Abstract
Rationale
Physiological functions of mitochondria in contractile arterial myocytes are poorly understood. Mitochondria can uptake calcium (Ca2+), but intracellular Ca2+ signals that regulate mitochondrial Ca2+ concentration ([Ca2+]mito) and physiological functions of changes in [Ca2+]mito in arterial myocytes are unclear.
Objective
Identify Ca2+ signals that regulate [Ca2+]mito, examine the significance of changes in [Ca2+]mito, and test the hypothesis that [Ca2+]mito controls functional ion channel transcription in myocytes of resistance-size cerebral arteries.
Methods and Results
Endothelin-1 (ET-1) activated Ca2+ waves and elevated global Ca2+ concentration ([Ca2+]i) via inositol 1,4,5-trisphosphate receptor (IP3R) activation. IP3R-mediated sarcoplasmic reticulum (SR) Ca2+ release increased [Ca2+]mito and induced mitochondrial depolarization, which stimulated mitochondrial reactive oxygen species (mitoROS) generation that elevated cytosolic ROS. In contrast, a global [Ca2+]i elevation did not alter [Ca2+]mito, mitochondrial potential, or mitoROS generation. ET-1 stimulated nuclear translocation of nuclear factor kappa B (NF-κB) p50 subunit and ET-1-induced IP3R-mediated mitoROS elevated NF-κB-dependent transcriptional activity. ET-1 elevated voltage-dependent Ca2+ (CaV1.2) channel expression, leading to an increase in both pressure (myogenic tone)- and depolarization-induced vasoconstriction. Baseline CaV1.2 expression and the ET-1-induced elevation in CaV1.2 expression were both reduced by IP3R inhibition, mitochondrial electron transport chain block, antioxidant treatment, and NF-κB subunit knockdown, leading to vasodilation.
Conclusions
IP3R-mediated SR Ca2+ release elevates [Ca2+]mito, which induces mitoROS generation. MitoROS activate NF-κB, which stimulates CaV1.2 channel transcription. Thus, mitochondria sense IP3R-mediated SR Ca2+ release to control NF-κB-dependent CaV1.2 channel expression in arterial myocytes, thereby modulating arterial contractility.
Keywords: mitochondria, calcium signaling, CaV1.2 expression, arterial smooth muscle, myogenic tone
Introduction
Intracellular calcium (Ca2+) signals modulate a wide variety of physiological functions in arterial myocytes, including contractility and gene expression 1. The spatial location of mitochondria nearby Ca2+ channels can expose these organelles to domains of elevated intracellular Ca2+ concentration ([Ca2+]i), leading to mitochondrial Ca2+ uptake 2,3. Mitochondrial Ca2+ sequestration can reduce the elevation and diffusion of cytosolic Ca2+ signals and feedback to alter the activity of Ca2+ channels that generate the signal 2–4. Mitochondria can also generate signaling molecules, including reactive oxygen species (ROS), which modulate local and global intracellular Ca2+ signals, leading to changes in arterial contractility 2,3,5. Although it is recognized that mitochondria can sequester Ca2+, intracellular Ca2+ signals that regulate mitochondrial Ca2+ concentration ([Ca2+]mito) and physiological functions of changes in [Ca2+]mito in arterial myocytes are poorly understood.
Arterial myocytes generate several distinct local and global Ca2+ signals 1,6. An elevation in global [Ca2+]i occurs in response to plasma membrane Ca2+ influx and sarcoplasmic reticulum (SR) Ca2+ release and directly regulates vascular contractility 1. Voltage-dependent Ca2+ (CaV1.2) channels are the major contributor to global [Ca2+]i in arterial myocytes and are essential for diameter regulation in resistance-size arteries that regulate blood pressure and regional blood flow 1,7,8. Ca2+ sparklets are subsarcolemmal Ca2+ signals generated by Ca2+ influx through CaV1.2 channels 6. Ca2+ sparklets contribute directly to global [Ca2+]i, thereby regulating contractility 6. Ca2+ sparks are local Ca2+ transients generated by SR ryanodine-sensitive Ca2+ release (RyR) channels 1. Ca2+ sparks activate large-conductance Ca2+-activated potassium channels, leading to membrane hyperpolarization and vasodilation 1. Ca2+ waves are propagating Ca2+ transients that can occur due to the activation of SR inositol 1,4,5-trisphosphate receptor (IP3R) channels and RyR channels 9. Physiological functions of Ca2+ waves are less clear with studies reporting that these Ca2+ signals either directly stimulate contraction or do not alter contractility 10–12.
Here, we investigated Ca2+ signaling mechanisms that regulate [Ca2+]mito in myocytes of resistance-size cerebral arteries and tested the hypothesis that changes in [Ca2+]mito control the expression of CaV1.2 channels, an ion channel whose transcriptional regulation in arterial myocytes is unclear. Our data indicate that mitochondria sense IP3R-mediated SR Ca2+ release to control mitochondrial ROS (mitoROS) generation, nuclear factor kappa B (NF-κB) activity, and functional CaV1.2 expression in arterial myocytes.
Methods
Tissue preparation
Animal protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center. All experiments were performed using Sprague-Dawley rat (~250 g) resistance-size (~50–200 µm diameter) cerebral arteries or myocytes isolated from these arteries as previously described 7.
Laser-scanning confocal Ca2+ imaging
Intracellular Ca2+ signals were imaged in myocytes of cerebral arteries using fluo-4 AM and a Noran Oz laser-scanning confocal microscope, as previously described 13.
Imaging of genetically-encoded fluorescent indicators
Vectors encoding 2mt8CG2, mt-cpYFP, or HyPer-CYTO were inserted into myocytes of intact cerebral arteries using reverse permeabilization. Expressed fluorescent indicators were imaged using a Zeiss LSM5 confocal microscope. Indicator localization was determined in myocytes isolated from arteries through colocalization with MitoTracker Orange CMTMRos using weighted colocalization.
Tetramethylrhodamine, Methyl Ester (TMRM) Imaging
Isolated arterial myocytes were loaded with TMRM and excited with 535 nm light. Background corrected TMRM fluorescence was collected at 610 nm using a Dage MTI iCCD camera.
NF-κB immunofluorescence
Paraformaldehyde-fixed arteries were incubated with antibodies against NF-κB p50 subunit (p50), followed by Cy3-conjugated secondary antibody. YOYO-1 was used to counterstain nuclei. Images were obtained using a Zeiss LSM5 confocal microscope. Weighted colocalization was used to quantify p50 nuclear localization.
NF-κB-dependent luciferase reporter gene activity
Vectors that express firefly luciferase under the control of an NF-κB promoter (NF-κB-p-Luc) were inserted into myocytes of intact arteries using reverse permeabilization. Promoter-deficient pGL3 Basic control vector containing a luciferase reporter gene was used as a control. Luciferase activity was quantified using a luminometer.
NF-κB p105 subunit (p105) knockdown
Two siRNAs directed against p105 (p105siRNA1 and p105siRNA2) were used, with scrambled siRNA (p105scrm) as control. siRNA was introduced into myocytes of intact arteries using reverse permeabilization.
Pressurized artery diameter measurements
Diameter was measured in endothelial-denuded arteries using edge-detection myography. Myogenic tone (%) was calculated as 100×(1-active diameter/passive diameter).
Statistical analysis
OriginLab and GraphPad InStat software were used for statistical analyses. Values are expressed as mean±SEM. Student’s t-test was used for comparing paired and unpaired data from two populations, and ANOVA with Student–Newman–Keuls post-hoc test used for multiple group comparisons. P<0.05 was considered significant. Power analysis was performed where P>0.05 to verify that sample size was sufficient to give a power value >0.8.
Detailed Methods is provided as Supplemental Material.
Results
ET-1 modifies local and global Ca2+ signals in cerebral artery myocytes
The regulation of local and global Ca2+ signals by endothelin-1 (ET-1), a phospholipase C (PLC)-coupled receptor agonist and vasoconstrictor, was studied in myocytes of endothelium-denuded cerebral artery segments. ET-1 reduced mean Ca2+ spark frequency to ~32% of control, elevated mean Ca2+ wave frequency to ~135% of control, and increased mean global [Ca2+]i to ~127% of control (Fig. 1A–D). Xestospongin C (XeC), an IP3R inhibitor, blocked ET-1-induced Ca2+ wave activation and reduced ET-1-induced global [Ca2+]i elevation, but did not alter ET-1-induced Ca2+ spark inhibition (Fig. 1D).
Figure 1.
ET-1 regulates local and global Ca2+ signals in arterial myocytes. A, Confocal images illustrating average fluo-4 fluorescence in myocytes in the same artery in control and ET-1 (10 nmol/L). White boxes illustrate locations where sparks occurred during 10 seconds of imaging. Colored boxes illustrate locations from where normalized fluorescence (F/F0) over time traces shown in C were determined. B, Two representative Ca2+ sparks that occurred at locations labeled in A for each condition. C, F/F0 over time traces illustrate ET-1-induced Ca2+ wave activation. D, Mean data (n=7 for each condition). P<0.05: * vs control; # vs ET-1.
ET-1-induced IP3R-mediated SR Ca2+ release elevates [Ca2+]mito and depolarizes mitochondria in cerebral artery myocytes
Regulation of [Ca2+]mito by ET-1-induced Ca2+ signals was measured in myocytes of intact arteries using 2mt8CG2 (Kd for Ca2+ ~5 µmol/L), a genetically-encoded, mitochondria-targeted, fluorescent Ca2+ indicator 14,15. Confocal imaging revealed punctate 2mt8CG2 fluorescence that exhibited ~95% pixel colocalization with Mito Tracker Orange, indicating mitochondrial localization (Fig. 2A). In myocytes of intact arteries, ionomycin, a Ca2+ ionophore, and CCCP, a protonophore that disrupts mitochondrial potential, caused fluorescence changes in 2mt8CG2 consistent with the range of this indicator 15 (Fig. 2C). ET-1 increased mean 2mt8CG2 fluorescence to ~140% of control, and this elevation was blocked by thapsigargin, a SR Ca2+ ATPase inhibitor, XeC, or Ru360, a mitochondrial Ca2+ uniporter blocker (Fig. 2B,C). Thapsigargin, XeC, or Ru360 alone, or membrane depolarization (60 mmol/L K+), which elevates global [Ca2+]i, did not alter 2mt8CG2 fluorescence (Fig. 2C).
Figure 2.
ET-1-induced IP3R-mediated SR Ca2+ release elevates [Ca2+]mito in arterial myocytes. A, Confocal images illustrate colocalization of punctate 2mt8CG2 fluorescence with MitoTracker Orange in a myocyte. Colocalization quantified using weighted colocalization was 94.8±0.8% (n=10, p<0.001). B, Confocal images of 2mt8CG2 fluorescence in myocytes in the same area of a cerebral artery in control and ET-1. Scale bars=10 µm. C, Mean data for ionomycin (10 µmol/L, n=7), ET-1 (n=8), thapsigargin (100 nmol/L, n=5), ET-1+thapsigargin (n=6), XeC (20 µmol/L, n=5), ET-1+XeC (n=6), Ru360 (10 µmol/L, n=4), ET-1+Ru360 (n=4), CCCP (10 µmol/L, n=4), and 60 mmol/L K+ (n=6). ET-1 concentration was 100 nmol/L in all experiments. Thapsigargin was applied 15 minutes prior to ET-1, a time course sufficient to deplete SR Ca2+ load 21. P<0.05: * vs control; # vs ET-1.
Mitochondria potential regulation by ET-1 was studied in isolated myocytes using TMRM. ET-1 caused reproducible concentration-dependent mitochondrial depolarization with an EC50 of 29 nmol/L (Fig. 3A–C). Thapsigargin and XeC abolished ET-1-induced mitochondrial depolarization, but did not alter TMRM fluorescence when applied alone (Fig. 3A,C). CCCP depolarized mitochondria, whereas 60 mmol/L K+ did not alter mitochondrial potential (Fig. 3A,C). These data indicate that ET-1-induced IP3R-mediated SR Ca2+ release elevates [Ca2+]mito and depolarizes mitochondria in arterial myocytes. In contrast, a global [Ca2+]i elevation does not alter [Ca2+]mito or mitochondrial potential.
Figure 3.
ET-1-induced IP3R-mediated SR Ca2+ release depolarizes mitochondria in arterial myocytes. A, ET-1 caused reproducible mitochondrial depolarization which was blocked by XeC (20 µmol/L). B, Concentration-dependent mitochondrial depolarization by ET-1 (n=4–7). C, Mean data for ET-1 (n=37), second ET-1 application (n=12), thapsigargin (100 nmol/L, n=18), ET-1+thapsigargin (n=18), XeC (20 µmol/L, n=12), ET-1+XeC (n=12), 60 mmol/L K+ (n=10), and CCCP (10 µmol/L, n= 65). ET-1 concentration was 30 nmol/L in all experiments. P<0.05: * vs control; # vs ET-1.
ET-1-induced IP3R-mediated SR Ca2+ release stimulates mitoROS generation
We tested the hypothesis that a [Ca2+]mito elevation may alter mitoROS generation. MitoROS was measured using mt-cpYFP, a genetically-encoded mitochondria-targeted fluorescent O2− indicator 16. mt-cpYFP exhibited ~95% pixel colocalization with MitoTracker Orange, indicating mitochondrial localization (Fig. 4A). In myocytes of intact arteries, ET-1 elevated mt-cpYFP fluorescence to ~199% of control, and this was blocked by XeC, Ru360, rotenone, a mitochondrial electron transport chain (ETC) complex I inhibitor, and micromolar CCCP (Fig. 4B, Supplemental Fig. I). Rotenone and micromolar CCCP reduced mt-cpYFP fluorescence when applied alone (Fig. 4B). In contrast, Ru360, XeC, and 60 mmol/L K+ did not alter mt-cpYFP fluorescence (Fig. 4B). 1 nmol/L CCCP, which induces a small mitochondrial depolarization in cerebral artery myocytes 5, elevated mt-cpYFP fluorescence (Fig. 4B).
Figure 4.
ET-1-induced IP3R-mediated SR Ca2+ release elevates mitoROS generation, leading to an increase in cytosolic ROS in arterial myocytes. A, Confocal images illustrating mt-cpYFP and MitoTracker Orange fluorescence in the same myocyte. Colocalization quantified using weighted colocalization was 94.7±1.2% (n=10, p<0.001). Scale bar=10 µm. B, Mean mt-cpYFP fluorescence changes in myocytes of intact arteries. ET-1, XeC (20 µmol/L), ET-1+XeC, Ru360 (10 µmol/L), ET-1+Ru360, rotenone (10 µmol/L), ET-1+rotenone, CCCP (10 µmol/L), ET-1+CCCP, CCCP (1 nmol/L), and 60 mmol/L K+. C, Mean HyPer-CYTO fluorescence. H2O2 (100 µmol/L), ET-1 (endothelium-intact), ET-1 (endothelium-denuded), rotenone (10 µmol/L), ET-1+rotenone, MnTMPyP (10 µmol/L), and ET-1+MnTMPyP. ET-1 concentration was 100 nmol/L in all experiments. n=5 for each condition. P<0.05: * vs control; # vs ET-1.
Cytosolic ROS was measured using HyPer-CYTO, a genetically-encoded fluorescent cytosolic hydrogen peroxide (H2O2) indicator (Supplemental Fig. IIA) 17. Exogenous H2O2 elevated HyPer-CYTO fluorescence (Fig. 4C, Supplemental Fig. IIB). ET-1 similarly increased HyPer-CYTO fluorescence in myocytes of endothelium-intact and -denuded arteries (Fig. 4C, Supplemental Fig. IIC). Rotenone and MnTMPyP, a superoxide dismutase and catalase mimetic, reduced HyPer-CYTO fluorescence when applied alone, and blocked ET-1-induced elevations in HyPer-CYTO fluorescence (Fig. 4C).
Similar results were also obtained using CM-H2DCFDA, an inorganic fluorescent ROS indicator (Supplemental Fig. IIIA–D). Oxypurinol, a xanthine oxidase inhibitor, 17-octadecanoic acid (17-ODYA), a cytochrome P450 blocker, or gp91ds-tat, a NADPH oxidase inhibitor, did not alter baseline dichlorofluorescin (DCF) fluorescence or ET-1-induced DCF fluorescence elevations (Supplemental Fig. IIIC,D). gp91ds-tat inhibited DCF fluorescence elevations induced by tumor necrosis factor-alpha (TNF-α), which stimulates NADPH oxidase-derived ROS in cerebral artery myocytes 18 (Supplemental Fig. IIID). Scrambled gp91-tat did not alter baseline DCF fluorescence, or ET-1- or TNF-α-induced DCF fluorescence elevations (Supplemental Fig. IIID).
These data indicate that mitochondria generate ROS in the absence of ET-1, and that ET-1-induced IP3R-mediated SR Ca2+ release elevates mitoROS generation, leading to an increase in cytosolic ROS in cerebral artery myocytes.
ET-1-induced IP3R-mediated mitoROS stimulate NF-κB
To examine physiological functions of an IP3R-mediated mitoROS elevation, we tested the hypothesis that mitochondria regulate the expression of genes that modulate myocyte contractility. Therefore, immunofluorescence was performed to study cellular localization of the p50 subunit of NF-κB, a ROS-sensitive transcription factor 19, in arterial myocytes. ET-1 increased nuclear translocation of p50, measured as an increase in mean pixel colocalization with YOYO-1 from ~12 to 22% (Fig. 5A–C).
Figure 5.
ET-1 stimulates p50 nuclear translocation and NF-κB-dependent transcription through SR Ca2+ release and mitoROS elevation in arterial myocytes. A, Immunofluorescence images of myocytes in arteries illustrating YOYO-1 (nuclear stain, green), p50 (red), overlay, and DIC (lumenally-inserted rectangular glass cannula can be seen). B, Enlarged images indicated by boxes in A illustrate ET-1-induced elevation in p50 and YOYO-1 pixel colocalization (purple). Scale bars=20 µm. C, Mean data (n=10 for each). D, Average NF-κB-p-Luc luciferase activity with TNF-α (100 ng/ml, n=4), ET-1 (n= 5), thapsigargin (100 nmol/L, n=5), ET-1+thapsigargin (n=5), XeC (20 µmol/L, n=5), ET-1+XeC (n=5), rotenone (1 µmol/L, n=5), ET-1+rotenone (n=5), MnTMPyP (10 µmol/L, n=4), ET-1+MnTMPyP (n=4), H2O2 (100 µmol/L, n=4), and H2O2+rotenone (n=4). ET-1 concentration was 100 nmol/L in all experiments. P<0.05: * vs control; # vs ET-1; § vs rotenone or MnTMPyP.
To investigate the regulation of NF-κB-dependent transcriptional activity, assays were performed using vectors that express firefly luciferase under the control of an NF-κB promoter. As a control, regulation of luciferase activity by TNF-α, which activates NF-κB was measured. TNF-α increased mean luciferase activity to ~547% of control (Fig. 5D). ET-1 increased luciferase activity to ~372% of control, and this was blocked by thapsigargin and XeC (Fig. 5D). Rotenone and MnTMPyP reduced the ET-1-induced elevation in luciferase activity (Fig. 5D). Rotenone also blocked the ET-1-induced ROS elevation over the 24-hour period used for these transcriptional measurements (Supplemental Fig. IV). Exogenous H2O2 elevated luciferase activity, and this effect was not altered by rotenone, indicating that rotenone did not cause general inhibition of luciferase expression (Fig. 5D). When applied alone, thapsigargin, XeC, rotenone, and MnTMPyP reduced luciferase activity by ~16, 22, 23, and 20%, respectively (Fig. 5D). TNF-α, ET-1, thapsigargin, XeC, rotenone, MnTMPyP, and H2O2 did not alter luciferase activity in arteries in which a promoter-deficient control vector was inserted (Supplemental Fig. V). These data indicate that IP3R-mediated SR Ca2+ release stimulates NF-κB primarily by elevating mitoROS generation, but also via a secondary mitochondria- and ROS-independent pathway in arterial myocytes.
IP3R-mediated SR Ca2+ release stimulates NF-κB and CaV1.2 expression
We sought to examine the functional significance of IP3R-mediated, mitochondrial-dependent NF-κB activation. CaV1.2 channels are the principal Ca2+ influx pathway in myocytes of resistance-size arteries and are essential for contractility regulation by a wide variety of stimuli, including intravascular pressure and membrane potential 8. However, mechanisms that regulate CaV1.2 gene (CACNA1C) transcription in arterial myocytes are unclear. Real-time PCR data indicated that ET-1 (6 hrs) increased CaV1.2 channel mRNA expression to ~244% of control in cerebral arteries (Fig. 6A, Supplemental Fig VI). Western blotting indicated that ET-1 (24 hrs) increased CaV1.2 channel (190 and 240 kD bands) protein to ~141% of control (Fig. 6B,C). XeC, rotenone, and MnTMPyP alone reduced basal CaV1.2 expression to ~87, 89 and 80% of control, respectively (Fig. 6B,C). XeC blocked, and rotenone and MnTMPyP reduced the ET-1-induced elevation in CaV1.2 expression (Fig. 6B,C). Exogenous H2O2 increased CaV1.2 expression, and this elevation was not altered by rotenone, indicating that rotenone did not induce non-specific inhibition of CaV1.2 expression (Fig. 6C). These data indicate that ET-1-induced IP3R-mediated SR Ca2+ release and mitoROS elevation stimulate CaV1.2 expression in cerebral arteries.
Figure 6.
ET-1-induced IP3R-mediated SR Ca2+ release and mitoROS elevation stimulate CaV1.2 expression in cerebral arteries. A, ET-1 elevated mean CaV1.2 mRNA (n=7). B, Western blot indicating that XeC (20 µmol/L) blocked ET-1-induced elevation in CaV1.2 expression. C, Mean data for ET-1 (n=12), XeC (20 µmol/L, n=7), ET-1+XeC (n=7), rotenone (1µmol/L, n=6), ET-1+rotenone (n=6), MnTMPyP (10 µmol/L, n=5), ET-1+MnTMPyP (n=5), H2O2 (100 µmol/L, n=5), and H2O2+rotenone (n=5). ET-1 concentration was 10 nmol/L in all experiments. P<0.05: * vs untreated; # vs ET-1; § vs rotenone or MnTMPyP.
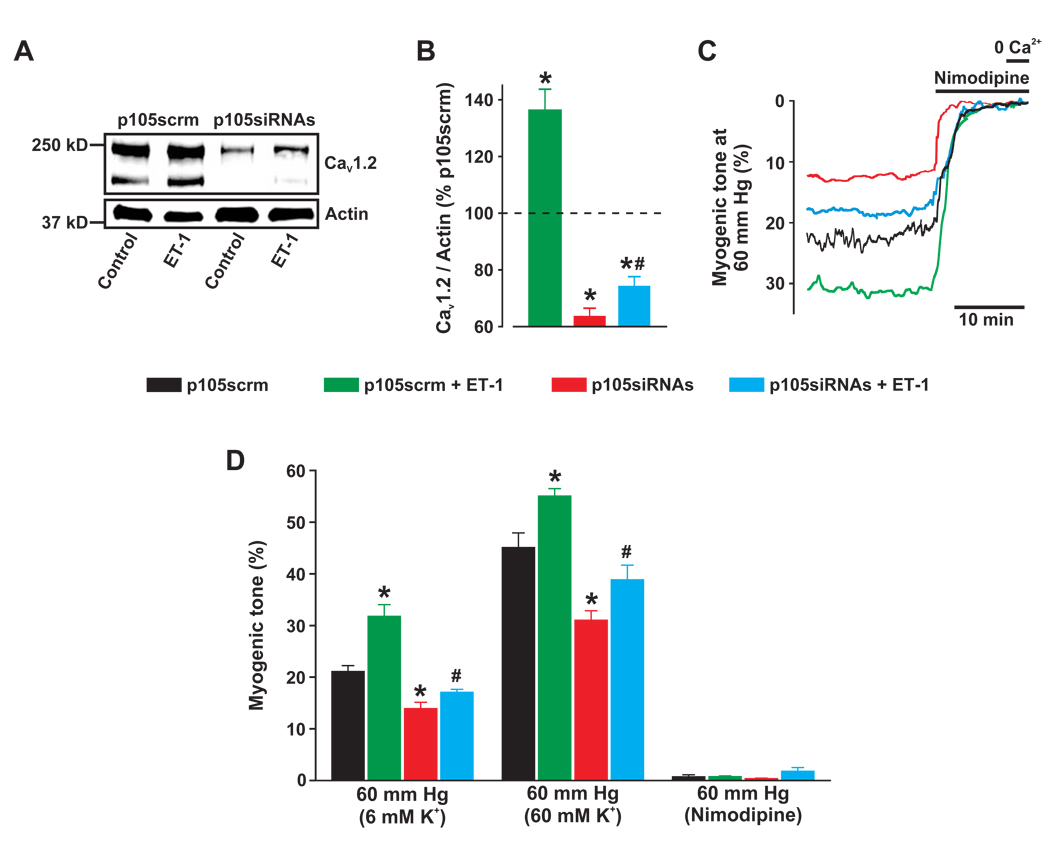
Two different siRNAs were used to knockdown expression of p105, the p50 precursor. p105siRNA1, p105siRNA2, and a combination of both siRNAs (p105siRNAs) reduced mean p105 protein to ~73, 80, and 57%, respectively, of that in arteries treated with scrambled siRNA (p105scrm) (Supplemental Fig. VIIA,B). p105siRNAs also reduced p50 expression to ~53% of p105scrm (Supplemental Fig. VIIIC,D). p105siRNAs reduced CaV1.2 expression to ~64% of p105scrm (Fig. 7A,B). In p105scrm-treated arteries, ET-1 increased CaV1.2 protein to ~136% of p105scrm (Fig. 7A,B). p105siRNAs reduced the ET-1-induced elevation in CaV1.2 expression by ~71% (Fig. 7A,B). These data indicate that NF-κB controls basal CaV1.2 expression and that IP3R-mediated SR Ca2+ release stimulates CaV1.2 expression via mitochondria-dependent NF-κB activation.
Figure 7.
NF-κB controls basal and ET-1-induced elevation in functional CaV1.2 expression in cerebral arteries. A, Western blot illustrating effects of p105siRNAs (10 µg/ml each) on basal and ET-1-induced CaV1.2 expression. B, Mean data. n=5 for each. C, Representative traces illustrating diameter in arteries pressurized to 60 mm Hg. Nimodipine (1 µmol/L) fully dilated p105scrm- and p105siRNAs-treated arteries. D, Mean myogenic tone at 60 mm Hg, in 60 mmol/L K+ at 60 mm Hg, and nimodipine at 60 mm Hg. n=5–6 for each. ET-1 concentration was 10 nmol/L in all experiments. P<0.05: * vs p105scrm; # vs p105siRNAs.
The p105 gene promoter contains an NF-κB-binding sequence, and p50 activation can elevate p105 expression 20. Therefore, mechanisms that regulate NF-κB subunit expression in cerebral arteries were investigated. XeC reduced basal p105 and p50 expression, whereas rotenone or MnTMPyP did not alter basal p105 or p50 expression (Supplemental Fig. VIIIA,B). ET-1 elevated p105 and p50 expression and XeC blocked this effect (Supplemental Fig. VIIIA,B). In contrast, rotenone or MnTMPyP had no effect on the ET-1-induced elevation in p105 and p50 subunit expression (Supplemental Fig. VIIIB). Exogenous H2O2 applied alone or in the presence of rotenone did not change p105 and p50 expression (Supplemental Fig. VIIIB). p105siRNAs did not alter the ET-1-induced relative increase in p105 and p50 expression (Supplemental Fig. VIIIC,D). These data indicate that IP3R-mediated SR Ca2+ release activates p105/p50 expression via a mitochondria-, ROS-, and NF-κB-independent pathway. Data also indicate that rotenone, MnTMPyP, and p105siRNAs do not cause general inhibition of gene transcription.
NF-κB stimulates functional CaV1.2 expression in cerebral artery myocytes
NF-κB regulation of functional CaV1.2 expression was examined in pressurized cerebral arteries. Arteries were treated with either p105scrm or p105siRNAs and then exposed to either no further treatment or to ET-1 for 24 hours. At 60 mm Hg, control arteries (p105scrm) developed ~21% myogenic tone and membrane depolarization with 60 mmol/L K+ elevated tone to ~45%. p105 knockdown (p105siRNAs) reduced myogenic tone and depolarization-induced vasoconstriction (Fig. 7C,D). A 24 hour exposure to ET-1 elevated mean myogenic tone and depolarization-induced tone in control arteries (Fig. 7C,D). p105 knockdown attenuated the ET-1-induced elevation in myogenic tone and depolarization-induced vasoconstriction (Fig. 7C,D). Nimodipine fully dilated arteries regardless of treatment, indicating that vasoconstriction occurred due to CaV1.2 channel activation (Fig. 7C,D). These data indicate that NF-κB is essential for functional basal CaV1.2 expression in cerebral artery myocytes and that ET-1-induced NF-κB activation elevates CaV1.2-dependent vasoconstriction.
Discussion
Here, we investigated physiological signaling mechanisms that regulate [Ca2+]mito and functional consequences of changes in [Ca2+]mito in myocytes of resistance-size cerebral arteries. A schematic diagram summarizing major findings of this study is provided in Figure 8. Our data indicate that ET-1-induced IP3R-mediated SR Ca2+ release increases Ca2+ wave frequency, elevates [Ca2+]mito and depolarizes mitochondria, leading to an increase in mitoROS generation. ET-1-induced IP3R-mediated mitoROS elevate cytosolic ROS that increase NF-κB nuclear translocation and transcriptional activity. ET-1-induced NF-κB activation leads to an increase in CaV1.2 transcription and CaV1.2-dependent pressure- and depolarization-induced vasoconstriction. IP3R-mediated SR Ca2+ release, mitoROS, and NF-κB control basal CaV1.2 expression and NF-κB knockdown reduces CaV1.2 expression, leading to vasodilation. Collectively, these data indicate that mitochondria sense IP3R-mediated SR Ca2+ release to control functional CaV1.2 channel transcription in arterial myocytes, thereby regulating arterial contractility.
Figure 8.
Proposed signaling pathways that control basal and ET-1-induced CaV1.2 expression in cerebral artery myocytes. Δψm indicates mitochondrial potential.
PLC-coupled receptor agonists elevate IP3, which stimulates IP3Rs, and diacylglycerol, which activates protein kinase C (PKC). Vasoconstrictor-induced IP3R activation elevates [Ca2+]i in arterial myocytes via two distinct mechanisms: 1) SR Ca2+ release, which produces Ca2+ waves 9,13, and 2) SR Ca2+ release-independent plasma membrane TRPC3 channel activation, which leads to depolarization-induced CaV1.2 channel activation and a global [Ca2+]i elevation 13,21,22. In contrast, vasoconstrictor-activated PKC inhibits Ca2+ sparks, leading to membrane depolarization, CaV1.2 channel activation, and a global [Ca2+]i elevation 9. The regulation of arterial myocyte [Ca2+]mito by these different intracellular Ca2+ signals was unclear. Electron microscopy indicates that mitochondria can be located in close proximity (~20 nm) to the SR membrane in many cell types, including cultured arterial myocytes 23,24. Such localization could place mitochondria within the vicinity of SR Ca2+ release channels that generate local micromolar Ca2+ transients necessary for mitochondrial Ca2+ uptake via the uniporter 25. Inorganic Ca2+ indicators, which cannot be targeted specifically to organelles, have been used to measure [Ca2+]mito in cultured vascular myocytes. In these studies, SR Ca2+ release elicited a [Ca2+]mito elevation 24,26. Ca2+ influx via the plasma membrane Na+/Ca2+ exchanger also elevated [Ca2+]mito in cultured vascular myocytes 27. In non-cultured colonic myocytes, uncaging IP3 leads to mitochondrial Ca2+ uptake that feeds back to regulate IP3R activity 28. To our knowledge, our study is the first to use a genetically-encoded mitochondria-targeted fluorescent indicator to measure [Ca2+]mito in contractile arterial myocytes. Our data indicate that in resting arterial myocytes, mitochondria contain Ca2+ and generate low levels of ROS through mechanisms that are independent of IP3R-mediated SR Ca2+ release. ET-1-induced IP3R-mediated SR Ca2+ release stimulated Ca2+ waves and elevated [Ca2+]mito, leading to mitochondrial depolarization and mitoROS generation. In contrast, a global [Ca2+]i elevation did not alter [Ca2+]mito, mitochondrial potential or mitoROS generation. These findings also raise the possibility that Ca2+ waves specifically regulate [Ca2+]mito in arterial myocytes. Given that Ca2+ waves are propagating Ca2+ signals, [Ca2+]mito may also oscillate. Here, [Ca2+]mito was simultaneously measured within multiple myocytes in the arterial wall. Asynchronous Ca2+ oscillations within individual mitochondria would have been averaged out by the imaging protocol. Future studies should be designed to examine spatial and temporal relationships between Ca2+ waves and mitochondrial Ca2+ signals within individual mitochondria in arterial myocytes.
The mechanism by which mitochondrial Ca2+ uptake induces mitochondrial depolarization and elevates ETC-generated mitoROS was not determined, but several possibilities exist. Mitochondrial depolarization has been demonstrated to increase or decrease ROS in different cell types, including vascular myocytes 5,16,17,29. Here, a small mitochondrial depolarization increased mitoROS generation, whereas a large mitochondrial depolarization inhibited mitoROS production, consistent with an earlier report in cerebral artery myocytes 5. A [Ca2+]mito elevation may stimulate mitoROS generation through multiple mechanisms, including stimulation of the tricarboxylic acid cycle 29. In addition, a [Ca2+]mito elevation and mitochondrial depolarization can open the mitochondrial permeability transition pore (mPTP) 29,30, which in turn can enhance depolarization 29. mPTP opening elevates mitoROS generation via several pathways, including dissipation of chemical gradients across the mitochondrial membrane and diversion of electrons in the ETC to ROS generation 16. Therefore, a [Ca2+]mito elevation and mitochondrial depolarization may stimulate mitoROS generation by opening mPTP. MitoROS production is also regulated by multiple additional factors, including redox status of respiratory substrates, and proton pumping by ETC complexes 31. Future investigations should examine the mechanisms by which a vasoconstrictor-induced [Ca2+]mito elevation and mitochondrial depolarization stimulate mitoROS generation.
Regulation and physiological functions of NF-κB in contractile arterial myocytes are poorly understood. Data here indicate that ET-1-induced NF-κB activation occurs primarily via IP3R-mediated mitoROS generation in cerebral artery myocytes. MitoROS activate NF-κB in several cell types, including cultured vascular myocytes 19,32. Alternate ROS-mediated mechanisms can also activate NF-κB in arterial myocytes. For example, following balloon injury NAD(P)H oxidase-derived ROS stimulate NF-κB in arterial myocytes 33. The mechanisms by which ROS activate NF-κB are unclear, with reports suggesting that ROS stimulate IκB kinase (IKK), leading to phosphorylation and proteasomal degradation of I kappa B (IκB) 32. Other studies indicate that ROS activate kinases other than IKK to phosphorylate IκB 34. Additional studies will be necessary to identify the specific ROS involved and the mechanisms by which ROS activate NF-κB in contractile arterial myocytes. Data here indicate that IP3R-mediated SR Ca2+ release also activates NF-κB via a secondary mitoROS-independent pathway. Supporting bi-modal activation, redox-dependent and -independent mechanisms activate NF-κB in U937 cells 35. Redox-independent NF-κB activation mechanisms may be mediated by calcineurin, PI3K/Akt, and/or protein kinase C, as demonstrated in neurons 36.
CaV1.2 channels are the principal functional Ca2+ influx pathway in myocytes of resistance-size arteries 1,7,8. Our data indicate that basal CaV1.2 expression is controlled through both an IP3R-mediated mitochondria-independent pathway and an IP3R-independent mitoROS pathway acting through NF-κB. ET-1-induced IP3R activation stimulates an NF-κB-dependent elevation in CaV1.2 expression primarily via a mitoROS-dependent pathway and via a secondary mitochondria-independent pathway. In the absence of PLC-coupled receptor ligands, intracellular IP3 concentration ([IP3]i) and thus, IP3R activity should be low. However, in the intact artery preparation studied here endothelial cell release of receptor ligands, including ET-1, may generate low levels of [IP3]i in myocytes. In our experiments, the degree of p105 knockdown and the reduction in basal CaV1.2 expression were similar, indicating that NF-κB is a major CaV1.2 gene transcription activator in arterial myocytes. In contrast, in colonic myocytes NF-κB p50 and p65 subunit activation reduced CaV1.2 expression 37. Opposing regulation by NF-κB in these different myocyte types may occur through interaction with different κB binding motifs, of which there are several upstream of the CaV1.2 gene 37. Furthermore, the presence or absence of additional transcriptional activators and/or repressors may explain differential regulation of CaV1.2 expression by NF-κB. Our data also indicate that ET-1-induced IP3R-mediated SR Ca2+ release stimulates NF-κB subunit expression through a mitochondria-, ROS- and NF-κB-independent pathway. The ET-1-induced elevation in NF-κB expression may serve to amplify CaV1.2 expression. In rat renal arteries, membrane depolarization increased CaV1.2 protein 38, whereas here IP3Rs were necessary for ET-1-induced elevation in CaV1.2 expression. Data from these studies raise several possibilities, including that local and global Ca2+ signals regulate CaV1.2 expression by different mechanisms in cerebral and renal artery myocytes. Conceivably, this could occur at many levels, including that global Ca2+ may regulate [Ca2+]mito in renal artery myocytes, leading to ROS generation and NF-κB activation.
Data here and previous studies suggest that vasoconstrictors cause a similar shift in local and global Ca2+ signals in myocytes of anatomically different arteries 9,39. Therefore, IP3R regulation of Ca2+ waves, [Ca2+]mito and mitoROS generation may be a common mechanism by which vasoconstrictors regulate myocyte NF-κB activity and functional CaV1.2 expression. IP3R-mediated SR Ca2+ release and local Ca2+ influx through CaV1.2 channels also stimulates calcineurin-dependent nuclear translocation of nuclear factor of activated T cell c3 in arterial myocytes 40,41. Data here not only indicate that IP3Rs control physiological CaV1.2 expression via a mitoROS/NF-κB pathway, but raise the possibility that disease-associated alterations in ion channel expression may also occur through activation of this pathway. Many vasoconstrictors, including ET-1, are elevated in cardiovascular diseases, including systemic hypertension 42. Hypertension is also associated with an increase in vascular ROS, NF-κB activity, and CaV1.2 protein and currents 43–45. Therefore, targeting this transcriptional pathway may be beneficial in treating cardiovascular diseases.
In summary, this study indicates that mitochondria sense IP3R-mediated SR Ca2+ release to control the activity of NF-κB, which stimulates functional CaV1.2 expression in cerebral artery myocytes.
Novelty and Significance.
What is known?
Mitochondria can sequester calcium (Ca2+) when exposed to elevated levels of intracellular Ca2+ ([Ca2+]i).
Mitochondria generate reactive oxygen species (ROS), which can regulate the activity of a variety of downstream targets, including several transcription factors.
Voltage-dependent Ca2+ (CaV1.2) channels are a major contributor to arterial smooth muscle cell global [Ca2+]i, a Ca2+ signal that regulates blood pressure and regional blood flow.
What new information does this article contribute?
In contractile arterial smooth muscle cells, sarcoplasmic reticulum (SR) Ca2+ released by inositol 1,4,5-trisphosphate receptors (IP3R) specifically elevates mitochondrial Ca2+ concentration ([Ca2+]mito), leading to mitochondrial depolarization.
An IP3R-mediated elevation in [Ca2+]mito induces the generation of mitochondrial ROS (mitoROS), which activate nuclear factor kappa B (NF-κB), a transcription factor.
NF-κB controls basal CaV1.2 expression and IP3R-mediated mitoROS-induced NF-κB activation elevates CaV1.2, leading to vasoconstriction.
Physiological functions of mitochondria in contractile arterial smooth muscle cells are poorly understood. Arterial smooth muscle cells generate several distinct local and global Ca2+ signals. Mitochondria can sequester Ca2+, but Ca2+ signals that regulate [Ca2+]mito and the significance of changes in [Ca2+]mito are unclear. We show that IP3R-mediated SR Ca2+ release, but not a global [Ca2+]i elevation, elevates [Ca2+]mito. An IP3R-mediated [Ca2+]mito elevation stimulates the generation of mitoROS, which activate NF-κB. IP3R-mediated mitoROS-induced NF-κB activation elevates functional CaV1.2 expression. This study indicates that mitochondria sense changes in IP3R-mediated SR Ca2+ release to alter NF-κB-mediated CaV1.2 expression, thereby modulating arterial contractility. Many cardiovascular diseases, including hypertension, are associated with altered ROS generation, NF-κB activity, CaV1.2 expression, and vascular contractility. The identification of this novel mitochondrial signaling pathway controlling arterial contractility may lead to the development of new approaches to treat cardiovascular diseases.
Supplementary Material
Acknowledgements
We thank Drs. John Bannister and Adebowale Adebiyi for comments on the manuscript and Drs. Lidia A. Gardner and John Cox for technical assistance with immunofluorescence analysis.
Sources of Funding
NIH grants HL67061, HL77678, and HL094378 to J.H.J.. D.N. is a recipient of a Predoctoral Fellowship from the American Heart Association Greater Southeast Affiliate (R079008156).
Non-standard Abbreviations and Acronyms
- Ca2+
Calcium
- [Ca2+]i
Intracellular Ca2+ concentration
- [Ca2+]mito
Mitochondrial Ca2+ concentration
- CaV1.2
Voltage-dependent Ca2+ channel
- CCCP
Carbonyl cyanide 3-chlorophenylhydrazone
- CT
fluorescence threshold
- DCF
Dichlorofluorescin
- ET-1
Endothelin-1
- ETC
Mitochondrial electron transport chain
- F
fluorescence
- F0
baseline fluorescence
- gp91ds-tat
gp91phox docking sequence peptide conjugated to tat
- gp91scrm-tat
scrambled gp91phox peptide conjugated to tat
- H2O2
Hydrogen peroxide
- IκB
I kappa B
- IKK
IκB kinase
- IP3
Inositol 1,4,5-trisphosphate
- [IP3]i
Intracellular IP3 concentration
- IP3R
IP3 receptor
- mitoROS
Mitochondrial reactive oxygen species
- MnTMPyP
Manganese (III) tetrakis(1-methyl-4-pyridyl)porphyrin
- mPTP
Mitochondrial permeability transition pore
- NF-κB
Nuclear factor kappa B
- NF-κB-p-Luc
Vectors that express firefly luciferase under the control of an NF-κB promoter
- 17-ODYA
17-octadecanoic acid
- p105
NF-κB p105 subunit
- p105siRNA
siRNA directed against NF-κB p105 subunit
- p105scrm
Scrambled siRNA
- p50
NF-κB p50 subunit
- PBS
phosphate-buffered saline
- PKC
Protein kinase C
- PLC
Phospholipase C
- PSS
physiological salt solution
- ROS
Reactive Oxygen Species
- Rps5
ribosomal protein S5
- RyR
Ryanodine-sensitive Ca2+ channel
- SR
Sarcoplasmic reticulum
- TMRM
Tetramethylrhodamine Methyl Ester
- TNF-α
Tumor necrosis factor-alpha
- XeC
Xestospongin C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: [136] Calcium cycling/excitation-contraction coupling, [138] Cell signalling/signal transduction, [142] Gene expression, [152] Ion channels/membrane transport.
Disclosures
None.
References
- 1.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 2.Chalmers S, Olson ML, MacMillan D, Rainbow RD, McCarron JG. Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium. 2007;42:447–466. doi: 10.1016/j.ceca.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Poburko D, Lee CH, van Breemen C. Vascular smooth muscle mitochondria at the cross roads of Ca2+ regulation. Cell Calcium. 2004;35:509–521. doi: 10.1016/j.ceca.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Cheranov SY, Jaggar JH. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol. 2004;556:755–771. doi: 10.1113/jphysiol.2003.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santana LF, Navedo MF. Molecular and biophysical mechanisms of Ca2+ sparklets in smooth muscle. J Mol Cell Cardiol. 2009;47:436–444. doi: 10.1016/j.yjmcc.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 8.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel CaV1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem. 2007;282:29211–29221. doi: 10.1074/jbc.M610623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–C1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- 10.Heppner TJ, Bonev AD, Santana LF, Nelson MT. Alkaline pH shifts Ca2+ sparks to Ca2+ waves in smooth muscle cells of pressurized cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2169–H2176. doi: 10.1152/ajpheart.00603.2002. [DOI] [PubMed] [Google Scholar]
- 11.Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol. 1999;518:815–824. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruehlmann DO, Lee CH, Poburko D, van Breemen C. Asynchronous Ca2+ waves in intact venous smooth muscle. Circ Res. 2000;86:E72–E79. doi: 10.1161/01.res.86.4.e72. [DOI] [PubMed] [Google Scholar]
- 13.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295:C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippin L, Abad MC, Gastaldello S, Magalhaes PJ, Sandona D, Pozzan T. Improved strategies for the delivery of GFP-based Ca2+ sensors into the mitochondrial matrix. Cell Calcium. 2005;37:129–136. doi: 10.1016/j.ceca.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang WZ, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 18.Cheranov SY, Jaggar JH. TNF-α dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation. Am J Physiol Cell Physiol. 2006;290:C964–C971. doi: 10.1152/ajpcell.00499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Seo KW, Yun MR, Bae SS, Lee WS, Hong KW, Kim CD. 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-κB signaling pathways. Free Radic Biol Med. 2008;45:1487–1492. doi: 10.1016/j.freeradbiomed.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Ten RM, Paya CV, Israel N, Le BO, Mattei MG, Virelizier JL, Kourilsky P, Israel A. The characterization of the promoter of the gene encoding the p50 subunit of NF-κB indicates that it participates in its own regulation. EMBO J. 1992;11:195–203. doi: 10.1002/j.1460-2075.1992.tb05042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res. 2008;102:1118–1126. doi: 10.1161/CIRCRESAHA.108.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res. 2010;106:1603–1612. doi: 10.1161/CIRCRESAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szado T, Kuo KH, Bernard-Helary K, Poburko D, Lee CH, Seow C, Ruegg UT, van Breemen C. Agonist-induced mitochondrial Ca2+ transients in smooth muscle. FASEB J. 2003;17:28–37. doi: 10.1096/fj.02-0334com. [DOI] [PubMed] [Google Scholar]
- 24.Pacher P, Sharma K, Csordas G, Zhu Y, Hajnoczky G. Uncoupling of ER-mitochondrial calcium communication by transforming growth factor-β. Am J Physiol Renal Physiol. 2008;295:F1303–F1312. doi: 10.1152/ajprenal.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 26.Monteith GR, Blaustein MP. Heterogeneity of mitochondrial matrix free Ca2+ : resolution of Ca2+ dynamics in individual mitochondria in situ. Am J Physiol. 1999;276:C1193–C1204. doi: 10.1152/ajpcell.1999.276.5.C1193. [DOI] [PubMed] [Google Scholar]
- 27.Poburko D, Liao CH, van Breemen C, Demaurex N. Mitochondrial regulation of sarcoplasmic reticulum Ca2+ content in vascular smooth muscle cells. Circ Res. 2009;104:104–112. doi: 10.1161/CIRCRESAHA.108.180612. [DOI] [PubMed] [Google Scholar]
- 28.Olson ML, Chalmers S, McCarron JG. Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1,4,5-trisphosphate receptor clusters in smooth muscle cells. J Biol Chem. 2010;285:2040–2050. doi: 10.1074/jbc.M109.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 30.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 31.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 32.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Souza HP, Souza LC, Anastacio VM, Pereira AC, Junqueira ML, Krieger JE, da Luz PL, Augusto O, Laurindo FR. Vascular oxidant stress early after balloon injury: evidence for increased NAD(P)H oxidoreductase activity. Free Radic Biol Med. 2000;28:1232–1242. doi: 10.1016/s0891-5849(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 34.Brzoska K, Szumiel I. Signalling loops and linear pathways: NF-κB activation in response to genotoxic stress. Mutagenesis. 2009;24:1–8. doi: 10.1093/mutage/gen056. [DOI] [PubMed] [Google Scholar]
- 35.Shatrov VA, Lehmann V, Chouaib S. Sphingosine-1-phosphate mobilizes intracellular calcium and activates transcription factor NF-κB in U937 cells. Biochem Biophys Res Commun. 1997;234:121–124. doi: 10.1006/bbrc.1997.6598. [DOI] [PubMed] [Google Scholar]
- 36.Lilienbaum A, Israel A. From calcium to NF-κB signaling pathways in neurons. Mol Cell Biol. 2003;23:2680–2698. doi: 10.1128/MCB.23.8.2680-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle CaV1.2 channels by p50 and p65 subunits of nuclear factor-κB. Gastroenterology. 2005;129:1518–1532. doi: 10.1053/j.gastro.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 38.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–e104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 39.Mauban JR, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am J Physiol Heart Circ Physiol. 2001;280:H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- 40.Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem. 2002;277:37756–37764. doi: 10.1074/jbc.M203596200. [DOI] [PubMed] [Google Scholar]
- 41.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdel-Sayed S, Nussberger J, Aubert JF, Gohlke P, Brunner HR, Brakch N. Measurement of plasma endothelin-1 in experimental hypertension and in healthy subjects. Am J Hypertens. 2003;16:515–521. doi: 10.1016/s0895-7061(03)00903-8. [DOI] [PubMed] [Google Scholar]
- 43.Touyz RM, Schiffrin EL. Reactive oxygen species and hypertension: a complex association. Antioxid Redox Signal. 2008;10:1041–1044. doi: 10.1089/ars.2007.2012. [DOI] [PubMed] [Google Scholar]
- 44.Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39:809–814. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- 45.Wang WZ, Saada N, Dai B, Pang L, Palade P. Vascular-specific increase in exon 1B-encoded Cav1.2 channels in spontaneously hypertensive rats. Am J Hypertens. 2006;19:823–831. doi: 10.1016/j.amjhyper.2006.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.