highly expressed in the posterior midgut (PMG; Fig. 6, arrows) (Tepass and Hartenstein, 1994). SGPs, as identified by the nuclear protein eyes absent (eya) (Boyle et al., 1997), are positioned very close to the PMG, beginning at stage 11 when they are initially specified and through the end of germ band retraction at late stage 12 (Fig. 6D). During this period, the SGPs passively move past the gut, and PMG cells and SGPs are found in the same focal plane (Fig. 6D). The SGPs closest to the PMG are only 3-6 μm away, well within the range of distances reported for productive Delta-Notch signaling (up to 15 μm) (de Joussineau et al., 2003). These data suggests that the PMG cells are close enough to activate Notch in SGPs.
Fig. 6.
The posterior midgut (PMG) is necessary for proper hub cell specification. (A,B) Lateral view of a z-section through stage 12 male embryos from (A) wild type and (B) fog showing Delta (red, PMG; arrows) and zfh-1 (green, somatic cells). In the fog mutant (B), the PMG develops on the exterior of the embryo. Scale bar: 100 μm. (C) Stage 16 fog mutant male embryo showing Sox100B (red, msSGPs) and Traffic jam (white, SGPs). Scale bar: 10 μm. (D,D′) Lateral view of a z-slice through a stage 12 wild-type male embryo showing Delta (red, PMG; arrows) and eyes absent (green, SGPs; encircled in white). z-slice: 0.7 μm. Scale bar (in A): 100 μm. (E,F) 1st larval instar male gonads from +/Y (E) and fog/Y (F). Filamin (green, hub cells) and Vasa (red, germ cells). One gonad is outlined in E; a second lies just up and to the right. Fewer germ cells contribute to the fog/Y larval gonad. Scale bars: 10 μm in D; 5 μm in E. (G) Distribution of the number of hub cells in +/Y (blue) and fog/Y (red) is shown (P<0.0001). The average number of hub cells per gonad ±s.e.m. and the number of gonads (n) observed is also shown. (H) Distribution of the number of Filamin-positive hub cells in Uas-Dl-RNAi (blue), Drm-Gal4 (yellow) and Drm-Gal4; Uas-Dl-RNAi (red) gonads is shown. Note the decreased hub cell number in Drm-Gal4; Uas-Dl-RNAi gonads (P<0.05) compared with controls, Drm-Gal4 and Uas-Dl-RNAi gonads. The average number of hub cells per gonad (±s.e.m.) and the number of gonads (n) observed is also shown. (I) Distribution of the number of hub cells in cyo;Uas-Dl (blue) and Endoderm-Gal4;Uas-Dl (red) is shown (P<0.005). The average number of hub cells per gonad (±s.e.m.) and the number of gonads (n) observed is also shown.
We first attempted an endoderm-specific knockdown of Delta. Driving Delta dsRNA using either a midgut (Fig. 6H) or an endoderm driver (data not shown) led to a reduction of ~20% in hub cell number. This small decrease was perhaps due to the inefficiency of knockdown, as we observed residual Delta protein on gut cells (data not shown). For example, embryos expressing dsRNA to Delta driven by Drm-Gal4 averaged 13±0.4 hub cells compared with 16±0.7 for Uas-Dl-RNAi alone and 15±0.6 for Drm-Gal4 alone (Fig. 6H; P<0.05).
As an independent test of whether Delta-expressing PMG cells contribute to hub cell specification, we assayed folded gastrulation (fog) mutants (Tepass and Hartenstein, 1994). In fog mutant embryos, the posterior midgut is not internalized and instead develops on the exterior of the embryo (Fig. 6B), although all other cell types develop normally. Such fog mutant gonads displayed a 70% decrease in hub cell number, scored using either Filamin or esg-lacZ (Fig. 6E-G; 12±0.4 versus 3±0.9, respectively; P<0.0001; data not shown). Importantly, the phenotype was selective for hub cells, as a distinct intragonadal cell type, msSGPs, were specified normally in fog mutants (Fig. 6C). In addition, normal numbers of SGPs were specified, as sibling controls and fog mutant embryos at stage 13 had a similar number of Traffic jam-positive SGPs (32±1.5 versus 31±0.9, respectively; P=0.71). Thus, the absence of hub cells in fog mutants was consistent with the proposal that the proximity of endoderm to the SGPs was essential for hub specification. Furthermore, over expressing Delta from the endoderm resulted in a 20% increase in hub cell number overcontrols (Fig. 6I; averaging 16±1.8 versus 13±1.6, respectively; P<0.005). This indicates that Delta specifically expressed from the PMG is not only necessary for hub cell specification, but its overexpression can cause an increase in hub cell number. Taken together, our findings implicate the endoderm in delivery of Delta to activate Notch for hub cell specification among SGPs.
DISCUSSION
Stem cell niches are inferred to exist for many tissues. However, the difficulty in unambiguously identifying niche cells has left unanswered when and how these niches are specified. Here, we have identified the Notch pathway as key in the specification of a crucial component of the Drosophila male testis niche: the hub cells. We find that hub cells are specified before gonad coalescence, earlier in development than previously appreciated. Furthermore, our data suggest that Delta-expressing endoderm cells are crucial for proper hub cell specification. This demonstrates tissue non-autonomous regulation of this niche.
The role of Notch signaling in hub cell specification
Our data reveal that Notch signaling is necessary to specify hub cell fate. A similar conclusion has recently been reached by Kitadate and Kobayashi (Kitadate and Kobayashi, 2010). It is interesting to note that in three well-characterized stem cell-niche systems in Drosophila, including the transient niche for adult midgut progenitors, the female gonad and now the developing male gonad, Notch signaling is directly responsible for niche cell specification (Mathur et al., 2010; Kitadate and Kobayashi, 2010; Ward et al., 2006; Song et al., 2007). Moreover, Notch has been found to play a role in the maintenance of various mammalian stem cell populations, including neural stem cells, HSCs and hair follicle stem cells (for reviews, see Chiba, 2006; Schmidt et al., 2009; Burns et al., 2005; Vauclair et al., 2005). However, owing to difficulty in performing lineage-specific knockouts in these systems, it remains unclear which cells require Notch activity. As the various cases in Drosophila all require direct Notch activation for niche cell specification, perhaps this reveals a conserved role for Notch signaling in other, more complex stem cell systems.
Notch signaling specifies niche cells in both the male and female Drosophila gonad; however, it is important to note that there are still some differences. For the ovary, only Delta is required to activate the Notch receptor for proper niche cell specification (Ward et al., 2006; Song et al., 2007). For the testis, we find that both ligands contribute to the process, although, here too, it appears that Delta is the dominant ligand employed (Fig. 4). Interestingly, depleting Delta or (genetically) separating the endoderm from SGPs both led to a 70% reduction in hub cell number, while depleting Serrate yielded a 30% reduction. Perhaps Delta-Notch signaling from the endoderm accounts for two-thirds of hub cell specification, while Serrate-Notch signaling accounts for only one-third of this process. Although we were unable to identify the source of Serrate, Kitadate and Kobayashi (Kitadate and Kobayashi, 2010) have shown that Serrate mRNA is expressed from SGPs after gonad coalescence. Perhaps, this late expression accounts for the modest role Serrate plays in hub specification. Those authors did not explore in detail a potential role for Delta in hub specification, and our data suggests that that role is carried out at earlier stages, and from outside the gonad proper.
Second, in the ovary, cells within the developing gonad appear to present the Notch-activating ligand, although it is unclear whether germ cells or somatic cells are the source of Delta (Ward et al., 2006; Song et al., 2007). Here, our data suggests that cells from a distinct germ layer, the endoderm, present Delta to SGPs in the male gonad. These differences may indicate distinct evolutionary control over gonadal niche development between the sexes.
Hub cell specification occurs early, before gonad coalescence
Although the gonad first forms during mid-embryogenesis, hub cells only become identifiable just prior to hatching of the larvae, some 6 hours later (Le Bras and Van Doren, 2006). At that time, hub cells begin to tightly pack at the anterior of the gonad, upregulate several cell adhesion and cytoskeletal molecules (Fascilin 3, Filamin, DN-Cadherin, DE-Cadherin) as well as induce Upd expression and other markers of hub fate (Le Bras and Van Doren, 2006; Tanentzapf et al., 2007). Surprisingly, our data reveal that most hub cells are specified well before these overt signs of hub cell differentiation, as judged by Notch reporter activation and Notch rescue (Figs 2 and 3). Although it was previously thought that SGPs were equivalent at the time of gonad coalescence (Le Bras and Van Doren, 2006) it is now clear that due to Notch activity, the SGPs are parsed into a group of either hub cells or cyst cells before gonad coalescence occurs.
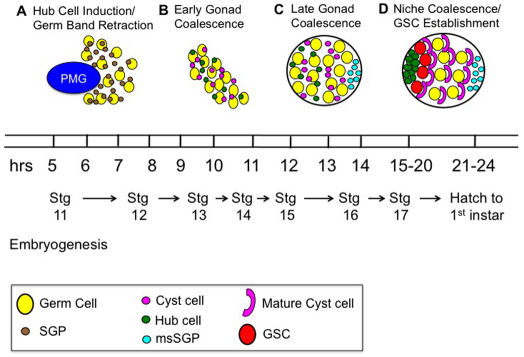
Thus, we believe that a series of steps must occur before the hub can function as a niche. First, the PMG presents Delta, leading to Notch activation in some SGPs as they are carried over these endodermal cells during germ band retraction (Fig. 7). Activation might be dependent on, for example, length of time in contact with passing PMG cells. At the present time, it is unclear whether all SGPs are activated for Notch (Kitadate and Kobayashi, 2010), or only some of them (this work). Second, after gonad coalescence, activated SGPs must then migrate anteriorly (this work) (LeBras and Van Doren, 2006; Kitadate and Kobayashi, 2010). Although it is known that integrin-mediated adhesion is required to maintain the hub at the anterior (Tanentzapf et al., 2007), no cues have been identified that could guide the migration of the Notch-activated SGPs. Third, as the cells reach the anterior of the gonad they must execute a mesenchymal-to-epithelial transition, as evidenced by the upregulation of cell-adhesion molecules and preferential associations between hub cells (DeFalco et al., 2003; Le Bras and Van Doren, 2006). This step occurs independently of the integrin-mediated anchoring at the anterior. Finally, the hub cells must induce Upd expression and recruit neighboring cells to adopt stem cell fate (Sheng et al., 2009). The apparent delay between the activation of the Notch pathway and the initiation of the hub cell gene expression program might suggest that initiating that hub program first requires that the cells coalesce into an epithelium. Such a mechanism would prevent precocious or erroneous stem cell specification within the gonad.
Fig. 7.
Model for hub cell specification in the male gonad. (A) SGPs (brown) become Notch-activated as they passively travel by Delta-expressing PMG cells (dark blue) during germ band retraction. (B) During early gonad coalescence as germ cells (yellow) and SGPs form a contiguous tissue, SGPs begin to differentiate into either hub (green) or cyst cells (purple). (C) During late gonad coalescence, Notch-activated hub cells must migrate towards the anterior. Sox100B-positive male-specific SGPs (light blue) join the gonad. (D) During the last stage of embryogenesis, stage 17, the niche cells execute a mesenchymal-to-epithelial transition, upregulate cell-adhesion molecules and induce Unpaired expression, establishing germline stem cells (GSCs, red).
Although our data reveal Notch-activated SGPs at all positions within the gonad and that some of these become hub cells, it is unclear how hub cell number is tightly regulated. Potentially, SGP migration over endodermal cells could induce Notch activation among SGPs throughout the forming gonad, potentiating these cells to become hub cells. However, solely relying on that mechanism could lead to the specification of too many hub cells. It appears, though, that specification is regulated by EGFR pathway activation (Kitadate and Kobayashi, 2010). The authors have recently shown that EGFR protein is observed on most SGPs throughout the embryonic gonad, beginning at gonad coalescence (stage 13). The EGFR ligand Spitz is expressed from all germ cells during gonad coalescence and activates EGFR among posterior SGPs. This activity antagonizes Notch and that appears to regulate final hub cell number. How EGFR activation is restricted or enhanced only among posterior SGPs is at present unclear (Kitadate and Kobayashi, 2010).
Given that we find that hub cell specification occurs prior to gonad coalescence, it is also possible that Notch and EGFR act in a temporal sequence. In this case, early Notch-activated SGPs, perhaps even those in the posterior will adopt hub cell fate. But, as EGFR becomes activated, further induction of the Notch pathway in the posterior is antagonized, prohibiting the specification of too many hub cells. Such a temporal inhibition might be important, as Serrate is expressed on the SGPs (Kitadate and Kobayashi, 2010) and both Delta and Serrate are robustly expressed on tracheal cells (Fig. 5), the activity of which might otherwise lead to excess hub cell induction. Last, perhaps during later stages of gonadogenesis (stages 14-16) a small number of anterior SGPs become Notch activated due to the activity of Serrate-Notch signaling from other SGPs, supplementing the hub cells previously specified by Delta-Notch signaling.
Endoderm induction of hub cells
Given that niche cells in the Drosophila ovary become activated via Delta-Notch signaling by neighboring somatic cells, we initially expected that Notch would be activated in a subset of SGPs by ligand presented from other SGPs (Song et al., 2007). However, we could not detect Delta nor Serrate expression among SGPs. Furthermore, although nearby tracheal cells expressed both ligands robustly, that expression appears later than our Notch rescue suggests would be necessary, and genetic ablation of tracheal cells did not influence hub cell number.
Instead, we found that a crucial signal for niche cell specification is presented from the endoderm, as Delta is expressed robustly on posterior midgut cells, at a time consistent with the requirement for Notch function. Furthermore, these endodermal cells are close enough to SGPs for productive Delta-Notch signaling to occur (Fig. 6D). Although visceral mesodermal cells are also close to the PMG and the SGPs (Azpiazu and Frasch, 1993; Tepass and Hartenstein, 1994; Boyle et al., 1997; Broihier et al., 1998), this tissue does not affect hub specification, as we found that brachyenteron mutants exhibited normal hub cell number (data not shown). By contrast, in mutants that do not internalize the gut (fog), and thus would not present Delta to SGPs, we found a drastic reduction in hub cell number.
Additionally, we note that absolute hub cell number varies among animals and according to genetic background (Kitadate et al., 2007; Wallenfang et al., 2006) (this work). We attribute this to normal biological variation, just as germline stem cell number varies (Wallenfang et al., 2006). Potentially, this variation could be caused by the robustness with which the Notch pathway is activated in SGPs, as they are carried over the midgut cells. It will be interesting to test this hypothesis by genetically manipulating the number of midgut cells or the time of contact between endoderm and SGPs. Additionally, the antagonistic effects of EGFR signaling might account for some of the observed variation. In fact, gonads heterozygous for Star, a component of the EGFR pathway, exhibit increased hub cell number (Kitadate and Kobayashi, 2010).
Finally, it is interesting to consider why the endoderm would be crucial for the proper specification of the GSC niche. In Drosophila, as in many animals, there is a special relationship between the gut and the germ cells. Primordial germ cells in mammals and in Drosophila must migrate through the endoderm to reach the gonadal mesoderm (for a review, see Richardson and Lehmann, 2010). In fact, in Drosophila, the gut exercises elaborate control over germ cell migration. As the germ cells begin their transepithelial migration and exit from the midgut pocket, tight connections between midgut cells are dissolved, allowing for easy germ cell passage (Jaglarz and Howard, 1994; Jaglarz and Howard, 1995). Germ cells then migrate on the basal surface of endodermal cells and midgut expression of wunens (which encodes lipid phosphate phosphatases) repels germ cells, driving them into the mesoderm (Starz-Gaiano et al., 2001; Zhang et al., 1997). Thus, the endoderm not only delivers germ cells to the somatic mesoderm, but our work reveals that the same endoderm specifies niche cells from among the somatic mesoderm wherein germ cells can subsequently develop into stem cells. In mammals, although the exact make-up of the spermatogonial stem cell niche has not been determined, it must (in part) derive from cells of the genital ridge. It will be interesting to determine whether proximity to the gut endoderm is important for the specification of this niche.
Acknowledgements
We thank members of the fly community, the Bloomington Stock Center and the DSHB for reagents. We are grateful to Mark van Doren, and members of the DiNardo and Ghabrial laboratories for helpful discussions and critical reading of the manuscript. This work was supported by the National Science Foundation pre-doctoral fellowship to T.C.O. and by NIH GM60804 to S.D. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Aboim A. N. (1945). Développement embryonnair et postembryonnair des gonads normale et agametiques de Drosophila melanogaster. Rev. Suisse Zool. 53, 53-154 [Google Scholar]
- Azpiazu N., Frasch M. (1993). tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 7, 1325-1340 [DOI] [PubMed] [Google Scholar]
- Berry L., Westlund B., Schedl T. (1997). Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development 124, 925-936 [DOI] [PubMed] [Google Scholar]
- Boyle M., DiNardo S. (1995). Specification, migration and assembly of the somatic cells of the Drosophila gonad. Development 121, 1815-1825 [DOI] [PubMed] [Google Scholar]
- Boyle M., Bonini N., DiNardo S. (1997). Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development 124, 971-982 [DOI] [PubMed] [Google Scholar]
- Broihier H. T., Moore L. A., Van Doren M., Newman S., Lehnmann R. (1998). zfh-1 is required for germ cell migration and gonadal mesoderm development in Drosophila. Development 125, 655-666 [DOI] [PubMed] [Google Scholar]
- Brookman J. J., Toosy A. T., Shashidhara L. S., White R. A. (1992). The 412 retrotransposon and the development of gonadal mesoderm in Drosophila. Development 116, 1185-1192 [DOI] [PubMed] [Google Scholar]
- Burns C. E., Traver D., Mayhall E., Shepard J. L., Zon L. I. (2005). Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 19, 2331-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega J. A., Hartenstein V. (1985). The Embryonic Development of Drosophila melanogaster. Berlin: Springer-Verlag; [Google Scholar]
- Chen H. W., Chen X., Oh S. W., Marinissen M. J., Gutkind J. S., Hou S. X. (2002). mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 16, 388-398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S. (2006). Course review: Notch signaling in stem cell systems. Stem Cells 24, 2437-2447 [DOI] [PubMed] [Google Scholar]
- Clark I. B., Jarman A. P., Finnegan D. J. (2007). Live imaging of Drosophila gonad formation reveals roles for Six4 in regulating germline and somatic cell migration. BMC Dev. Biol. 7, 52-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Joussineau C., Soulé J., Martin M., Anguille C., Montcourrier P., Alexandre D. (2003). Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature 426, 555-559 [DOI] [PubMed] [Google Scholar]
- DeFalco T., Camara N., Le Bras S., Van Doren M. (2005). Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Dev. Cell 14, 275-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T. J., Verney G., Jenkins A. B., McCaffery J. M., Russell S., Van Doren M. (2003). Sex-specific apoptosis regulates sexual dimorphism in the Drosophila embryonic gonad. Dev. Cell 5, 205-216 [DOI] [PubMed] [Google Scholar]
- Go M. J., Eastman D. S., Artavanis-Tsakonas S. (1998). Cell proliferation control by Notch signaling in Drosophila development. Development 125, 2031-2040 [DOI] [PubMed] [Google Scholar]
- Gönczy P., DiNardo S. (1996). The germ line regulates somatic cyst proliferation and fate during Drosophila spermatogenesis. Development 122, 2437-2447 [DOI] [PubMed] [Google Scholar]
- Gönczy P., Viswanathan S., DiNardo S. (1992). Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development 114, 89-98 [DOI] [PubMed] [Google Scholar]
- Green R. B., Hatini V., Johansen K. A., Liu X. J., Lengyel J. A. (2002). Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development 129, 3645-3656 [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Tokuyasu K. T., Lindlsey D. L., Garavito M. (1979). The germinal proliferation center in the testis of Drosophila melanogaster. J. Ultrastruct. Res. 69, 180-190 [DOI] [PubMed] [Google Scholar]
- Issigonis M., Tulina N., de Cuevas M., Brawley C., Sandler L., Matunis E. (2009). JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science 326, 153-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglarz M. K., Howard K. R. (1994). Primordial germ cell migration in Drosophila melanogaster is controlled by somatic tissue. Development 120, 83-89 [DOI] [PubMed] [Google Scholar]
- Jaglarz M. K., Howard K. R. (1995). The active migration of Drosophila primordial germ cells. Development 121, 3495-3503 [DOI] [PubMed] [Google Scholar]
- Jenkins A. B., McCaffery M., Van Doren M. (2003). Drosophila E-cadherin is essential for proper germ cell-soma interaction during gonad morphogenesis. Development 130, 4417-4426 [DOI] [PubMed] [Google Scholar]
- Kawase E., Wong M. D., Ding B. C., Xie T. (2004). Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131, 1365-1375 [DOI] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B., Fuller M. T. (2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542-2545 [DOI] [PubMed] [Google Scholar]
- Kimble J. E., White J. G. (1981). On the control of germ cell development in C. elegans. Dev. Biol. 81, 208-219 [DOI] [PubMed] [Google Scholar]
- Kitadate Y., Kobayashi S. (2010). Notch and EGFR signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc. Natl. Acad. Sci. USA 107, 14241-14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitadate Y., Shigenobu S., Arita K., Kobayashi S. (2007). Boss/Sev signaling from the germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonads. Dev. Cell 13, 151-159 [DOI] [PubMed] [Google Scholar]
- Lam N., Chesney M., Kimble J. (2006). Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Curr. Biol. 16, 287-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras S., Van Doren M. (2006). Development of the male germline stem cell niche in Drosophila. Dev. Biol. 294, 92-103 [DOI] [PubMed] [Google Scholar]
- Leatherman J. L., DiNardo S. (2008). Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3, 44-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman J. L., DiNardo S. (2010). Germline self-renewal requires cyst stem cells but stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 12, 806-811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D., Bost A., Driver I., Ohlstein B. (2010). A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327, 210-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. A., Broihier H. T., Van Doren M., Lunsford L. B., Lehmann R. (1998). Identification of genes controlling germ cell migration and embryonic gonad formation in Drosophila. Development 125, 667-678 [DOI] [PubMed] [Google Scholar]
- Morrison S., Spradling A. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parody T. R., Muskavitch M. A. (1993). The pleitropic function of Delta during postembryonic development of Drosophila melanogaster. Genetics 135, 527-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. E., Lehmann R. (2010). Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat. Rev. Mol. Cell Biol. 11, 37-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. H., Bicker F., Nikolic I., Meister J., Babuke T., Picuric S., Müller-Esterl W., Plate K. H., Dikic I. (2009). Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signaling and affects neural stem cell renewal. Nat. Cell Biol. 11, 873-880 [DOI] [PubMed] [Google Scholar]
- Sheng X. R., Posenau T., Gumulak-Smith J. J., Matunis E., Van Doren M., Wawersik M. (2009). Jak-STAT regulation of male germline stem cell establishment during Drosophila embryogenesis. Dev. Biol. 334, 335-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani A. A., Ingham P. W. (2003). Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr. Biol. 13, 2065-2072 [DOI] [PubMed] [Google Scholar]
- Song X., Call G. B., Kirilly D., Xie T. (2007). Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 134, 1071-1080 [DOI] [PubMed] [Google Scholar]
- Sonnenblick B. P. (1941). Germ cell movements and sex differentiation of the gonads in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 26, 373-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starz-Gaiano M., Cho N. K., Forbes A., Lehmann R. (2001). Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development 128, 983-991 [DOI] [PubMed] [Google Scholar]
- Struhl G., Adachi A. (1998). Nuclear access and action of Notch in vivo. Cell 93, 649-660 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Devenport D., Godt D., Brown N. H. (2007). Integrin-dependent anchoring of a stem-cell niche. Nat. Cell Biol. 12, 1413-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. (1994). Epithelium formation in the Drosophila midgut depends on the interaction of endoderm and mesoderm. Development 120, 579-590 [DOI] [PubMed] [Google Scholar]
- Terry N., Tulina N., Matunis E., DiNardo S. (2006). Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev. Biol. 294, 246-257 [DOI] [PubMed] [Google Scholar]
- Tulina N., Matunis E. (2001). Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546-2549 [DOI] [PubMed] [Google Scholar]
- Vässin H., Campos-Ortega J. A. (1987). Genetic analysis of Delta, a neurogenic gene of Drosophila melanogaster. Genetics 116, 433-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauclair S., Nicolas M., Barrandon Y., Radtke F. (2005). Notch1 is essential for postnatal hair follicle development and homeostasis. Dev. Biol. 284, 184-193 [DOI] [PubMed] [Google Scholar]
- Wallenfang M. R., Nayak R., DiNardo S. (2006). Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell 5, 397-304 [DOI] [PubMed] [Google Scholar]
- Ward E. J., Shcherbata H. R., Reynolds S. H., Fischer K. A., Hartfield S. D., Ruohola-Baker H. (2006). Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr. Biol. 16, 2352-2358 [DOI] [PubMed] [Google Scholar]
- Wawersik M., Milutinovich A., Casper A. L., Matunis E., Williams B., Van Doren M. (2005). Somatic control of germline sexual development is mediated by the JAK/STAT pathway. Nature 436, 563-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zhang J., Purcell K. J., Cheng Y., Howard K. (1997). The Drosophila protein Wunen repels migrating germ cells. Nature 385, 64-67 [DOI] [PubMed] [Google Scholar]