Abstract
Rationale
Inositol 1,4,5-trisphosphate (IP3)-induced vasoconstriction can occur independently of intracellular Ca2+ release and via IP3 receptor (IP3R) and canonical transient receptor potential (TRPC) channel activation, but functional signaling mechanisms mediating this effect are unclear.
Objectives
Study mechanisms by which IP3Rs stimulate TRPC channels in myocytes of resistance-size cerebral arteries.
Methods and Results
Immunofluorescence resonance energy transfer (immuno-FRET) microscopy using isoform-selective antibodies indicated that endogenous type 1 IP3Rs (IP3R1) are in close spatial proximity to TRPC3, but distant from TRPC6 or TRPM4 channels in arterial myocytes. Endothelin-1 (ET-1), a phospholipase C-coupled receptor agonist, elevated immuno-FRET between IP3R1 and TRPC3, but not between IP3R1 and TRPC6 or TRPM4. TRPC3, but not TRPC6, co-immunoprecipitated with IP3R1. TRPC3 and TRPC6 antibodies selectively inhibited recombinant channels, but only the TRPC3 antibody blocked IP3-induced non-selective cation current (ICat) in myocytes. TRPC3 knockdown attenuated immuno-FRET between IP3R1 and TRPC3, IP3-induced ICat activation, and ET-1 and IP3-induced vasoconstriction, whereas TRPC6 channel knockdown had no effect. ET-1 did not alter total or plasma membrane-localized TRPC3, as determined using surface biotinylation. RT-PCR demonstrated that C-terminal calmodulin and IP3R binding (CIRB) domains are present in myocyte TRPC3 and TRPC6 channels. A peptide corresponding to the IP3R N-terminal region that can interact with TRPC channels activated ICat. A TRPC3 CIRB domain peptide attenuated IP3- and ET-1-induced ICat activation and vasoconstriction.
Conclusions
IP3 stimulates direct coupling between IP3R1 and membrane-resident TRPC3 channels in arterial myocytes, leading to ICat activation and vasoconstriction. Close spatial proximity between IP3R1 and TRPC3 establishes this isoform-selective functional interaction.
Keywords: Inositol 1,4,5-trisphosphate; canonical transient receptor potential channel; coupling; vasoconstriction
Introduction
Activation of plasma membrane phospholipase C (PLC)-coupled receptors by vasoconstrictor agonists leads to phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis and the generation of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol.1 In vascular myocytes, diacylglyceroI (DAG) activates protein kinase C (PKC), leading to the phosphorylation of a wide variety of proteins, including ion channels.2 IP3 binds to sarcoplasmic reticulum (SR) IP3 receptors (IP3Rs), resulting in SR Ca2+ release, an elevation in intracellular Ca2+ concentration ([Ca2+]i), and vasoconstriction.3 Recent evidence also indicates that IP3-induced vasoconstriction can occur independently of SR Ca2+ release and via the activation of type 1 IP3 receptors (IP3R1) and type 3 canonical transient receptor potential (TRPC) channels.4, 5 However, the functional signaling mechanisms by which IP3Rs and TRPC channels communicate in arterial myocytes are unclear.
The mammalian TRP channel superfamily is encoded by at least 28 different genes that are subdivided into 7 families.6 These families encode ion channels with diverse ion selectivity, modes of regulation, and physiological functions.6 Vascular myocytes express at least four TRP families, including TRPC, TRPM, TRPV, and TRPP.7–10 These channels regulate arterial myocyte membrane potential, [Ca2+]i, contractility, and proliferation, and are implicated in the etiology of vascular diseases.4, 8–12 Given the diversity of vascular myocyte TRP channels, it has become important to identify signaling pathways that specifically regulate individual channel isoforms and to determine whether individual TRP channel isoforms perform distinct physiological functions. For instance, arterial myocytes express multiple TRPC isoforms, including 1, 3, and 6, but whether signaling pathways specifically regulate individual members and what the mechanisms are that mediate such effects are poorly understood.9, 10 In cerebral artery myocytes, vasoconstrictors activate TRPC3,4, 9, 13, 14 whereas intravascular pressure stimulates TRPC6.9, 10 Thus, TRPC3 and TRPC6 channels perform distinct physiological functions, but signaling pathways that mediate this differential regulation are unclear.
Here, we studied mechanisms by which IP3R1, the principal molecular and functional arterial myocyte IP3R isoform,5 stimulates TRPC currents in resistance-size cerebral arteries. Data suggest that IP3R1 is in close spatial proximity to, and associates with, TRPC3, but not TRPC6 or TRPM4 channels. Endothelin-1 (ET-1), a PLC-coupled receptor agonist, and IP3 alter the interaction between the IP3R N-terminus and the TRPC3 channel C-terminus, leading to channel activation and vasoconstriction. Data indicate that IP3R1 selectively couples to TRPC3 channels due to the close spatial proximity of these proteins and that this mechanism is essential for mediating ET-1 and IP3-induced vasoconstriction.
Materials and Methods
Tissue Preparation
Animal protocols used were reviewed and approved by the Animal Care and Use Committee at the University of Tennessee Health Science Center. Sprague–Dawley rat (~250 g) resistance-size cerebral arteries and myocytes from these arteries were isolated as previously described.4
Immunofluorescence resonance energy transfer (immuno-FRET)
Paraformaldehyde-fixed myocytes were incubated with primary antibodies: mouse monoclonal anti-IP3R1 and rabbit polyclonal anti-TRPC3, rabbit polyclonal anti-TRPC6, or rabbit polyclonal anti-TRPM4. Cells were then labeled with secondary antibodies: Cy3-conjugated donkey anti-mouse for IP3R1 and Cy2-conjugated goat anti-rabbit for TRPC3, TRPM4, or TRPC6. Fluorescent images, acquired using a Zeiss LSM 5 Pascal confocal microscope, were background-subtracted and N-FRET calculated using the Xia method.15
TRPC channel knockdown
Silencing vectors that express TRPC3- (TRPC3shV), TRPC6- (TRPC6shV), or scrambled control (scrm) short hairpin RNA (shRNA) were inserted into cerebral arteries using reverse permeabilization, as previously described.16
Co-immunoprecipitation (co-IP)
Arterial lysate was incubated with control mouse IgG or IP3R1 monoclonal antibody and then incubated with protein A sepharose beads. Protein samples were then analyzed by Western blotting using mouse monoclonal anti-IP3R1, mouse polyclonal anti-TRPC3, rabbit polyclonal anti-TRPC6, and horseradish peroxidase-conjugated secondary antibodies.
Surface Biotinylation
Membrane expression of TRPC3 channels was measured using surface biotinylation of intact arteries, as previously described 17.
Cell culture and Transfection
HEK293 cells were transfected with vectors encoding recombinant TRPC3 or TRPC6, kindly provided by Dr. James Putney (NIEHS) and Dr. Jochen Reiser (University of Miami), respectively. Electrophysiology and Western blotting experiments were performed 36–72 h after transfection.
Polymerase chain reaction (PCR)
Reverse transcription PCR (RT-PCR) was performed on pure populations of ~100 selected cerebral artery myocytes, as previously described.18
Patch-Clamp Electrophysiology
Membrane cation currents were measured in arterial myocytes and HEK293 cells using the conventional whole cell patch-clamp configuration, as done previously.4, 5
Pressurized Artery Diameter Measurement
Arterial diameter was measured using pressurized artery myography, as previously described.18
Statistical Analysis
Data are expressed as mean ± standard error of the mean. Statistical significance was calculated by using Student’s t-tests for paired or unpaired data or ANOVA followed by Student–Newman–Keuls test for multiple data sets. P<0.05 was considered significant.
Expanded Materials and Methods are available as supplement documentation.
Results
IP3R1 is in close proximity to TRPC3 channels in cerebral artery myocytes
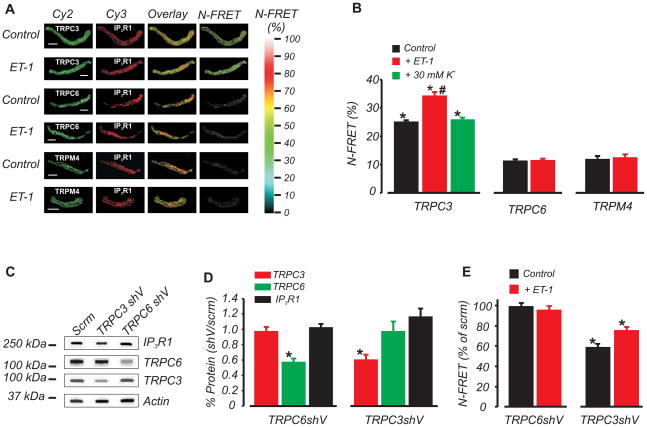
IP3R1 is the principal molecular and functional IP3R isoform that is expressed in myocytes of cerebral arteries and aorta.5, 19 To explore spatial proximity between IP3R1 and three different TRP channel isoforms in cerebral artery myocytes, we used immuno-FRET. Specifically, spatial localization between IP3R1 and TRPC3, TRPC6, or TRPM4 channels was examined. Cy3- and Cy2-labeled secondary antibodies bound to primary antibodies targeting IP3R1 (monoclonal) and TRPC3 (polyclonal), respectively, produced mean N-FRET of ~25 % (Fig. 1A,B). In contrast, fluorescent antibodies bound to IP3R1 and TRPM4 (polyclonal), or IP3R1 and TRPC6 (polyclonal), generated mean N-FRET of ~11 % and ~12 %, respectively (Fig. 1A,B). ET-1 increased mean N-FRET between IP3R1 and TRPC3-bound antibodies to ~34 %. In contrast, N-FRET between IP3R1 and TRPC3-bound antibodies was not altered by membrane depolarization with 30 mmol/L K+ (Fig. 1B). ET-1 did not change N-FRET between antibodies bound to IP3R1 and TRPC6 or IP3R1 and TRPM4 (Fig. 1A, B). Antigenic peptides for TRPC3, TRPC6, and TRPM4 specifically blocked immunofluorescence produced by each antibody, but did not alter immunofluorescence detection by the other two antibodies (Online Fig. IA).
Figure 1. TRPC3, but not TRPM4 or TRPC6, is in close spatial proximity to IP3R1 channels in arterial myocytes.
A, Fluorescent images of individual Cy2 and Cy3 labels, pixel overlay, and N-FRET for indicated TRP channel primary antibody combinations. Scale bar=10 μm; B, Mean data illustrating control and ET-1 (100 nmol/L)-induced N-FRET generated by Cy3 bound IP3R1 and Cy2-bound TRPC3, TRPM4, or TRPC6 antibodies, and effects of ET-1 and 30 mmol/L K+. * P<0.05 compared with TRPC6 or TRPM4; # P<0.05 compared with IP3R1-TRPC3 in control. n for columns from left to right are=27, 11, 15, 20, 28, 17, and 13, respectively. C, Representative Western blot illustrating that TRPC3shV causes selective knockdown of TRPC3 and TRPC6shV induces selective knockdown of TRPC6. D, Mean data illustrating effects of TRPC3shV and TRPC6shV on TRPC3 (n=4 and 6), TRPC6 (n=4 and 6), and IP3R1 (n=4 each), respectively (* P<0.05). E, TRPC6 knockdown did not reduce the N-FRET signal between IP3R1 and TRPC6 in control (n=15) or ET-1 (n=10), whereas TRPC3 knockdown reduced the N-FRET signal between IP3R1 and TRPC3 in control (n=10) and ET-1 (n=15). * P<0.05 compared with scrm.
Next, N-FRET was measured in myocytes in which TRPC3 or TRPC6 expression was reduced using shRNA and quantified using Western blotting. The selectivity of antibodies for Western blotting experiments was examined. Whole Western blots for IP3R1, TRPC3 and TRPC6 antibodies on arterial lysate are illustrated in Online Fig. IB. The monoclonal IP3R1 antibody detected only one band corresponding to IP3R1 (Online Fig. IB), and this band is reduced by IP3R1 knockdown, indicating specificity.5 Antigenic peptides abolished appropriate size bands for each TRPC isoform, but did not alter detection of the other TRPC protein (Online Fig. IC). The TRPC3 antibody detected recombinant TRPC3 expressed in HEK293 cells, but did not detect recombinant TRPC6 (Online Fig. IIA). Similarly, the TRPC6 antibody detected recombinant TRPC6, but did not detect recombinant TRPC3 (Online Fig. IIA). Endogenous TRPC3 and TRPC6 proteins were detected in HEK293 cells, consistent with a previous report.20 Taken together, these data further indicate that the TRPC3 and TRPC6 antibodies are selective for their respective TRPC channel isoforms.
Western blotting indicated that TRPC3shV and TRPC6shV reduced mean TRPC3 and TRPC6 protein by ~40 and 43%, respectively, of that in arteries treated with scrm (Fig. 1C,D). TRPC3shV did not alter TRPC6 and IP3R1 expression and TRPC6shV did not alter TRPC3 and IP3R1 expression (Fig. 1C,D). TRPC3 knockdown reduced control N-FRET and the ET-1-induced elevation in N-FRET between IP3R1 and TRPC3 (Fig. 1E). In contrast, TRPC6 knockdown had no effect on N-FRET between IP3R1 and TRPC6 in control or ET-1 (Fig. 1E), indicating that this signal represents background N-FRET.
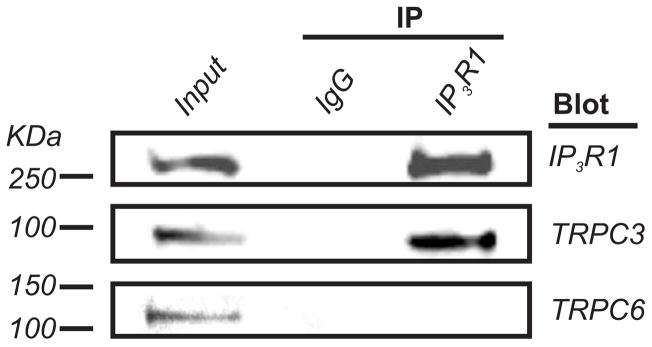
Co-IP was used to examine whether IP3R1 physically associates with TRPC3 and TRPC6 channels in small cerebral arteries. Due to the small size of the resistance-size vessels used in this study, arteries collected from ~20 rats were required for each experiment. The IP3R1 monoclonal antibody co-immunoprecipitated both IP3R1 and TRPC3 channels from lysate (Fig. 2). In contrast, TRPC6 was not detected in the same IP3R1 immunoprecipitate (Fig. 2).
Figure 2. IP3R1 interacts with TRPC3 but not TRPC6 channels in cerebral arteries.
IP3R1 monoclonal antibody co-immunoprecipitates IP3R1 (~270 kDa) and TRPC3 (~90 kDa), but not TRPC6 (~110 kDa) in cerebral arteries. Lysate supernatant (20 μg protein) was used as the input control. Mouse IgG was used instead of the IP3R1 monoclonal antibody as a negative co-IP control.
In summary, data indicate that TRPC3, but not TRPM4 or TRPC6, is in close spatial proximity to, and associates with, IP3R1 channels in myocytes of resistance-size cerebral arteries. Data also demonstrate that ET-1 modifies the molecular relationship between IP3R1 and TRPC3.
TRPC3 mediates IP3-induced ICat activation in cerebral artery myocytes
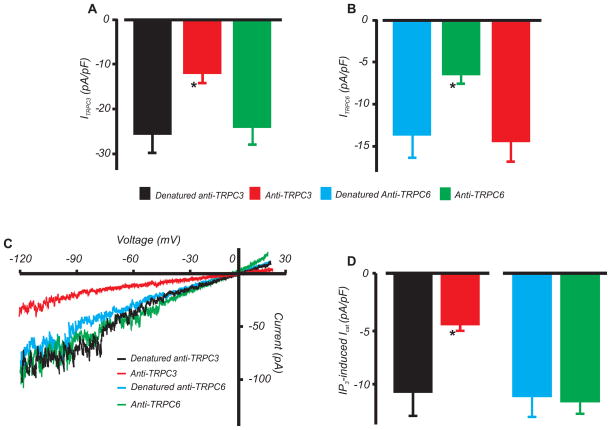
The contribution of TRPC3 and TRPC6 channels to IP3-induced ICat was examined. The TRPC3 antibody blocked whole-cell currents generated by recombinant TRPC3 expressed in HEK293 cells (Fig. 3A,B, Online Fig. IIB). In contrast, the TRPC3 antibody did not alter currents produced by recombinant TRPC6 channels. Similarly, the TRPC6 antibody blocked recombinant TRPC6 channel currents, but did not alter TRPC3 currents (Fig. 3A,B, Online Fig. IIB). Therefore, the antibodies selectively inhibit their respective TRPC currents. The TRPC3 antibody reduced mean IP3-induced ICat density by ~57 % in arterial myocytes (Fig. 3C,D). In contrast, the TRPC6 antibody had no effect on IP3-induced ICat density in arterial myocytes (Fig. 3C,D).
Figure 3. TRPC3 mediates IP3-induced ICat in arterial myocytes.
A,B, Mean data for current density generated in HEK293 cells in response to transfection with recombinant TRPC3 or TRPC6 channels, and selective inhibition by TRPC3 and TRPC6 antibodies (2 μg/mL each). Whole cell currents were essentially absent in cells transfected with only GFP. n for columns from left to right are = 7, 7, 6, 6, 6, and 4, respectively. C, Exemplary recordings illustrating inhibition of IP3 (10 μmol/L in pipette solution)-induced ICat by TRPC3 antibody (2 μg/mL), but no effect of TRPC6 antibody (2 μg/mL), in arterial myocytes. D, Mean data for active and denatured TRPC3 (n=8 each) and TRPC6 antibody (n=7 each) in arterial myocytes. *P<0.05 vs. denatured control.
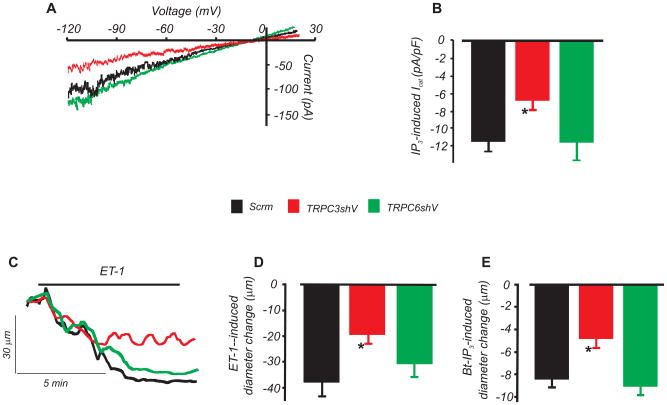
IP3-induced ICat activation and vasoconstriction were studied in myocytes and arteries in which TRPC3 or TRPC6 expression was reduced using shRNA. TRPC3 knockdown reduced IP3-induced ICat density by ~41 %. In contrast, TRPC6 knockdown did not alter IP3-induced ICat density (Fig. 4A,B). TRPC6 knockdown reduces myogenic tone at intravascular pressures >40 mmHg.10 Therefore, diameter regulation was measured in arteries pressurized to 20 mmHg to avoid differences in baseline tone between arteries in which TRPC3 and TRPC6 were knocked down. TRPC3 knockdown attenuated IP3- and ET-1-induced vasoconstriction by ~43 and 49 %, respectively (Fig. 4C,D,E). In contrast, TRPC6 knockdown had no effect on IP3- and ET-1-induced vasoconstriction (Fig. 4C,D,E). These data indicate that TRPC3, but not TRPC6, channels mediate IP3-induced ICat activation and contraction in cerebral artery myocytes.
Figure 4. TRPC3 knockdown attenuates IP3-induced ICat activation and vasoconstriction whereas TRPC6 knockdown has no effect.
A, Exemplary traces of IP3 (10 μmol/L)-induced ICat in arterial myocytes treated with scrm, TRPC3shV, or TRPC6shV. B, Mean ICat density (n: scrm, 6; TRPC3shV; 10, TRPC6shV, 8). C, Exemplary recordings of ET-1-induced vasoconstriction in pressurized (20 mmHg) arteries treated with scrm, TRPC3shV, or TRPC6shV. D and E, Mean data for ET-1- (300 pmol/L) and Bt-IP3 (10 nmol/L)-induced vasoconstriction (n=5, 4, and 4 for scrm, TRPC3shV, and TRPC6shV, respectively). * P<0.05 versus scrm). Baseline tone was similar in scrm- (12 ± 4%, n=5), TRPC3shV- (10 ± 1%, n=4), and TRPC6shV- (11 ± 2%, n=4) treated arteries (P>0.05 for each).
ET-1 does not increase surface expression of TRPC3 channels in myocytes
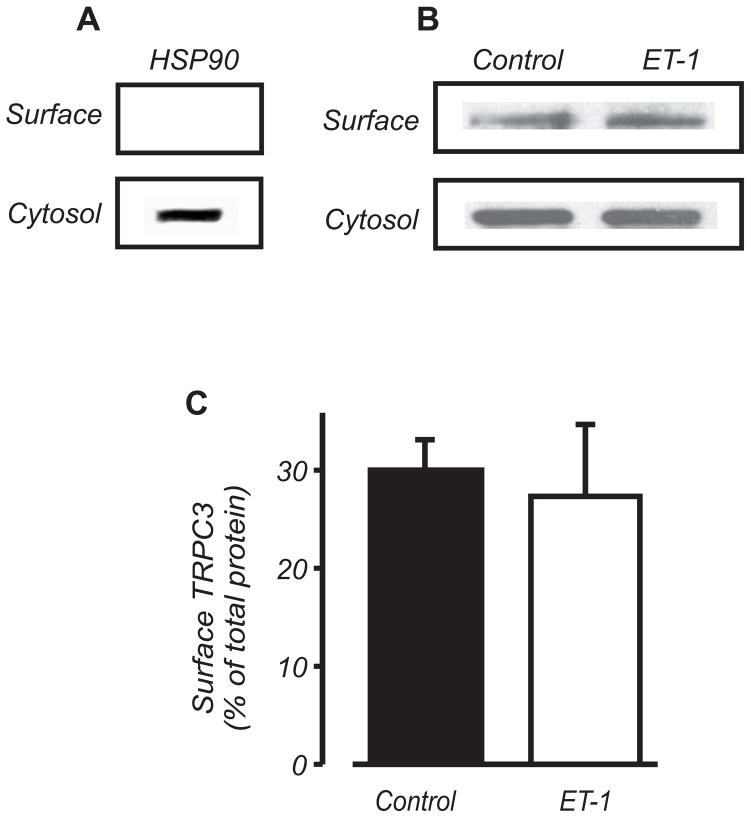
An increase in ion channel activity (NPo) can occur due to both an elevation in the number of channels (N) and an increase in channel open probability (Po). To investigate whether ET-1 alters plasma membrane TRPC3 channel protein, we used surface biotinylation.17
Confirmation that biotin specifically labels surface proteins was obtained using biotin-treated arterial segments exposed to Texas Red-conjugated streptavidin (Online Fig. III). In addition, heat shock protein 90 (HSP90), an intracellular protein, was detected only in the non-biotinylated (cytosolic) fraction (Fig. 5A). Biotinylation indicated that in control, ~30 % of total TRPC3 protein was present in the arterial plasma membrane (Fig. 5B,C). ET-1 did not alter the percentage of plasma membrane-localized TRPC3 (Fig. 5B,C). These data indicate that a significant proportion of arterial TRPC3 is cytosolic and that ET-1 elevates myocyte ICat by stimulating plasma membrane-resident TRPC3 channels.
Figure 5. ET-1 does not increase surface expression of TRPC3 channels in cerebral arteries.
A, Western blot illustrating that HSP90 is detected only in the non-biotinylated (cytosolic) fraction in cerebral arteries. B,C, Western blot and mean data (n=3) indicating that ET-1 (10 nmol/L) does not increase plasma membrane-localized TRPC3.
Calmodulin and IP3R binding domain (CIRB) domains are present in myocyte TRPC3 and TRPC6 channels
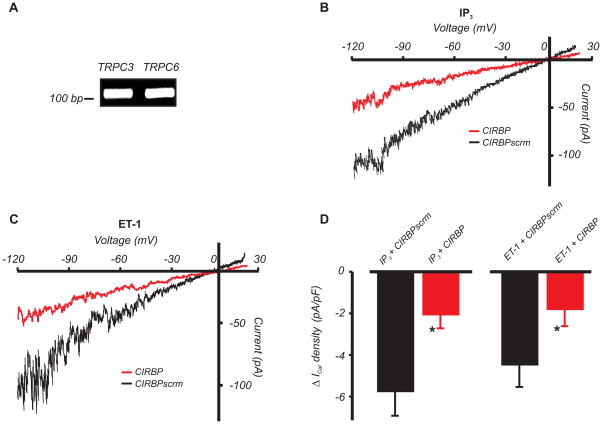
Recombinant IP3R N-termini contain a conserved amino acid sequence that can interact with a conserved C-terminal CIRB domain found on all on TRPC isoforms.21, 22 TRPM4 does not contain a CIRB domain (Genbank Accession number: XM_574447). Our data indicate that isoform-selective association of TRPC3 channels to IP3R1 occurs in arterial myocytes (Figs. 1–4). Therefore, we used RT-PCR to investigate whether myocyte TRPC channels contain CIRB domains. Primers used were selective for TRPC3 and TRPC6 CIRB domains (Online Fig. IV). RT-PCR was performed on manually selected arterial myocytes to prevent message contamination from other vascular wall cell types.18 Data indicated that the C-termini of arterial myocyte TRPC3 and TRPC6 channels contain CIRB domains (Fig. 6A).
Figure 6. IP3R to TRPC physical coupling is essential for ET-1- and IP3-induced ICat activation in arterial myocytes.
A, Cerebral artery myocyte TRPC3 and TRPC6 channels both contain CIRB domains, as determined using nested RT-PCR. B and C, Exemplary recordings illustrating attenuation of IP3 (10 μmol/L) and ET-1 (100 nmol/L)-induced ICat activation by CIRBP (1 μmol/L via pipette) in arterial myocytes. D, Mean IP3 and ET-1-induced change in ICat density in CIRBP or CIRBPscrm (1 μmol/L each) -treated myocytes (*P<0.05 vs. CIRBPscrm; n=9, 9, 10, and 8 for ET-1+CIRBPscrm, ET-1+CIRBP, IP3+CIRBPscrm, and IP3+CIRBP, respectively).
TRPC3-IP3R1 physical coupling is required for ET-1- and IP3-induced ICat activation
We tested the hypothesis that IP3-induced ICat activation in arterial myocytes occurs through an interaction between the conserved IP3R interaction domain and the TRPC CIRB domain. Synthetic peptides corresponding to a region in the TRPC3 CIRB domain (QIMKRLIKRYVLKAQVDKEND, CIRBP),21–24 and a scrambled control peptide (RDLKKAQNLVEIQKKYDRMVI, CIRBPscrm) were generated. CIRBP and CIRBPscrm alone did not alter mean baseline ICat density (pA/pF: no peptide, −3.5±0.5, n=5; CIRBPscrm, −3.7±0.9, n=10; CIRBP, −2.9±0.6, n=11; P>0.05 for each) in arterial myocytes. In contrast, CIRBP reduced IP3 and ET-1-induced ICat activation by ~63 % and 60 %, respectively, when compared with CIRBPscrm (Fig. 6B,C,D).
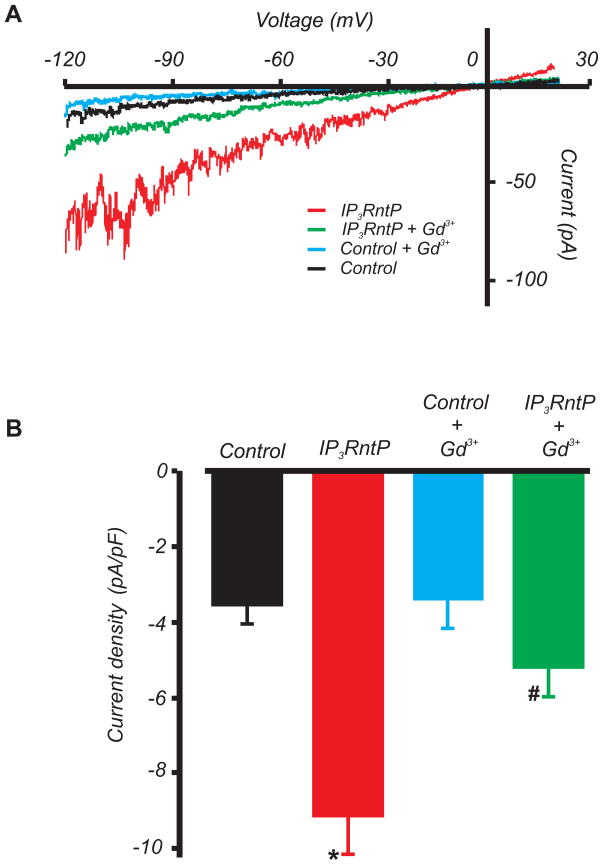
To study physiological functions of the IP3R N-terminus, a peptide was constructed corresponding to the IP3R N-terminal sequence containing the conserved TRPC-binding region (EEVWLFWRD, IP3RntP). IP3RntP increased mean ICat density by ~260 % (Fig. 7A,B). Gadolinium (Gd3+), a non-selective cation channel blocker, had no significant effect on baseline currents, but reduced mean IP3RntP-induced ICat densityby ~70% (Fig. 7A,B). These data indicate that physical association between the IP3R1 N-terminus and the TRPC C-terminus CIRB domain leads to ICat activation in arterial myocytes.
Figure 7. IP3RntP activates non-selective cation currents in arterial myocytes.
A, Exemplary recordings illustrating activation of Gd3+-sensitive ICat by IP3RntP (50 μmol/L via pipette). B, Mean ICat density. *P<0.05 vs. control; #P<0.05 vs. IP3RntP (n=5 and 6 for control and IP3RntP respectively).
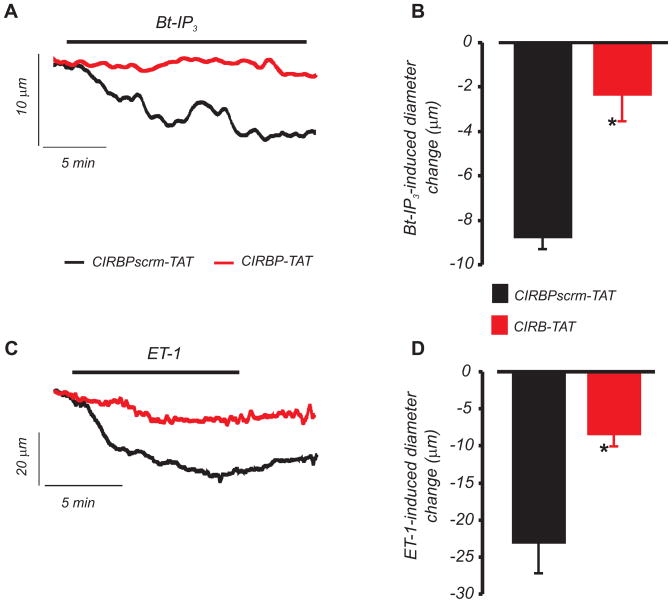
CIRBP attenuates ET-1 and IP3-induced vasoconstriction
The physiological function of molecular coupling between IP3R and TRPC3 channels was investigated by measuring diameter regulation of pressurized (60 mmHg) arteries. Membrane-permeant CIRBP (CIRBP-TAT) and scrambled control (CIRBPscrm-TAT) peptides were each generated by conjugation to a HIV-1 TAT sequence (RRRQRRKKRGY).25 CIRBP-TAT reduced vasoconstriction caused by Bt-IP3, a membrane permeant IP3 analog, and ET-1 by ~78 % and ~65 %, respectively, when compared with CIRBPscrm-TAT (Figs. 8A, B, C, and D). These data indicate that interaction between the IP3R1 N-terminus and the TRPC3 channel CIRB domain contributes to IP3 and ET-1-induced vasoconstriction.
Figure 8. IP3R to TRPC physical coupling contributes to ET-1 and IP3-induced vasoconstriction.
A, B, CIRBP-TAT (3 μmol/L, 20 min application) attenuates Bt-IP3 (1 nmol/L, n=4)-induced vasoconstriction in pressurized (60 mmHg) cerebral arteries. C, D, CIRBP-TAT (3 μmol/L, 20 min application) attenuates ET-1 (1 nmol/L, n=7)-induced vasoconstriction (*P<0.05 vs. CIRBPscrm-TAT). When applied alone, CIRBP-TAT and CIRBPscrm-TAT similarly reduced mean baseline diameter by ~15 and 13 μm, respectively, consistent with a previous report for the TAT sequence.25
Discussion
Here, we identify a novel vasoregulatory signaling mechanism whereby IP3Rs couple to plasma membrane TRPC3 channels in arterial myocytes (Online Fig. V). Novel findings of this study are that in cerebral artery myocytes: 1) IP3R1 is located in close proximity to TRPC3, but not TRPC6 or TRPM4 channels, 2) ET-1 elevates FRET between fluorescent antibodies bound to IP3R1 and TRPC3 channels, but does not alter FRET between IP3R1 and TRPC6 or TRPM4 channels, 3) TRPC3 channels co-IP with IP3R1, whereas TRPC6 channels do not, 4) TRPC3, but not TRPC6, channels underlie IP3-induced ICat, 5) ET-1 does not alter total or plasma membrane-localized TRPC3 channel protein, 6) TRPC3 and TRPC6 both contain CIRB domains, 7) a TRPC3 CIRB domain peptide reduces IP3- and ET-1-induced ICat activation and a peptide corresponding to the IP3R1 N-terminal sequence that interacts with the TRPC CIRB domain activates ICat, 8) CIRBP attenuates IP3-and ET-1-induced vasoconstriction. Taken together, these data indicate that IP3R1 activation causes physical coupling of the IP3R1 N-terminus to the TRPC3 channel C-terminus, leading to ICat activation and vasoconstriction. Physical coupling between all IP3R and TRPC channel isoforms occurs with recombinant channels,21–23, 26 To our knowledge, this is the first time that molecular coupling between IP3R1 and TRPC channels has been demonstrated to occur in a native cell type, to perform a physiological function, and to exhibit TRPC channel isoform-selectivity. Our data also suggest that close spatial proximity of IP3R1 to TRPC3 channels establishes selective molecular coupling of these proteins. In contrast, although TRPC6 contains a CIRB domain, spatial separation appears to prevent this channel from coupling to IP3R1 in arterial myocytes. Our study demonstrates that arterial myocyte IP3R channels, previously considered to function solely by releasing SR Ca2+, directly activate TRPC3 channels, thereby regulating arterial contractility. This study also illustrates that manipulating physical coupling between IP3R1 and TRPC3 channels is a novel mechanism that could be exploited to regulate arterial contractility.
Rat cerebral artery myocytes express several TRP isoforms, including TRPC1, -3, -6, TRPM4, and TRPV4.7, 9–11 Mammalian TRPC channel isoforms share >30% amino acid sequence identity,27 with all TRPC channel isoforms containing a C-terminal CIRB domain.21, 22 Cerebral artery myocytes express all three IP3R isoforms, with IP3R1 being the principal molecular and functional isoform.5 All recombinant IP3R isoforms contain an N-terminal TRPC channel interaction domain.21, 22 Coupling of the IP3R TRPC binding domain to the TRPC channel CIRB domain displaces inhibitory calmodulin, leading to channel activation.22 The CIRB domain may also be a TRPC channel trafficking sequence.24 These previous studies were performed primarily by studying recombinant channels. It has been proposed that the physical interaction between IP3Rs and TRPC channels may have occurred in response to TRPC channel overexpression.21–23, 26 Therefore, it was unclear whether physical coupling between IP3Rs and TRPC channels would occur in native cell types. Here, we demonstrate that this coupling mechanism does occur in a native cell type, selectively regulates TRPC3 currents in arterial myocytes, and leads to vasoconstriction.
In cerebral artery myocytes, IP3R stimulation leads to the activation of a ICat to which TRPC3 channels contribute.4, 9 IP3R-mediated ICat activation leads primarily to Na+ influx, resulting in membrane depolarization, voltage-dependent Ca2+ channel activation, an [Ca2+]i elevation, and vasoconstriction.4 The mechanism by which IP3Rs stimulate TRPC3 channels occurs independently of SR Ca2+ release and appears to be a major mechanism by which PLC-coupled receptor agonists cause vasoconstriction.4 IP3R1 and TRPC3 channels do not contribute to the cerebral artery myogenic response.4, 9 In contrast, TRPC6 channels do not contribute to ET-1 or UTP-induced cerebral artery constriction, but are essential for the myogenic response.4, 9, 10 The different mechanisms by which vasoconstrictors and IP3 activate TRPC3, whereas intravascular pressure stimulates TRPC6 were unclear. Fluorescent antibodies bound to endogenous IP3R1 and TRPC3 generated FRET that was elevated by ET-1 and higher than for TRPC6 or TRPM4. TRPC3 knockdown reduced basal and ET-1-induced IP3R1/TRPC3 FRET. The calculated Forster distance between Cy2 and Cy3 is 5–6 nm, supporting other evidence in this study that IP3R1 and TRPC3 are close enough to physically interact. In contrast, the FRET signal between IP3R1 and TRPC6 and TRPM4 was consistent with background,28 was not elevated by ET-1, and was not altered by TRPC6 knockdown. These data indicate that that TRPC6 and TRPM4 are not in close proximity to IP3R1. Mechanisms that position TRPC3 nearby IP3R1, but TRPC6 and TRPM4 away from IP3R1 are unclear. Several possibilities exist, including differential localization by local lipid environments, including caveolae. It is also possible that IP3R1 and TRPC3 are maintained by scaffolding proteins in a macromolecular complex. Scaffolding proteins, including Homer, establish close proximity of TRPC channels to other proteins, including IP3Rs.29 The ET-1-induced elevation in immuno-FRET may occur due to an IP3-induced conformational change in IP3R1 that locates the Cy3-tagged antibody closer to Cy2-bound TRPC3. Alternatively, ET-1 may cause IP3R clustering,30 thereby locating more Cy3-tagged IP3R1 channels in the vicinity of Cy2-bound TRPC3. The FRET method cannot differentiate between these potential mechanisms, but these possibilities deserve future investigation.
Co-IP data indicated that IP3R1 and TRPC3 are located in the same arterial macromolecular complex that does not contain TRPC6. The small arteries used for co-IP experiments inevitably contained endothelium. Complementary data, including those obtained using FRET, patch-clamp electrophysiology, RNA interference, antibodies and peptides, indicate that close spatial and functional interaction occurs between IP3R1 and TRPC3 in isolated myocytes. Arterial myocyte TRPC3 and TRPC6 channels both contained CIRB domains. Thus, although both TRPC3 and TRPC6 could interact molecularly with IP3R1, spatial proximity determines their ability to communicate. The CIRBP sequence used here as a competitive antagonist is identical to the sequence in TRPC3, and between 11 (TRPC1) and 89 (TRPC7) % homologous to sequences in other TRPC channel isoforms.24 The CIRBP peptide did not alter ICat or diameter in the absence of ET-1 or IP3, but reduced ET-1 and IP3-induced ICat activation and vasoconstriction. Additional support that the CIRB domain is functional in arterial myocytes was data indicating that IP3RntP stimulated a Gd3+-sensitive ICat. The IP3RntP should activate all TRP channels that contain a CIRB domain, including TRPC3, regardless of their proximity to IP3Rs. However, TRPC isoforms also have variable affinities for the IP3R N-terminus.21 Taken together our data indicate that: 1) IP3R1 and TRPC3 proteins are maintained in close proximity in the absence of receptor agonists; 2) physical interaction between the IP3R1 N-terminus and TRPC3 channel C-terminus is weak in the absence of IP3, indicating that the interaction domains do not maintain the close proximity; and 3) IP3 enhances physical interaction between IP3R1 and TRPC3. Although IP3R1 did not activate TRPC6, IP3R1 may activate TRPC isoforms other than TRPC3 in arterial myocytes. Given that there are seven TRPC isoforms and three IP3R isoforms, it was beyond the scope of the current study to determine which of the potential twenty-one interactions may occur molecularly and functionally in arterial myocytes. TRPC channel heteromultimers have been reported in cultured vascular myocytes and A7r5 cells31, but it is unclear whether TRPC channels that couple to IP3R1 in arterial myocytes are homomultimers or heteromultimers. TRPC3 knockdown reduced TRPC3 protein by ~40% and attenuated IP3-induced ICat by ~41%. A TRPC3-selective antibody also similarly inhibited recombinant TRPC3 currents and IP3-induced ICat in arterial myocytes. Therefore, any IP3-induced current mediated by channels other than TRPC3 would be small in arterial myocytes. In other native cell types, IP3Rs and TRPC channels may exhibit different isoform-dependent coupling patterns, including promiscuous coupling similar to that with recombinant channels.
Several stimuli, including receptor agonists, increase plasma membrane trafficking of recombinant TRPC3 channels.32 Biotinylation indicated that over the same time course that ET-1 activated ICat in arterial myocytes, total TRPC3 or plasma membrane-localized TRPC3 did not change. These data indicate that ET-1 elevates ICat by increasing the Po of membrane-resident TRPC3 channels. Biotinylation also indicated that a large proportion of TRPC3 protein is intracellular. One explanation for this data is that TRPC3 channels may be retained, for example by RNF24,33 and that signaling pathways other than those activated by acute ET-1 application may stimulate plasma membrane insertion. TRPC3 channels are also present on intracellular organelle membranes.29 Given that FRET was detected at the plasma membrane and intracellularly, our data indicate that intracellular TRPC3 channels are also in close proximity to IP3R1. Protein complexes containing both IP3R1 and TRPC3 may also form prior to plasma membrane insertion of TRPC3. 29
Phospholipase C not only generates IP3, but also elevates DAG, which activates PKC. CIRBP reduced ET-1- and IP3-induced ICat activation by >60 %. These data indicate that ET-1 and IP3 stimulate a ICat primarily through molecular coupling of IP3Rs to TRPC channels, rather than through DAG and PKC activation. This conclusion is consistent with previous evidence that IP3R1 antibodies and IP3R1 knockdown also inhibit UTP-induced ICat activation in arterial myocytes.5 DAG and PKC can regulate TRPC channels, but in vascular myocytes isolated from a variety of different blood vessels, regulation is complex with studies reporting PKC-mediated activation, inhibition, or no effect.14, 34–36 In cerebral artery myocytes, PKC stimulates a ICat by elevating TRPM4 channel apparent micromolar Ca2+ sensitivity.37, 38 Here, in patch clamp experiments, free intracellular Ca2+ was strongly buffered using EGTA and ET-1-induced PKC activation would not stimulate TRPM4 channels.37, 38 Conceivably, a proportion of the CIRBP-insensitive ET-1-induced vasoconstriction may occur due to PKC activation of other TRP channels, including TRPM4. Agonists also stimulate vasoconstriction via additional mechanisms, including activation of Ca2+ waves and voltage-dependent Ca2+ channels, and elevation of myofilament Ca2+ sensitivity.2,3
In summary, we describe a novel mechanism by which IP3Rs regulate arterial diameter. Our data indicate that ET-1 and IP3 activate membrane-resident TRPC3 channels in arterial myocytes by causing an interaction between the IP3R1 N-terminal TRPC channel interaction sequence and the C-terminal TRPC3 channel CIRB domain. We also provide a mechanism by which IP3R1 activation selectively stimulates TRPC3, but not TRPC6, channels and indicate that this occurs due to the close spatial proximity of these proteins which allows physical interaction. Physical coupling between IP3R1 and TRPC3 channels could be manipulated therapeutically to regulate arterial contractility, and thus, brain blood flow.
Novelty and Significance
What Is Known?
Phospholipase C-coupled receptor agonists elevate inositol 1,4,5-trisphosphate (IP3) in arterial smooth muscle cells, leading to the activation of sarcoplasmic reticulum (SR) type 1 IP3 receptors (IP3Rs), an increase in intracellular calcium ([Ca2+]i) concentration, and vasoconstriction.
Canonical transient receptor potential (TRPC) channels are molecularly and functionally diverse proteins that regulate smooth muscle cell plasma membrane cation influx and vascular contractility.
The conventional view has been that IP3Rs stimulate vasoconstriction by releasing SR Ca2+, although recent evidence indicates that IP3Rs also induce vasoconstriction via an SR Ca2+ release-independent mechanism that involves TRPC channel activation; the mechanism is unidentified.
What New Information Does This Article Contribute?
Type 1 IP3 receptors are located in very close spatial proximity to plasma membrane TRPC3 channels in arterial smooth muscle cells.
Vasoconstrictor agonists and IP3 induce physical interaction between the IP3R1 N-terminus and the TRPC3 channel C-terminal calmodulin and IP3R binding domain, leading to TRPC3 channel activation and vasoconstriction.
In arterial smooth muscle cells, IP3R1 coupling to TRPC3 channels is isoform-selective since IP3R1 does not activate spatially separated TRPC6 channels.
Many vasoconstrictors elevate intracellular IP3, but the mechanisms by which this second messenger stimulates vasoconstriction are poorly understood. We utilized a combination of molecular, cellular, and physiological approaches to examine IP3R regulation of TRPC channels in cerebral artery smooth muscle cells. We found that IP3R1, the predominant functional IP3R isoform in arterial smooth muscle cells, physically interacts with and directly activates plasma membrane TRPC3, but not TRPC6, channels. To our knowledge this is the first demonstration that: 1) native IP3Rs physically interact with and activate native TRPC channels, and 2) IP3Rs directly regulate the activity of a plasma membrane ion channel in arterial smooth muscle cells. Physical coupling of IP3R1 to TRPC3 channels occurs due close spatial proximity of these proteins. In contrast, TRPC6 channels are not located nearby IP3R1 and do not couple. Our findings identify a novel mechanism of arterial contractility regulation whereby agonist-induced IP3 stimulates coupling of IP3Rs to TRPC3 channels, resulting in plasma membrane cation influx, and vasoconstriction. We also show that manipulating the interaction between IP3R1 and TRPC3 channels is a novel mechanism that could be exploited to regulate arterial contractility.
Supplementary Material
Acknowledgments
We thank Dr. Lidia A. Gardner for technical advice with FRET measurements.
Sources of Funding
NHLBI grants R01 HL67061 and HL77678 to J.H.J. and K01 HL096411 to A.A. D. Narayanan is a recipient of an AHA Predoctoral Fellowship. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Non-standard Abbreviations and Acronyms
- Scrm
vectors encoding scrambled short hairpin RNA
- TRPC3shV
vectors encoding TRPC3 short hairpin RNA
- TRPC6shV
vectors encoding TRPC6 short hairpin RNA
- PLC
phospholipase C
- IP3R
IP3 receptors
- CIRB
calmodulin and IP3R binding
- Immuno-FRET
Immunofluorescence resonance energy transfer
- ET-1
endothelin-1
- IP3RntP
IP3R1 N-terminal sequence peptide
- ICat
non-selective cation current
Footnotes
Disclosures
None.
References
- 1.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 3.Sanders KM. Invited review: mechanisms of calcium handling in smooth muscles. J Appl Physiol. 2001;91:1438–1449. doi: 10.1152/jappl.2001.91.3.1438. [DOI] [PubMed] [Google Scholar]
- 4.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res. 2008;102:1118–1126. doi: 10.1161/CIRCRESAHA.108.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295:C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 7.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 8.Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2055–H2061. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- 10.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 11.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118:337–351. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Crossland RF, Noorani MM, Marrelli SP. Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. Am J Physiol Heart Circ Physiol. 2009;297:H417–H424. doi: 10.1152/ajpheart.01130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peppiatt-Wildman CM, Albert AP, Saleh SN, Large WA. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol. 2007;580:755–764. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J. 2001;81:2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesh RE, Somlyo AP, Owens GK, Somlyo AV. Reversible permeabilization. A novel technique for the intracellular introduction of antisense oligodeoxynucleotides into intact smooth muscle. Circ Res. 1995;77:220–230. doi: 10.1161/01.res.77.2.220. [DOI] [PubMed] [Google Scholar]
- 17.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell α2δ-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea receptor-dependent and -independent pathways mediate vasodilation induced by ATP-sensitive K+ channel openers. Mol Pharmacol. 2008;74:736–743. doi: 10.1124/mol.108.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou H, Nakamura T, Matsumoto N, Hisatsune C, Mizutani A, Iesaki T, Daida H, Mikoshiba K. Predominant role of type 1 IP3 receptor in aortic vascular muscle contraction. Biochem Biophys Res Commun. 2008;369:213–219. doi: 10.1016/j.bbrc.2007.12.194. [DOI] [PubMed] [Google Scholar]
- 20.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci U S A. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wedel BJ, Vazquez G, McKay RR, St JB, Putney JW., Jr A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process. J Biol Chem. 2003;278:25758–25765. doi: 10.1074/jbc.M303890200. [DOI] [PubMed] [Google Scholar]
- 25.Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Iα inhibit NO-induced cerebral dilation. Proc Natl Acad Sci U S A. 2000;97:14772–14777. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 27.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Chen J, Saraf A, Cassar S, Han P, Rogers JC, Brioni JD, Sullivan JP, Gopalakrishnan M. Association of Catsper1 or -2 with Cav3.3 leads to suppression of T-type calcium channel activity. J Biol Chem. 2006;281:22332–22341. doi: 10.1074/jbc.M511288200. [DOI] [PubMed] [Google Scholar]
- 29.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- 30.Taufiq UR, Skupin A, Falcke M, Taylor CW. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villereal ML. Mechanism and functional significance of TRPC channel multimerization. Semin Cell Dev Biol. 2006;17:618–629. doi: 10.1016/j.semcdb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandyopadhyay BC, Ong HL, Lockwich TP, Liu X, Paria BC, Singh BB, Ambudkar IS. TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1,4,5-trisphosphate receptor and RACK1. J Biol Chem. 2008;283:32821–32830. doi: 10.1074/jbc.M805382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lussier MP, Lepage PK, Bousquet SM, Boulay G. RNF24, a new TRPC interacting protein, causes the intracellular retention of TRPC. Cell Calcium. 2008;43:432–443. doi: 10.1016/j.ceca.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J Biol Chem. 2003;278:29031–29040. doi: 10.1074/jbc.M302751200. [DOI] [PubMed] [Google Scholar]
- 35.Trebak M, Hempel N, Wedel BJ, Smyth JT, Bird GS, Putney JW., Jr Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol Pharmacol. 2005;67:558–563. doi: 10.1124/mol.104.007252. [DOI] [PubMed] [Google Scholar]
- 36.Albert AP, Large WA. Inhibitory regulation of constitutive transient receptor potential-like cation channels in rabbit ear artery myocytes. J Physiol. 2004;560:169–180. doi: 10.1113/jphysiol.2004.071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol. 2007;292:H2613–H2622. doi: 10.1152/ajpheart.01286.2006. [DOI] [PubMed] [Google Scholar]
- 38.Slish DF, Welsh DG, Brayden JE. Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283:H2196–H2201. doi: 10.1152/ajpheart.00605.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.