Abstract
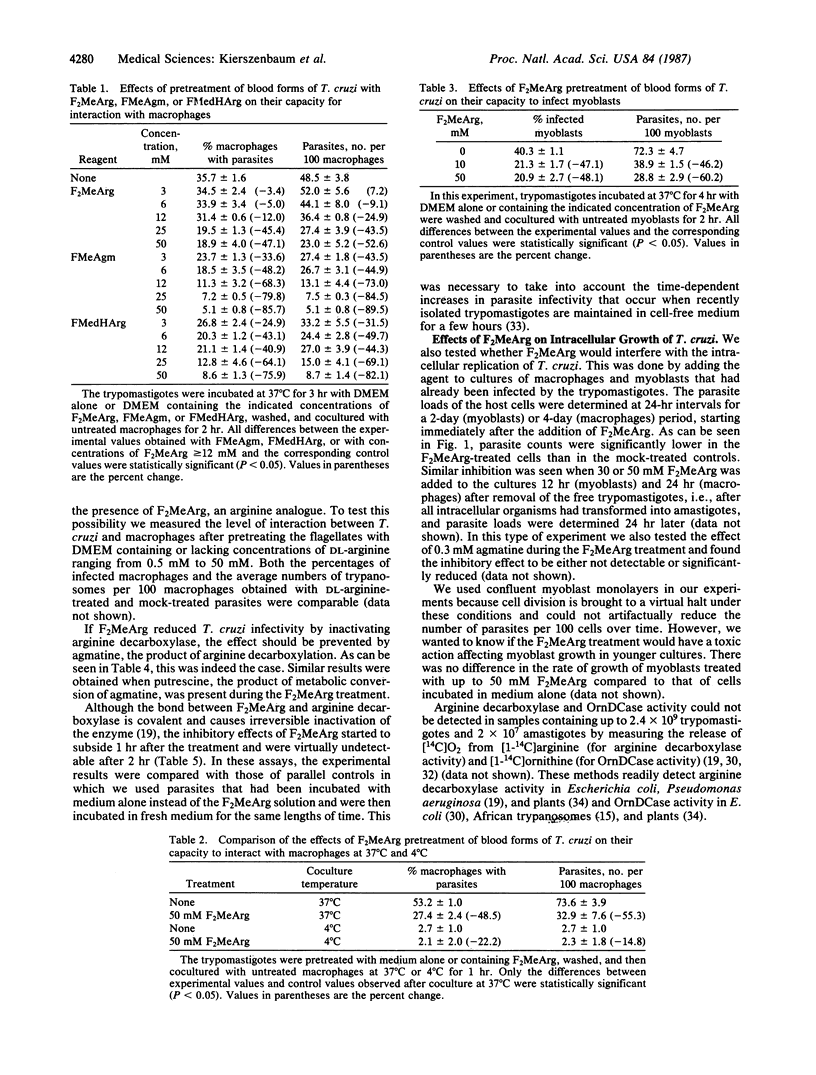
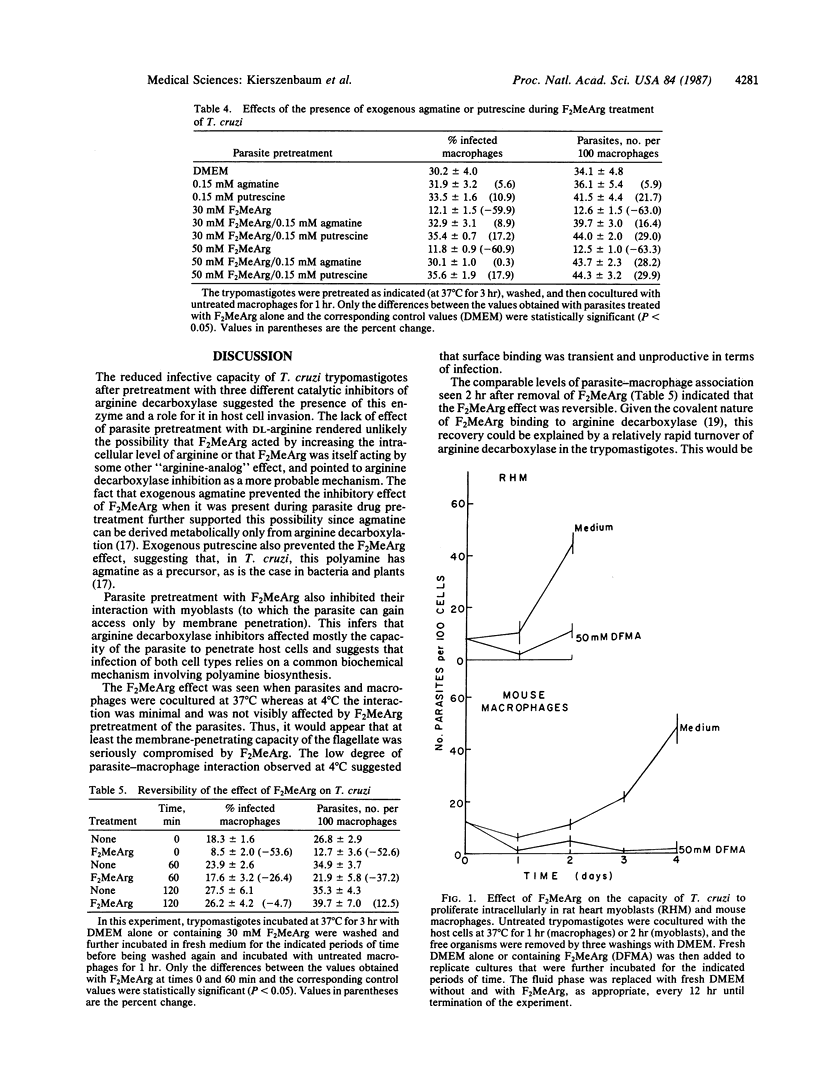
The capacity of blood (trypomastigote) forms of Trypanosoma cruzi to infect mouse peritoneal macrophages or rat heart myoblasts in vitro was inhibited by treatment of the trypomastigotes with DL-alpha-difluoromethylarginine (F2Me Arg), monofluoromethylagmatine, or (E)-alpha-monofluoromethyl-3-4-dehydroarginine--all irreversible inhibitors of arginine decarboxylase. Similar results were obtained when F2MeArg-treated parasites were incubated with rat heart myoblasts. The inhibitory effects were characterized by marked reductions in both the proportion of infected cells and the number of parasites per 100 host cells. The concentrations of the arginine decarboxylase inhibitors that affected infectivity had no detectable effect on either the concentration or motility of the parasite and, therefore, could not have affected the collision frequency. F2MeArg appeared to inhibit the ability of T. cruzi to penetrate the host cells since the drug had no significant effect on the extent of parasite binding to the surface of the host cells. The inhibitory effect of F2MeArg was markedly reduced or abrogated in the presence of either agmatine or putrescine, as would have been expected if F2MeArg acted by inhibiting arginine decarboxylase. Addition of F2MeArg to macrophage or myoblast cultures immediately after infection or at a time when virtually all of the intracellular parasites had transformed into the multiplicative amastigote form, resulted in a markedly reduced parasite growth rate. This effect was also prevented by exogenous agmatine. These results indicate the importance of polyamines and polyamine biosynthesis in the following two important functions of T. cruzi: invasion of host cells and intracellular multiplication. Furthermore, concentrations of the inhibitors tested that affected the parasite did not alter the viability of the host cells, the cellular density of the cultures, or the ability of uninfected myoblasts to grow. Thus, arginine decarboxylase inhibitors may have a potential application in chemotherapy against T. cruzi infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. W., Colli W. Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J Protozool. 1982 May;29(2):264–269. doi: 10.1111/j.1550-7408.1982.tb04024.x. [DOI] [PubMed] [Google Scholar]
- Bacchi C. J. Content, synthesis, and function of polyamines in trypanosomatids: relationship to chemotherapy. J Protozool. 1981 Feb;28(1):20–27. doi: 10.1111/j.1550-7408.1981.tb02798.x. [DOI] [PubMed] [Google Scholar]
- Birecka H., Bitonti A. J., McCann P. P. Assaying ornithine and arginine decarboxylases in some plant species. Plant Physiol. 1985 Oct;79(2):509–514. doi: 10.1104/pp.79.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., Bacchi C. J., McCann P. P., Sjoerdsma A. Catalytic irreversible inhibition of Trypanosoma brucei brucei ornithine decarboxylase by substrate and product analogs and their effects on murine trypanosomiasis. Biochem Pharmacol. 1985 May 15;34(10):1773–1777. doi: 10.1016/0006-2952(85)90648-3. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Casara P. J., McCann P. P., Bey P. Catalytic irreversible inhibition of bacterial and plant arginine decarboxylase activities by novel substrate and product analogues. Biochem J. 1987 Feb 15;242(1):69–74. doi: 10.1042/bj2420069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Brener Z. Present status of chemotherapy and chemoprophylaxis of human trypanosomiasis in the Western Hemisphere. Pharmacol Ther. 1979;7(1):71–90. doi: 10.1016/0163-7258(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Kierszenbaum F. Isolation of Trypanosoma cruzi from blood. J Parasitol. 1974 Dec;60(6):1037–1038. [PubMed] [Google Scholar]
- Connelly M. C., Kierszenbaum F. Increased host cell-Trypanosoma cruzi interaction following phospholipase D treatment of the parasite surface. Mol Biochem Parasitol. 1985 Nov;17(2):191–202. doi: 10.1016/0166-6851(85)90018-0. [DOI] [PubMed] [Google Scholar]
- Crane M. S., Dvorak J. A. Influence of monosaccharides on the infection of vertebrate cells by Trypanosoma cruzi and Toxoplasma gondii. Mol Biochem Parasitol. 1982 May;5(5):333–341. doi: 10.1016/0166-6851(82)90040-8. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985 Mar 22;227(4693):1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Henriquez D., Piras R., Piras M. M. The effect of surface membrane modifications of fibroblastic cells on the entry process of Trypanosoma cruzi trypomastigotes. Mol Biochem Parasitol. 1981 Apr;2(5-6):359–366. doi: 10.1016/0166-6851(81)90087-6. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Lima M. F., Kierszenbaum F. Biochemical requirements for intracellular invasion by Trypanosoma cruzi: protein synthesis. J Protozool. 1982 Nov;29(4):566–570. doi: 10.1111/j.1550-7408.1982.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Mercado T. I., Katusha K. Isolation of Trypanosoma cruzi from the blood of infected mice by column chromatography. Prep Biochem. 1979;9(1):97–106. doi: 10.1080/00327487908061675. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Osuna A., Ortega G., Gamarro F., Castanys S., Mascaro M. C. Some factors affecting the in vitro invasion of HeLa cells by Trypanosoma cruzi. Int J Parasitol. 1984 Jun;14(3):253–257. doi: 10.1016/0020-7519(84)90076-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras R., Piras M. M., Henriquez D. The effect of inhibitors of macromolecular biosynthesis on the in vitro infectivity and morphology of Trypanosoma cruzi trypomastigotes. Mol Biochem Parasitol. 1982 Aug;6(2):83–92. doi: 10.1016/0166-6851(82)90067-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Adiga P. R. Arginine decarboxylase from Lathyrus sativus seedlings. Purification and properites. Eur J Biochem. 1975 Nov 15;59(2):377–386. doi: 10.1111/j.1432-1033.1975.tb02465.x. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Growth of isolated amastigotes of Trypanosoma cruzi in cell-free medium. J Protozool. 1982 Nov;29(4):570–576. doi: 10.1111/j.1550-7408.1982.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of surface N-acetylglucosamine residues on host cell infection by Trypanosoma cruzi. Biochim Biophys Acta. 1985 May 30;845(2):216–222. doi: 10.1016/0167-4889(85)90179-x. [DOI] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. The effect of swainsonine on the association of Trypanosoma cruzi with host cells. Mol Biochem Parasitol. 1985 Jun;16(1):1–10. doi: 10.1016/0166-6851(85)90044-1. [DOI] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol. 1984 Jul;133(1):460–464. [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Modulatory effect of guanosine-3':5' cyclic monophosphate on macrophage susceptibility to Trypanosoma cruzi infection. J Immunol. 1983 Dec;131(6):3028–3031. [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]


