Abstract
Purpose
To assess the maximum-tolerated dose (MTD), dose-limiting toxicity (DLT), safety, and tolerability of the 24-hour continuous intravenous (CIV) infusion of MK-0457, a novel pan-Aurora kinase inhibitor, in patients with advanced solid tumors and to determine the bioavailability of an oral dose of 100 mg MK-0457.
Study Design
MK-0457 was administered as a 24-hour CIV infusion every 21 days. Dose escalation proceeded per toxicity criteria. A 100 mg oral dose was administered to 7 patients 48 hours prior to the CIV infusion dose of 64 mg/m2/hr.
Results
Twenty-seven patients received a total of 86 infusions of MK-0457. Dose limiting toxicity at 96 mg/m2/hr included grade 4 neutropenia and grade 3 herpes zoster. The MTD was identified as 64 mg/m2/hr. The most common adverse events were nausea, vomiting, diarrhea and fatigue. Pharmacokinetic analyses revealed that CIV infusion MK-0457 had an estimated mean terminal half-life of approximately 6.6-10.2 hours and that end of infusion concentrations and mean AUCs were approximately dose proportional. The estimated mean oral bioavailability of MK-0457 was 7.9%. One patient with advanced ovarian cancer attained prolonged stable disease for 11 months.
Conclusions
MK-0457 was well tolerated in this schedule. Almost half the patients attained stable disease. Further development of this class of agents will likely occur in combination with other anti-cancer treatments.
Keywords: Phase I, Aurora kinase, serine/threonine protein kinases, BCR-ABL mutations
Introduction
The Aurora kinases are a family of 3 highly homologous serine/threonine protein kinases that regulate multiple processes in cell division, leading to high-fidelity progression through mitosis (1-6). Aurora A, encoded by AURKA on chromosome 20q13.2-q13.3, is responsible for centrosome maturation and separation, and for regulation of the microtubule network that determines mitotic spindle function. Aurora B, encoded by AURKB on chromosome 17p13.1, serves as the catalytic component of the chromosomal passenger complex. This complex plays critical roles in the condensation of chromosomes, formation of the bipolar spindle, attachment of the chromosomes to the mitotic spindle, regulation of the spindle checkpoint, and completion of cytokinesis (3). The function of Aurora C remains largely unknown.
Interest in inhibiting Aurora kinase function in cancer therapeutics derives from evidence that links their activity to the progression of human cancer, although neither Aurora A nor B, alone, have consistently been shown to be potent inducers of cellular transformation (5). Elevated Aurora kinase function gives rise to aneuploid cells containing multiple centrosomes and multipolar spindles, resulting in genetic instability (7,8). In addition, Aurora kinase RNA and protein overexpression has been observed in many cancers and serves as a negative prognostic factor (9-11). Preclinical pharmacologic inhibition of Aurora kinase activity has enhanced the anti-tumor activity of cytotoxic chemotherapeutics, molecularly targeted agents, and radiation therapy (12-14).
MK-0457 is potent and selective small molecule inhibitor of all 3 Aurora kinases, with Ki values of 0.6, 18, and 4.6 nM for Aurora kinases A, B, and C, respectively. It inhibits Aurora kinase activity by a competitive and reversible mechanism at the ATP binding site. MK-0457 demonstrates significant selectivity for the Aurora kinase family over 190 kinases tested, although it does have some cross reactivity with Flt-3 and Abl kinases, showing a Ki of 30 nM against each of these (3). Harrington and colleagues demonstrated that MK-0457 inhibited the proliferation of transformed MCF-7 cells (IC50 15-113 nM). Following exposure of MCF-7 cells to MK-0457, 4N DNA content accumulated and histone H3 phosphorylation of Ser10 declined, indicative of Aurora kinase B inhibition. Treatment with MK-0457 resulted in marked tumor regression in nude mice xenografts of leukemia, pancreatic cancer, and colon cancer, and in 4 of 7 HCT116 colon cancer nude rat models. Data from these nude rat models suggested that the target plasma concentration of MK-0457 for achieving efficacy is 2 μM (1). Exposure to MK-0457 yielded a marked increase in G2-M arrest, aneuploidy, and apoptosis in acute myelogenous leukemia (AML), anaplastic thyroid, and ovarian cancer cell lines, while treatment of orthotopic murine models of metastatic ovarian cancer with either MK-0457 alone or MK-0457 combined with docetaxel resulted in reductions in tumor volume (6,15,16).
MK-0457 is hepatically metabolized, primarily by Cyp3A4, Cyp2C8, and flavin monooxygenase. Oxidation of the N-methylpiperazine group is the major route of metabolism, with the principle metabolites being the N-oxide and N-demethylated analogues (3).
Pan-Aurora kinase inhibitors and inhibitors of Aurora kinase A have entered early clinical trials in oncology therapeutics (17-21). Common toxicities have included neutropenia, with and without fever, gastrointestinal distress (nausea, vomiting, anorexia, constipation, diarrhea), stomatitis, and fatigue. Anti-tumor activity has been seen in a patient with liposarcoma and in patients with AML and chronic myelogenous leukemia (CML) (17,20). Evidence of Aurora kinase inhibition has been confirmed with a decrease in bipolar spindles in mitotic cells from tumor biopsies (17) and from the reduction in phosphorylation of histone H3 in peripheral blood mononuclear cells (20) and in skin biopsies (18).
Here, we report a phase 1 study conducted to determine the safety and tolerability of the pan-Aurora kinase inhibitor, MK-0457, administered over 24 hours every 21 days in patients with refractory solid tumors. Based on the regulation of multiple processes in cell division by the Aurora kinases, early clinical development of MK-0457 utilized continuous intravenous (CIV) infusion in an attempt to maximize exposure to cycling cancer cells. Rubin and colleagues conducted a phase I study of MK-0457 infused over 5 days every 28 days to patients with refractory solid tumors. Preliminary pharmacokinetic data from this study demonstrated that MK-0457 had a high clearance and short half-life, and that MK-0457 concentrations declined biexponentially after the end of infusion (22). This rapid clearance of MK-0457 further supported clinical administration via CIV infusion. A 24-hour duration of administration was selected in this study since preclinical data from MK-0457 suggested that the minimal duration of treatment for maximal antiproliferative effects, cell cycle effects, and apoptosis was between 24 and 48 hours. Therefore, comparison of the safety, efficacy, and pharmacokinetic results from a 5 day CIV infusion and from this study's 24-hour CIV infusion would help determine the lower and upper limits of infusion duration for Phase II studies. No other schedules of administration are under evaluation.
Patients and Methods
Patient selection
Eligible patients were at least 18 years of age and had a histologically documented, advanced solid tumor that was refractory to standard therapy or for which no curative therapy was available. Other inclusion criteria included: Eastern Cooperative Oncology Group (ECOG) performance status of 0-2; adequate bone marrow function (absolute neutrophils ≥ 1,500 cells/μl, platelets ≥ 100,000/μl); adequate hepatic function (total bilirubin ≤ 1.5 mg/dL, aspartate transaminase (AST) ≤ 3 × institutional upper limit of normal (IULN) or alanine aminotransferase (ALT) ≤ 3 × the IULN, or AST or ALT ≤ 5 × the IULN if hepatic metastases were present); adequate renal function (serum creatinine ≤ 2.0 mg/dL, or calculated creatinine clearance ≥ 40 mL/min); and life expectancy greater than 12 weeks. If central nervous system metastases were present, patients must have completed a course of radiotherapy and been on a stable dose of glucocorticoids for at least 4 weeks.
Exclusion criteria included the following: concurrent hematologic malignancy including leukemia; concurrent second malignancy, other than non-melanoma skin cancer; carcinomatous meningitis or primary brain tumors; < 3 weeks since prior chemotherapy, radiation, surgical, or biological anti-neoplastic therapy (< 6 weeks if prior chemotherapy had been mitomycin-C or a nitrosurea); < 30 days since prior investigational therapy; pregnant or lactating women; uncontrolled intercurrent illness, including history of myocardial infarction within the past 3 months, unstable angina, uncontrolled heart failure, ongoing or active infection, or psychiatric/substance abuse disorders that would limit compliance with study requirements; history of symptomatic pleural effusions or ascites; history of allogeneic stem cell or bone marrow transplantation; history of hepatitis B or C, or treatment for such; history of HIV positivity; or history of hyperensitivity to the study drug or its analogs.
All patients had to practice effective birth control. Before entering the study, each patient gave written informed consent indicating that they were aware of the investigational nature of the study, according to institutional and federal guidelines. The protocol was approved by the Institutional Review Boards at the two participating centers.
Study Design
This was a multicenter, open-label, non-randomized, accelerated dose escalation trial designed to determine the safety, tolerability, and maximum tolerated dose (MTD) of MK-0457 administered as a 24-hour CIV infusion in patients with advanced solid tumors. The starting dose of MK-0457 was 4 mg/m2/hr. This study also evaluated the oral bioavailability of MK-0457. Patients were treated with CIV MK-0457 every 21 days. Plasma samples were collected at defined intervals for pharmacokinetic analyses. The prophylactic use of hematologic growth factors was not permitted in this study. Adverse events were evaluated using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 guidelines.
Drug Administration
MK-0457 was supplied by Merck & Co., Inc., in 75-mg and 500-mg vials of lyophilized drug (to achieve a reconstituted concentration of 13 mg/mL in sterile water) and in 75-mg and 500-mg vials of liquid solution. It was administered as a 24-hour CIV infusion in 21-day cycles (+/- 24 hours). Patients at dose levels of 45 mg/m2/hr or higher utilized a 500-mL bag of D5W delivered via a 1.2 micron filtered administration set. The total 24-hour dose was given at a rate of 10 mL/hr utilizing a 250-mL bag of D5W, or at a rate of 20 mL/hr utilizing a 500-mL bag of D5W. Each bag of solution was infused via a PICC line or centrally placed catheter using a filtered administration set, and the rate of infusion was the same regardless of the dose infused. In the bioavailability part of this study, a 100-mg capsule of MK-0457 was administered orally at least 48 hours before beginning the 24-hour infusion at cycle 1 at a pre-set dose of CIV MK-0457 of 64 mg/m2/hr.
Dose escalation
Cohorts consisting of at least 1 patient were treated sequentially at escalating dose levels of 4, 8, 16, 32, 64, and 96 mg/m2/hr. This escalation scheme continued until the occurrence of any first-cycle dose-limiting toxicity (DLT) or the second instance of a first-cycle ≥ grade 2 toxicity. At that point, cohorts were expanded to 3 patients (due to the second occurrence of ≥ grade 2 toxicity) or 6 patients (due to a DLT). If ≥2 of 6 subjects at a given dose level experienced a DLT, or if 2 subjects experienced a DLT prior to completing the cohort, dose escalation was halted, and the dose level below the DLT level was considered the MTD. If only 1 of 6 patients in the expanded cohort experienced a DLT, further dose escalation continued. During the conduct of the trial, an intervening dose level could be evaluated to more fully characterize the MTD level. Investigator teleconferences were held throughout the study to monitor patient safety and dose escalation.
Dose limiting toxicities at least possibly related to MK-0457 occurring during cycle 1 of treatment were defined as follows: grade 4 neutropenia for at least 5 days; grade 3 or 4 neutropenia with fever > 38.5°C; grade 4 thrombocytopenia; any ≥ grade 3 non-hematologic toxicity, except alopecia and inadequately treated diarrhea, nausea, and vomiting; grade 3 transaminase elevation lasting at least 1 week; the withholding of treatment for greater than 3 weeks from the scheduled dose due to toxicity.
Patients were removed from protocol treatment for disease progression, unacceptable adverse events, intercurrent illness that prevented further treatment, subject's withdrawal of consent, investigator's discretion, greater than 2 dose reductions, if the patient became pregnant or failed to use adequate birth control, or was lost to follow-up. Dose modifications and delays were specified in the protocol. Participants were followed for a minimum of 30 days following their termination from the study.
Pretreatment and follow-up studies
History, physical examination, assessment of ECOG performance status, CBC, PT/INR, PTT, and serum chemistries were obtained from all patients at baseline and at the beginning of subsequent cycles. Additional pre-registration tests included serum pregnancy testing for women of childbearing age, an ECG, and a urinalysis. Vital signs, a CBC, and serum chemistries were measured twice weekly during cycles 1 and 2. Electrocardiograms were performed 4 hours pre-dose on cycle 1, day 1 (C1D1) and immediately following the completion of the 24-hour infusion on C1D2. Tumor assessments were obtained by imaging at baseline and at the completion of every other cycle of treatment. Patients with measurable disease had their tumor response assessed using the Response Evaluation Criteria in Solid Tumors (RECIST). All patients with responding tumors were required to have response confirmed no sooner than 4 weeks after the first documented response.
All patients who received at least 1 dose of study drug were evaluable for safety/toxicity. All patients who received at least 90% of one dose of MK-0457 and underwent one post-treatment efficacy assessment were considered evaluable for efficacy.
Pharmacokinetics
Patient blood samples were collected in heparin-containing tubes and centrifuged. Plasma samples were stored at -20°C until processing. Blood samples were collected on the following schedule on C1D1 – C1D3 for the 24 hour CIV infusion of MK-0457: pre-infusion, 0.5, 1, 2, 4, 8, 23, 23.5, 24.33 (20 minutes post the completion of infusion), 24.67 (40 minutes post the completion of infusion), 25, 26, 28, 32, and 48 hours post the start of the infusion.
Blood samples for MK-0457 following oral administration of the 100-mg capsule were drawn on C1D1 – C1D3: pre-dose, 0.5, 1, 2, 4. 6. 8. 32, and 48 hours following oral administration.
The determination of MK-0457 in human plasma was based on double liquid-liquid extraction in the 96-well plate format using a Tomtec Quadra 96 workstation. The stable isotope labeled MK-0457 was used as internal standard. Both analytes were chromatographed by HPLC and detected by MS/MS in the positive ionization mode using a turbo-ion spray probe and monitoring their precursor → product ion combinations in multiple-reaction monitoring (MRM) mode. The limit of reliable quantification was 0.486 nM when 0.25 mL of human plasma was processed. Standard curve range was 0.86 – 861 nM.
Pharmacodynamics
Paired tumor biopsies were planned for this clinical trial in order to document the molecular impact of MK-0457, including measurements of phosphohistone H3, proliferative index (Ki-67), mitotic index, TUNEL-positive cells, p53 and p21 levels, Aurora kinase levels, and RNA expression profiling. However, these analyses were not conducted due to the investigation of MK-0457 in other clinical trials.
Statistical methods
The primary outcome of this study was assessment of safety and tolerability of MK-0457 when administered as a 24-hour CIV infusion and determination of the MTD in this population. Secondary objectives included assessment of pharmacokinetics of MK-0457, estimation of anti-tumor activity per RECIST criteria, and determination of the oral bioavailability and safety of MK-0457 when administered as a 100-mg capsule. Exploratory analyses, limited to summary statistics on duration, intensity, and time to onset of toxicity with respect to dose, were used to assess the adverse effects of MK-0457. Summary statistics were provided by dose for the pharmacokinetic parameters (AUC, end of infusion plasma concentration, clearance, Vdss [volume of distribution at steady state], half-life, and oral bioavailability) obtained after the first treatment cycle.
Role of the funding source
The design of the study protocols was the responsibility of Merck Research Laboratories in collaboration with various health authorities. All analyses were conducted by scientists at Merck Research Laboratories. All authors had full access to the study data.
Results
Patient Characteristics
Twenty-seven patients enrolled between May 2005 and October 2006. Pretreatment characteristics are outlined in Table 1. Eighty-six infusions of MK-0457 were administered. The average number of cycles of MK-0457 administered per patient was 2.42 (range 1 - 8 cycles). Twenty-three patients (85%) completed 2 cycles of treatment, and 3 patients (11%) completed 6 cycles.
Table 1. Patient demographics.
| N | |
|---|---|
| Number of patients | 27 |
| Median age (range) | 61 (22-80) |
| Sex | |
| Male | 14 |
| Female | 13 |
| ECOG Performance status | |
| 0 | 4 |
| 1 | 21 |
| 2 | 1 |
| Unknown | 1 |
| Primary tumor type | |
| Colon | 6 |
| Bladder | 3 |
| Ovarian | 3 |
| Renal cell | 3 |
| Prostate | 2 |
| Breast | 1 |
| Germ cell | 1 |
| Melanoma | 1 |
| Non-small cell lung | 1 |
| Pancreas | 1 |
| Leiomyosarcoma | 1 |
| Chondrosarcoma | 1 |
| Rectal | 1 |
| Lung | 1 |
| Adenocarcioma, NOS | 1 |
| Prior lines of cytotoxic chemotherapy | |
| 1 | 1 |
| 2 | 2 |
| ≥ 3 | 23 |
| Unknown | 1 |
Dose limiting toxicities and maximum tolerated dose
All 27 patients were evaluable for assessment of DLTs. The starting dose (level 1) was MK-0457, 4 mg/m2/hr, administered as a 24-hour CIV infusion every 21 days. No DLTs were observed in this cohort, and subsequent doses were escalated according to Table 2. At dose level 7 (MK-0457 at 96 mg/m2/hr), a 69-year-old male patient with colon cancer experienced asymptomatic grade 4 neutropenia exceeding 5 days. This cohort was expanded due to this DLT. A second DLT then occurred when a 70-year-old female patient with rectal cancer developed neutropenia and grade 3 herpes zoster. As such, dose escalation was stopped, and dose level 6 (MK-0457 at 64 mg/m2/hr) was identified as the MTD for patients with solid tumors.
Table 2. Dose escalation schema with number of patients and cycles administered.
| Dose Levels | MK-0457 (mg/m2/day) | Number of Patients | Number of Cycles |
|---|---|---|---|
| 1 | 4 | 1 | 2 |
| 2 | 8 | 2 | 6 |
| 3 | 16 | 2 | 4 |
| 4 | 32 | 3 | 7 |
| 5* | 45 | 4 | 23 |
| 6 | 64 | 2 | 5 |
| 6 + oral dose | 64 | 7 | 19 |
| 7 | 96 | 6 | 20 |
Intermediate dose level added due to toxicity in dose level 4.
Seven patients were treated with a single capsule of 100 mg of MK-0457 48 hours prior to C1D1 of MK-0457 at 64 mg/m2/hr administered as a 24-hour CIV infusion (dose level 6). As such, 9 patients, total, underwent treatment at the MTD, thus satisfying the need to expand this cohort for further safety evaluation.
Dose escalation, toxicity, and safety
All 27 patients were evaluable for assessments of safety and toxicity. MK-0457 was well tolerated overall. There were no deaths on study in patients who received MK-0457 or within 30 days of follow-up. Very few grade 4 toxicities occurred. Only 4 patients (15%) had their treatment delayed or dose reduced due to toxicity.
Serious adverse events (SAEs) were defined regardless of attribution, and were documented in 9 (33%) patients throughout the study. The most commonly reported SAE was grade 3 vomiting, seen in 2 patients. All other SAEs reported were noted in single patients and included: grade 4 neutropenia; grade 3 anemia, nausea, diarrhea, intestinal obstruction, herpes zoster, abdominal wall abscess, fatigue, muscular weakness, and peripheral motor neuropathy. Although the particular SAE cited was not necessarily the medical indication for admission, all 9 patients were hospitalized while on study, and the toxicities were thus recorded as SAEs. All SAEs resolved by each the end of each patient's treatment, except for the patient with peripheral motor neuropathy. That adverse event was still active at the time of that patient's discontinuation from the study, so the duration of the neuropathy is not known.
Three patients (11%) had their MK-0457 dose reduced due to toxicity, including the 2 patients described above who experienced DLTs. In addition, a 61-year-old female patient with renal cell carcinoma on dose level 6 (64 mg/m2/hr) was dose-reduced secondary to asymptomatic grade 3 hyponatremia during cycle 2. A 54-year-old patient with prostate cancer on dose level 6 (64 mg/m2/hr) with the oral 100-mg dose of MK-0457 delayed treatment during cycle 6 due to fatigue and worsening peripheral sensory neuropathy; he subsequently chose to stop MK-0457 with stable disease due to these symptoms.
Grade 3 and 4 hematologic toxicities that occurred in any cycle are listed in Table 3a. There were no cases of febrile neutropenia. Grade 2, 3, and 4 non-hematologic toxicities that occurred in at least 3 (11%) patients are listed in Table 3b. There was no evidence of cumulative toxicity, though relatively few patients remained on therapy beyond two cycles, limiting the ability to determine if such an effect might occur. The most common grade 2, 3, and 4 non-hematologic toxicities that occurred in any cycle were nausea, vomiting, diarrhea and fatigue. Two patients developed 3 grade 4 non-hematologic toxicities. One patient on dose level 4 (32 mg/m2/hr) experienced asymptomatic elevated lipase on cycle 1, day 15. This grade 4 elevation was believed to be related to a concomitant medication, rather than to MK-0457; as such, it was not a DLT. This same patient also developed grade 4 hyperuricemia (cycle 2). Due to these 2 grade 4 toxicities, an intermediate dose level, 45 mg/m2/hr, was investigated. No significant grade 3 or 4 toxicity was seen at this intermediate dose level. Therefore, dose escalation proceeded according to the original plan described in the Patients and Methods section. In addition, 1 patient on dose level 6 (64 mg/m2/hr) with the oral 100-mg dose of MK-0457 developed grade 4 arthralgia (cycle 1). Prolongation of the QTc was not observed in this population.
Table 3.
| Table 3a. Grade 3 and 4 hematologic toxicities (N = 27) occurring in any cycle of treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Description | Dose Level 1 | Dose Level 2 | Dose Level 3 | Dose Level 4 | Dose Level 5 | Dose Level 6 | Dose Level 6 (oral 100 mg) | Dose Level 7 |
| N | 1 | 2 | 2 | 3 | 4 | 2 | 7 | 6 |
| Neutropenia | ||||||||
| Grade 3 | 1 | 1 | 1 | 1 | ||||
| Grade 4 | 1 | |||||||
| Leukopenia | ||||||||
| Grade 3 | 1 | 1 | 3 | |||||
| Grade 4 | ||||||||
| Anemia | ||||||||
| Grade 3 | 1 | |||||||
| Grade 4 | ||||||||
| Table 3b. Grade 2, 3, and 4 non-hematologic toxicities that occurred in at least 3 patients (11%), regardless of attribution (N = 27) during any cycle of treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Description | Dose Level 1 | Dose Level 2 | Dose Level 3 | Dose Level 4 | Dose Level 5 | Dose Level 6 | Dose Level 6 (oral 100 mg) | Dose Level 7 |
| N | 1 | 2 | 2 | 3 | 4 | 2 | 7 | 6 |
| Nausea | ||||||||
| Grade 2 | 1 | 1 | 2 | |||||
| Grade 3 | 1 | 1 | ||||||
| Vomiting | 1 | 1 | ||||||
| Grade 2 | ||||||||
| Grade 3 | 1 | 1 | 2 | |||||
| Diarrhea | ||||||||
| Grade 2 | 1 | 1 | 1 | |||||
| Grade 3 | 2 | |||||||
| Fatigue | ||||||||
| Grade 2 | 1 | 1 | 1 | 2 | 5 | 3 | ||
| Grade 3 | 1 | |||||||
| Urinary tract infection | ||||||||
| Grade 2 | 1 | 1 | 1 | 1 | ||||
| Dehydration | ||||||||
| Grade 2 | 1 | 2 | ||||||
| Arthralgia | ||||||||
| Grade 2 | 1 | 1 | 1 | |||||
| Grade 4 | 1 | |||||||
| Back pain | ||||||||
| Grade 2 | 1 | 1 | 1 | |||||
| Dyspnea | ||||||||
| Grade 2 | 1 | 2 | ||||||
| Grade 3 | 1 | |||||||
| Increased alk phos | ||||||||
| Grade 2 | 1 | 1 | 1 | |||||
| Hypoalbuminemia | ||||||||
| Grade 2 | 1 | 1 | 1 | |||||
| Grade 3 | 1 | |||||||
| Hypophosphatemia | ||||||||
| Grade 2 | 1 | 2 | ||||||
| Grade 3 | 1 | 1 | ||||||
| Hyperglycemia | ||||||||
| Grade 2 | 1 | |||||||
| Grade 3 | 1 | 1 | ||||||
Abbreviations: alk phos (alkaline phosphatase)
No adverse events attributed as at least possibly related to study drug were observed between administration of the oral dose of MK-457 and initiation of the CIV infusion in the 7 patients who ingested a single capsule of 100 mg of MK-0457 48 hours prior to C1D1 of MK-0457 at 64 mg/m2/hr (dose level 6).
Twenty-two patients were removed from study treatment due to progressive disease. Three patients came off study due to the investigator's discretion; all these patients were experiencing symptoms suggestive of disease progression. A 69 year old male patient with pancreas cancer died from a massive pulmonary embolism following study registration but prior to receiving MK-0457. Lastly, 2 patients withdrew consent for study participation, 1 patient with worsening fatigue and peripheral sensory neuropathy and 1 patient for unknown reasons.
Efficacy
There were no objective anti-tumor responses to MK-0457. A total of 12 patients experienced stable disease (i.e. defined as 2 or more cycles without progression), with a median duration of 75.5 days (range 38 – 328 days). A 60-year-old female patient with ovarian cancer on dose level 6 (45 mg/m2/hr) attained stable disease as her best response to treatment and received 15 cycles of MK-0457 over her 11 months on study. In addition, a 70-year-old female patient with rectal cancer on dose level 7 (96 mg/m2/hr) attained stable disease and received 9 cycles of MK-0457.
Pharmacokinetics
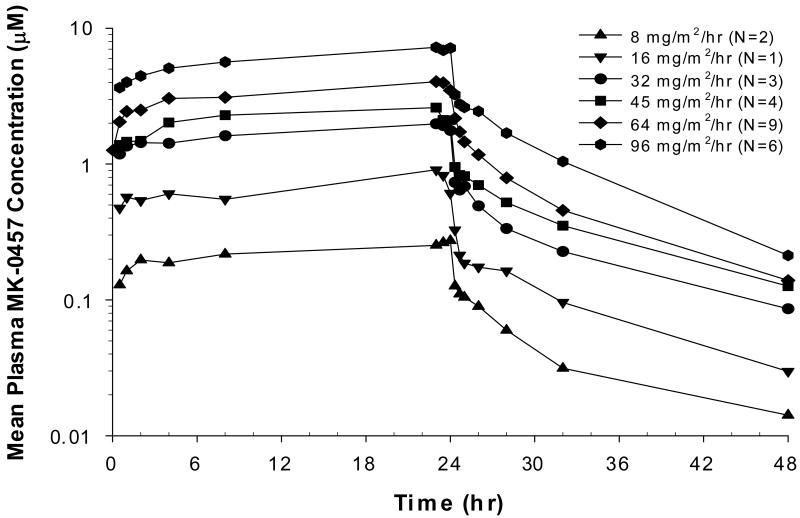
Concentrations of MK-0457 were measured in plasma before dosing and at multiple times during C1D1 – C1D3 as detailed in Patients and Methods, in 25 patients who received MK-0457 dosed at 8 mg/m2/hr, or higher, including the 7 patients who received the oral 100-mg capsule of MK-0457. The pharmacokinetic parameters of CIV infusion MK-0457 are described in Table 4 and Figure 1. MK-0457 concentrations rose rapidly following the start of infusion and appeared to decline bi-exponentially after the end of infusion, with an estimated mean terminal half-life of approximately 6.6-10.2 hours (Figure 1). The end-of-infusion concentration was approximately dose proportional and averaged from 0.274 to 7.635 μM. The estimated mean clearance ranged from 54 to 139 L/hr. The mean AUCs ranged from 6.4 to 167 μM·hr and were also approximately dose proportional. The dose-adjusted AUC averaged 0.0315 μM·hr/mg. Pharmacokinetic results of MK-0457 following single oral 100-mg doses are available for 7 of those patients who received 64 mg/m2/hr IV doses. The estimated mean oral bioavailability of MK-0457 was 7.9%. Pharmacokinetic data from 1 patient at 16 mg/m2/hr were not available.
Table 4. Summary of pharmacokinetic parameters.
| Dose (mg/m2/hr) | Infusion rate (mg/hr) (Range) | Total dose (mg) (Range) | Apparent t1/2 (hr) (Pseudo-SD) | Cmax (μM) (CV%) | Total AUC (μM*hr) (CV%) | CL (L/hr) (CV%) | Vdss (L) (CV%) |
|---|---|---|---|---|---|---|---|
| 8 (N=2) | 17.2 (16.8-17.6) | 413 (403-422) | 9.3 (2.7) | 0.274 (10.8) | 6.40 (6.6) | 139.3 (9.8) | 735 (7.2) |
| 16 (N=1) | 36.8 | 883 | 8.5 | 0.911 | 18.8 | 101.2 | 514 |
| 32 (N=3) | 61.5 (54.4-67.2) | 1477 (1306-1613) | 10.2 (1.4) | 2.170 (8.1) | 47.2 (19.2) | 69.5 (26.1) | 328 (16.3) |
| 45 (N=4) | 99.6 (94.5-112.5) | 2390 (2268-2700) | 10.2 (0.5) | 2.666 (18.4) | 62.9 (16.6) | 83.6 (18.7) | 429 (33.7) |
| 64 (N=9) | 120.5 (96-134.4) | 2893 (2304-3226) | 8.0 (1.5) | 4.015 (22.9) | 90.3 (25.4) | 76.2 (46.7) | 301 (30.0) |
| 96 (N=6) | 175.5 (130.6-220.8) | 4212 (3133-5299) | 6.6 (1.2) | 7.635 (31.8) | 167.4 (24.3) | 53.6 (32.8) | 248 (41.2) |
Cmax=Cend-of-infusion
CV = Coefficient of Variation
Harmonic mean calculated for t1/2
Figure 1. Pharmacokinetic profiles of MK-0457.
Mean plasma concentrations (μM) of MK-0457 following a 24-hour CIV infusion in patients with advanced solid tumors. Dose is in mg/m2/hr according to symbols shown above.
Discussion
Inhibition of Aurora kinase activity is a potential therapeutic target for the treatment of cancer (1-5). Disruption of high-fidelity mitosis mediated by Aurora kinases selects for rapidly cycling cells, as shown by the toxicities seen to date with the early clinical use of Aurora kinase inhibitors (marrow, gut epithelium). Evidence of anti-cancer activity in the preliminary results from single-agent studies has been sparse, despite some pharmacodynamic evidence of target inhibition (17-22).
MK-0457 is small molecule pan-Aurora kinase inhibitor that does have cross reactivity with Flt-3 and Abl kinases. Rubin et al. reported the preliminary results from their phase I study of MK-0457 infused over 5 days every 28 days to patients with refractory solid tumors. Toxicities included neutropenia, with and without fever, and a grade 2 allergic reaction. The MTD with this schedule of administration was 10 mg/m2/hr. One patient, each, with pancreas cancer and non-small cell lung cancer attained stable disease on treatment (22).
As with the experience from the 5-day continuous infusion, we found that MK-0457 was well-tolerated when administered over 24 hours. Neutropenia, an expected mechanism-based toxicity, was observed to be dose-limiting, and adverse effects were also seen on the rapidly cycling cells of the gut epithelium. However, drug-related effects on the platelet count were not observed, in keeping with the clinical experience from other Aurora kinase inhibitor studies, and possibly related to down-regulation of Aurora A and B during megakaryocyte maturation (5). No unexpected patterns of toxicities were seen in our heavily pretreated population, 85% of whom completed 2 cycles of treatment. There were no deaths on study in patients who received MK-0457, minimal grade 4 toxicities, and no clear-cut discontinuations of study treatment due to toxicity. Toxicities related to oral MK-0457 were not detected, likely due to its poor bioavailability.
Our pharmacokinetic results indicated that doses above 32 mg/m2/hr of the 24 hour CIV infusion surpassed the target efficacy concentration of 2 μM (1). Further, MK-0457 pharmacokinetic characteristics of high clearance, short half-life, and biexponentially declining concentrations after the completion of the infusion in our 24-hour duration of administration mirrored those identified by Rubin and colleagues using a 5 day CIV infusion (22).
Two patients in this trial attained prolonged stable disease exceeding 7 months. However, no anti-tumor responses were noted, also consistent with prior clinical use of Aurora kinase inhibitors. Similar patterns of attaining prolonged survival despite an absence of objective anti-tumor activity have been noted with the use of other molecularly targeted inhibitors (23,24). Further development of this class of agents in patients with solid tumors will likely depend on their use in combination with cytotoxic chemotherapeutics, molecularly targeted agents, and radiation therapy (12-14), and upon the identification of predictive biomarkers needed to identify enriched patient populations for treatment. For example, Gizatullin et al. found that lung, breast, and colon cancer cell lines with defective p53-p21 pathways were more sensitive to apoptotic death following exposure to MK-0457 (2,25). Moreover, it remains unclear as to which Aurora kinase, A or B, is the more critical target to inhibit. For example, preclinical evidence suggests that overexpression of Aurora A exerts a more important role in tumorigenesis than Aurora B (5). However, through serial exposure of HCT-116 cells to the selective Aurora kinase B inhibitor ZM447439, Girdler and colleagues found that HCT-116 cells resistant to Aurora B inhibition were also resistant to MK-0457, implying that MK-0457 cytotoxicity is most likely mediated by Aurora B (26).
Off-target effects may also contribute to the successful development of Aurora kinase inhibitors. MK-0457 is able to bind with high affinity to both wild-type and mutant Abl kinases, and is a potent inhibitor of the T315I mutant with a Ki of 42 nM (3). The BCR-ABL T315I mutation mediates clinical resistance to imatimib, nilotinib, and dasatinib. Giles et al. reported in 2007 that 3 patients with T315I Abl-mutated chronic myelogenous leukemia (CML) or Philadelphia chromosome-positive acute lymphocytic leukemia (Ph+ ALL) achieved hematologic responses to treatment with MK-0457 (27). Subsequent treatment of 3 patients with imatinib-resistant Ph+ ALL and CML with the sequential or concurrent combination of dastatinib and MK-0457 yielded complete hematologic responses without significant hematologic toxicity (28). Exposure of leukemia cells with and without BCR-Abl T135I mutations to the combination of MK-0457 and the histone deacetylase inhibitor vorinostat greatly potentiated the lethality of MK-0457 (29,30). Thus, the off-target activity of MK-0457, such as BCR-ABL T315I, may augment its clinical development.
In summary, MK-0457 was fairly well tolerated in these doses and schedule in this population of patients with advanced solid tumors. Toxicities and pharmacokinetic results were in keeping with the prior limited clinical experience with this agent (22,27,28). Prolonged stable disease was seen in a patient with ovarian cancer and a patient with rectal cancer. Although no objective tumor responses were observed in this trial, the absence of such findings is not uncommon for this class of targeted therapy. Alternative trial designs could be considered to assess efficacy of MK-0457 as a single agent or combined with other anti-cancer therapeutics.
Acknowledgments
The authors thank our patients, their families, and our clinic and research staffs for the completion of this clinical trial. We also thank Robert Crane and Elizabeth Reilly for their programming work on the MK-0457 program and acknowledge Lingling Xue, Alan Xiao, and Cynthia Chavez-eng for their analytical support. Melinda Baker and Jennifer Pawlowski provided logistical support for this manuscript.
This trial was registered with ClinicalTrials.gov NCT00104351
References
- 1.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 2.Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN, Jr, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14:1639–48. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 3.Pollard JR, Mortimore M. Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem. 2009;52:2629–51. doi: 10.1021/jm8012129. [DOI] [PubMed] [Google Scholar]
- 4.Keen N, Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat Rev Cancer. 2004;4:927–36. doi: 10.1038/nrc1502. [DOI] [PubMed] [Google Scholar]
- 5.Boss DS, Beijnen JH, Schellens JH. Clinical experience with aurora kinase inhibitors: a review. Oncologist. 2009;14:780–93. doi: 10.1634/theoncologist.2009-0019. [DOI] [PubMed] [Google Scholar]
- 6.Lin YG, Immaneni A, Merritt WM, Mangala LS, Kim SW, Shahzad MM, et al. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin Cancer Res. 2008;14:5437–46. doi: 10.1158/1078-0432.CCR-07-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda A, Kawai H, Suto S, Kitajima S, Sato S, Takata T, et al. Aurora-B/AIM-1 kinase activity is involved in Ras-mediated cell transformation. Oncogene. 2005;24:7266–72. doi: 10.1038/sj.onc.1208884. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Hirota T, Marumoto T, Shimizu M, Kunitoku N, Sasayama T, et al. Cre-loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene. 2004;23:8720–30. doi: 10.1038/sj.onc.1208153. [DOI] [PubMed] [Google Scholar]
- 9.Tanner MM, Tirkkonen M, Kallioniemi A, Holli K, Collins C, Kowbel D, et al. Amplification of chromosomal region 20q13 in invasive breast cancer: prognostic implications. Clin Cancer Res. 1995;1:1455–61. [PubMed] [Google Scholar]
- 10.Kurai M, Shiozawa T, Shih HC, Miyamoto T, Feng YZ, Kashima H, et al. Expression of Aurora kinases A and B in normal, hyperplastic, and malignant human endometrium: Aurora B as a predictor for poor prognosis in endometrial carcinoma. Hum Pathol. 2005;36:1281–8. doi: 10.1016/j.humpath.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Vischioni B, Oudejans JJ, Vos W, Rodriguez JA, Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5:2905–13. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- 12.Nair JS, de Stanchina E, Schwartz GK. The topoisomerase I poison CPT-11 enhances the effect of the aurora B kinase inhibitor AZD1152 both in vitro and in vivo. Clin Cancer Res. 2009;15:2022–30. doi: 10.1158/1078-0432.CCR-08-1826. [DOI] [PubMed] [Google Scholar]
- 13.Cha TL, Chuang MJ, Wu ST, Sun GH, Chang SY, Yu DS, et al. Dual degradation of aurora A and B kinases by the histone deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of renal cancer cells. Clin Cancer Res. 2009;15:840–50. doi: 10.1158/1078-0432.CCR-08-1918. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y, Zhang P, Girdler F, Frascogna V, Castedo M, Bourhis J, et al. Enhancement of radiation response in p53-deficient cancer cells by the Aurora-B kinase inhibitor AZD1152. Oncogene. 2008;27:3244–55. doi: 10.1038/sj.onc.1210990. [DOI] [PubMed] [Google Scholar]
- 15.Arlot-Bonnemains Y, Baldini E, Martin B, Delcros JG, Toller M, Curcio F, et al. Effects of the Aurora kinase inhibitor VX-680 on anaplastic thyroid cancer-derived cell lines. Endocr Relat Cancer. 2008;15:559–68. doi: 10.1677/ERC-08-0021. [DOI] [PubMed] [Google Scholar]
- 16.Huang XF, Luo SK, Xu J, Li J, Xu DR, Wang LH, et al. Aurora kinase inhibitory VX-680 increases Bax/Bcl-2 ratio and induces apoptosis in Aurora-A-high acute myeloid leukemia. Blood. 2008;111:2854–65. doi: 10.1182/blood-2007-07-099325. [DOI] [PubMed] [Google Scholar]
- 17.Cervantes-Ruiperez A, Elez ME, Rosello T, Macarulla T, Rodriguez-Braun E, Lee Y, et al. Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of MLN8237, a novel selective aurora A kinase (AAK) inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2009;27:124s. [Google Scholar]
- 18.Robert F, Verschraegen C, Hurwitz H, Uronis H, Advani R, Chen A, et al. A phase I trial of sns-314, a novel and selective pan-aurora kinase inhibitor, in advanced solid tumor patients. J Clin Oncol. 2009;27:117s. [Google Scholar]
- 19.Jones SF, Burris HA, I, Dumez H, Infante JR, Fowst C, Gerletti P, et al. Phase I accelerated dose-escalation, pharmacokinetic (PK) and pharmacodynamic study of PF-03814735, an oral aurora kinase inhibitor, in patients with advanced solid tumors: Preliminary results. J Clin Oncol. 2008;26:116s. [Google Scholar]
- 20.Foran JM, Ravandi F, O'Brien SM, Borthakur G, Rios M, Boone P, et al. Phase I and pharmacodynamic trial of AT9283, an aurora kinase inhibitor, in patients with refractory leukemia. J Clin Oncol. 2008;26:116s. [Google Scholar]
- 21.Cohen RB, Jones SF, von Mehren M, Cheng J, Spiegel DM, Laffranchi B, et al. Phase I study of the pan aurora kinases (AKs) inhibitor PHA-739358 administered as a 24 h infusion without/with G-CSF in a 14-day cycle in patients with advanced solid tumors. J Clin Oncol. 2008;26:117s. [Google Scholar]
- 22.Rubin EH, Shapiro GI, Stein MN, Watson P, Bergstrom D, Xiao A, et al. A phase I clinical and pharmacokinetic (PK) trial of the aurora kinase (AK) inhibitor MK-0457 in cancer patients. J Clin Oncol. 2006;24:123s. [Google Scholar]
- 23.Schiller JH, Larson T, Ou SH, Limentani S, Sandler A, Vokes E, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27:3836–41. doi: 10.1200/JCO.2008.20.8355. [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 25.Gizatullin F, Yao Y, Kung V, Harding MW, Loda M, Shapiro GI. The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Cancer Res. 2006;66:7668–77. doi: 10.1158/0008-5472.CAN-05-3353. [DOI] [PubMed] [Google Scholar]
- 26.Girdler F, Sessa F, Patercoli S, Villa F, Musacchio A, Taylor S. Molecular basis of drug resistance in aurora kinases. Chem Biol. 2008;15:552–62. doi: 10.1016/j.chembiol.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Giles FJ, Cortes J, Jones D, Bergstrom D, Kantarjian H, Freedman SJ. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109:500–2. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- 28.Papayannidis C, Iacobucci I, Soverini S, Paolini S, Cilloni D, Messa F, et al. Innovative phase I study of concomitant and consecutive treatment with dasatinib and MK-0457 in refractory Ph+ CML and ALL patients. J Clin Oncol. 2009;27:375s. [Google Scholar]
- 29.Dai Y, Chen S, Venditti CA, Pei XY, Nguyen TK, Dent P, et al. Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood. 2008;112:793–804. doi: 10.1182/blood-2007-10-116376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiskus W, Wang Y, Joshi R, Rao R, Yang Y, Chen J, et al. Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res. 2008;14:6106–15. doi: 10.1158/1078-0432.CCR-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]