Abstract
Scrapie in sheep and goats has been known for more than 250 years and belongs nowadays to the so-called prion diseases that also include e.g. bovine spongiform encephalopathy in cattle (BSE) and Creutzfeldt-Jakob disease in humans. According to the prion hypothesis, the pathological isoform (PrPSc) of the cellular prion protein (PrPc) comprises the essential, if not exclusive, component of the transmissible agent. Currently, two types of scrapie disease are known - classical and atypical/Nor98 scrapie. In the present study we examine 24 cases of classical and 25 cases of atypical/Nor98 scrapie with the sensitive PET blot method and validate the results with conventional immunohistochemistry. The sequential detection of PrPSc aggregates in the CNS of classical scrapie sheep implies that after neuroinvasion a spread from spinal cord and obex to the cerebellum, diencephalon and frontal cortex via the rostral brainstem takes place. We categorize the spread of PrPSc into four stages: the CNS entry stage, the brainstem stage, the cruciate sulcus stage and finally the basal ganglia stage. Such a sequential development of PrPSc was not detectable upon analysis of the present atypical/Nor98 scrapie cases. PrPSc distribution in one case of atypical/Nor98 scrapie in a presumably early disease phase suggests that the spread of PrPSc aggregates starts in the di- or telencephalon. In addition to the spontaneous generation of PrPSc, an uptake of the infectious agent into the brain, that bypasses the brainstem and starts its accumulation in the thalamus, needs to be taken into consideration for atypical/Nor98 scrapie.
Introduction
Scrapie in sheep and goats, which has been reported for more than 250 years [1], belongs to the transmissible spongiform encephalopathies (TSEs) - also known as prion diseases. This group of fatal diseases includes bovine spongiform encephalopathy (BSE) in cattle, chronic wasting disease (CWD) in deer and Creutzfeldt-Jakob disease (CJD) in humans. TSEs are characterized by the accumulation of protein aggregates, which are relatively stable against proteolysis. According to the prion hypothesis, a misfolded protein is the relevant part of the infectious agent [2]. It is widely accepted that this "proteinaceous infectious particle" is the pathological isoform of the physiological prion protein (PrPc) which is encoded by a cellular gene [3]. Recently, it has been shown that infectivity can be generated from a synthetic misfolded form of the prion protein [4]. Depending on the kind of prion disease, the pathological prion protein (PrPSc) is detectable solely in the central nervous system (CNS) or may also be found in other tissues, especially in those of the lymphoreticular system (LRS) [5].
In the worldwide population of small ruminants, BSE and scrapie are considered to be the relevant TSEs affecting sheep and goats. Scrapie, however, is not a homogenous disease form, as demonstrated by the existence of several strains upon transmission to rodents [6] and the peculiar molecular properties of the sheep-passaged scrapie isolate CH1641 [7,8]. The discovery of a novel type of scrapie in Norway in 1998 (Nor98) that was clearly distinguishable from all previously reported forms of scrapie [9], and that was soon after detected in several other countries, added to the diversity of this TSE [10]. In our present work we concentrate on scrapie field cases that include cases of "classical" scrapie as well as "atypical"/Nor98 scrapie. Obvious differences exist between the two scrapie forms with regard to the epidemiology of the disease and the properties of the proteinaceous particle. The latter include Western blot profiles and the stability against denaturation and proteases [11-13]. The two forms of sheep scrapie also differ with regard to the genotypes affected. Amino acids at codon 136 (A/V), 154 (H/R) and 171 (H/Q/R) are considered to markedly influence susceptibility to classical scrapie; the most susceptible alleles are V136R154Q171 (VRQ) and A136R154Q171 (ARQ), while the A136R154R171 allele (ARR) seems to confer a certain resistance against the disease [14,15]. Atypical/Nor98 scrapie affects a number of genotypes, including the ARR allele, and animals with the AHQ allele or a Phenylalanin (F) instead of Leucin (L) at codon 141 in the ARQ allele are proportionally overrepresented [16-18].
The results of a number of case reports and studies have shown that the deposition form and distribution of PrPSc aggregates in atypical/Nor89 scrapie sheep are clearly distinct from classical scrapie; immunohistochemical methods and recently the sensitive PET blot method have been used for the detection of PrPSc in the ovine brain [9,19-23]. Formerly, the PET blot had only been used for the sensitive detection of PrPSc in extra-cerebral organs of classical scrapie sheep [24-27]. Surprisingly, the anatomical distribution of PrPSc in the ovine brain found in the literature is more thoroughly documented for atypical/Nor98 scrapie than for classical scrapie. Although the pathogenesis of classical scrapie is well-studied [28,29], detailed descriptions on how the infectious agent spreads once it has reached the brain seem to be lacking for both scrapie types. For classical scrapie, numerous reports exist on the different forms of PrPSc that can be found in the brain tissue and the presence of PrPSc aggregates in peripheral neural and non-neural tissues - at least in sheep carrying susceptible PrP genotypes. Also, the entry of the infectious agent into the CNS has been described thoroughly for field classical scrapie infections and has been shown to agree with the oral infection of sheep with BSE and scrapie as well as the oral infection of rodent models infected with scrapie [29-32]. The infectious agent apparently enters the CNS via the intermediolateral column of the thoracic spinal cord (Th8 - Th10 in natural scrapie infection) and the dorsal motor nucleus of the vagus nerve (DMNV) in the brainstem. Unfortunately, reports on the spread of ovine PrPSc from the brainstem into the brain are usually not very detailed. In atypical/Nor98 scrapie, most of the PrPSc load in affected sheep is found in the cerebellum and cerebrum. It still needs to be determined whether this novel disease is a sporadic prion disease or not. If sheep could acquire the disease from their environment, where would the infectious agent enter the CNS? The pattern of PrPSc deposition is apparently reproduced when atypical/Nor98 scrapie is transmitted from one sheep to another via intracerebral inoculation [33].
In this study the PrPSc deposition pattern in the CNS of 24 classical and 25 atypical/Nor98 field scrapie sheep was determined using the sensitive and specific PET blot method. Different amounts of PrPSc in the CNS of classical scrapie have been assigned to different stages of PrPSc spread into the brain, depending on the affected neuroanatomical structures.
Materials and methods
Material
The brains and, if available, the spinal cords as well as lymphatic tissue (tonsils and/or retropharyngeal lymph nodes) were collected from 49 scrapie field cases and 6 further sheep from scrapie-free flocks as controls. Scrapie positivity was diagnosed either ante mortem by tonsil biopsy or post mortem using the respective methods stipulated by the EU VO999/2001 at that time (samples were collected during a time span of 12 years). The scrapie-positive group included 19 German and 5 Norwegian sheep diagnosed with classical scrapie and 24 Norwegian atypical/Nor98 scrapie cases, plus one German atypical/Nor98 case. The control group was made up of six German sheep derived from scrapie-free flocks. The PrP genotypes were determined either by PCR and melting curve analysis [34] or by automated sequencing as described previously [9]. Further information on the individual animals including age, breed, genotype, presence of clinical signs and availability of LRS and spinal cord is listed in Table 1.
Table 1.
Genotype, age, breed, the presence of clinical signs and the availability of lymphatic tissue and spinal cord of the individual sheep
| Genotype | Breed | Age in months | Lymphatic tissue available | Spinal cord available | Clinical signs present | |
|---|---|---|---|---|---|---|
| Classical scrapie cases | ||||||
| ARQ/ARQ | G.M./B.M. crossbreed | ~42 | yes | yes | no | |
| ARQ/ARQ | G.M./B.M. crossbreed | ~24 | yes | yes | no | |
| ARQ/ARQ | G.M./B.M. crossbreed | >48 | yes | yes | no | |
| ARQ/ARQ | Black headed Mutton | ~72 | yes | yes | no | |
| ARQ/ARQ | German Merino | ~60 | yes | yes | yes | |
| ARQ/ARQ | German Merino | >48 | yes | yes | yes | |
| ARQ/ARQ | G.M./B.M. crossbreed | >48 | yes | yes | yes | |
| ARQ/ARQ | G.M./B.M. crossbreed | unknown | yes | no | unknown | |
| ARQ/ARQ | G.M./B.M. crossbreed | ~48 | yes | no | unknown | |
| ARQ/ARQ | Black headed Mutton | unknown | yes | no | unknown | |
| ARQ/ARQ | B.M./Mountain sheep crossbreed | 27 | yes | yes | yes | |
| ARQ/ARQ | G.M./B.M./Mountain sheep crossbreed | 25 | yes | yes | yes | |
| ARQ/ARQ | B.M./Mountain sheep crossbreed | unknown | yes | yes | yes | |
| ARQ/ARQ | B.M./Mountain sheep crossbreed | 29 | yes | yes | no | |
| ARQ/ARQ | B.M./Mountain sheep crossbreed | 38 | yes | yes | yes | |
| VRQ/ARQ | Texel | unknown | yes | no | unknown | |
| VRQ/ARQ | Norwegian pelt sheep | ~24 | yes | no | unknown | |
| VRQ/ARQ | Steigar sheep | ~42 | no | no | unknown | |
| VRQ/ARQ | Texel | unknown | yes | no | unknown | |
| VRQ/ARQ | Steigar sheep | unknown | no | no | unknown | |
| VRQ/ARQ | Texel/Mountain sheep crossbreed | 32 | yes | yes | yes | |
| VRQ/ARH | Texel | 30 | yes | yes | no | |
| VRQ/ARH | Steigar sheep | unknown | yes | no | unknown | |
| VRQ/ARH | Texel | unknown | yes | no | unknown | |
| Atypical/Nor98 scrapie cases | ||||||
| AFRQ/ARQ | Spæl sheep | ~78 | no | no | uncertain | |
| AFRQ/AHQ | Suffolk/Rygja/Steigar crossbreed | ~72 | no | yes | yes | |
| ARQ/AHQ | German Merino | unknown | yes | no | unknown | |
| AHQ/AHQ | Steigar sheep | ~48 | yes | yes | yes | |
| AHQ/AHQ | Spæl sheep | ~72 | yes | no | yes | |
| AHQ/AHQ | Norwegian white sheep | ~84 | yes | no | yes | |
| AHQ/AHQ | Spæl sheep | ~42 | yes | yes | uncertain | |
| AHQ/AHQ | Spæl sheep | ~48 | yes | no | yes | |
| AHQ/AHQ | Spæl sheep | ~72 | no | no | yes | |
| AHQ/ARH | Norwegian white sheep | ~120 | yes | no | yes | |
| AHQ/AFRQ | Dala sheep | ~84 | no | yes | yes | |
| AHQ/AFRQ | Steigar sheep | ~60 | no | no | yes | |
| AFRQ/AFRQ | Norwegian white sheep | ~60 | yes | no | uncertain | |
| AFRQ/AFRQ | Dala sheep | ~36 | yes | yes | yes | |
| AFRQ/AFRQ | Norwegian white sheep | ~78 | yes | no | yes | |
| AFRQ/AFRQ | Rygja/Dala crossbreed | ~84 | yes | no | uncertain | |
| AFRQ/AFRQ | Dala sheep | ~108 | yes | no | no | |
| AHQ/ARR | Spæl sheep | ~72 | no | no | yes | |
| AHQ/ARR | Norwegian white sheep | ~96 | yes | no | no | |
| AHQ/ARR | Dala sheep | ~72 | yes | no | uncertain | |
| AHQ/ARR | Dala sheep | ~84 | no | yes | no | |
| ARR/AFRQ | Norwegian white sheep | ~96 | no | no | unknown | |
| ARR/AFRQ | Norwegian white sheep | ~66 | yes | no | no | |
| ARR/ARR | Steigar | ~84 | no | yes | no | |
| unknown | Rygja/Dala crossbreed | unknown | no | no | no | |
| Control cases | ||||||
| ARQ/ARQ | Skudde | unknown | yes | no | no | |
| ARR/ARH | Leine | 30 | yes | no | no | |
| ARR/ARR | German Merino | ~132 | yes | no | no | |
| ARR/ARR | Leine | ~60 | yes | no | no | |
| ARR/ARR | Leine | ~96 | yes | no | no | |
| ARR/ARR | Leine | >48 | yes | no | no | |
B.M. = blackheaded mutton, G.M. = German Merino
Depending on the circumstances under which the samples were collected, the post mortem times of tissues varied between 2 h and 4 days. Usually one half of the brain/tonsil/lymph node was fixed in 4% buffered formaldehyde, cut into slices and embedded in paraffin within five to seven days, while the other half was frozen and stored at -80 °C.
Histopathology
One to three μm-thick CNS/lymphatic tissue sections were cut, collected on silane-coated glass slides and stained with haematoxylin and eosin (H&E). Brain sections were also stained with Luxol Fast Blue then counterstained by periodic acid Schiff` reagent (LFB/PAS) for the orientation and discrimination of neuronal nuclei and neural tracts.
PET blot
The PET blot procedure followed the protocol as described previously [23,35] using the monoclonal antibody (mAb) P4 (R-Biopharm, Darmstadt, Germany), which had proved to give the best results regarding sensitivity and specificity for the detection of PrPSc in classical and atypical/Nor98 sheep scrapie [23]. In brief, immunolabeling of PrPSc was performed after a 1-3 μm tissue section had been placed on a nitrocellulose membrane (0.45 μm, Bio-Rad, Hercules, CA, USA) which was then deparaffinized and rehydrated. This was followed by treatment with proteinase K (250 μg/mL; Sigma-Aldrich, MO, USA) overnight at 56 °C and the decontamination of the membranes in 4 M guanidine thiocyanate (GdnSCN) for 30 min. Membranes were blocked with 0.2% casein in PBS containing 1% Tween before the primary antibody (mAbP4) was applied 1:5000 in TBST. An alkaline phosphatase-coupled goat-anti-mouse antibody (Dako, Glostrup, Denmark) and the formazan-reaction with NBT/BCIP were used to visualize the result. Thorough rinsing of the membranes with TBST was required between the different steps.
Immunohistochemistry
Tissue sections on silane-coated glass slides were stained with one of the primary mAbs P4, L42 (R-Biopharm, Darmstadt, Germany), F89/160.1.5 (Veterinary Medical Research and Developement, Pullman, WA, USA), and 12F10 (kindly provided by W Bodemer and D Motzkus, German Primate Center), which were used 1:500 in combination with an alkaline phosphatase-coupled goat anti-mouse antibody (Dako) and neufuchsine as chromogene as described previously [23]. Alternatively, a commercially available kit from Dako (Envision AEC, Glostrup, Denmark) was applied by using mAb F89/160.1.5 at a dilution of 1:2000 in combination with the mAb 2G11 (1:200, kindly provided by J Grosclaude (INRA, Jouy-en-Josas, France)).
Examination and evaluation of immunolabelled sections
From each sheep all available sections of the CNS and the LRS were examined with the PET blot, and the intensity of the PrPSc staining as well as the forms and distribution of the PrPSc deposition were evaluated. The presence of PrPSc deposits and the deposition forms in CNS and LRS sections were verified by immunohistochemistry. This was usually done using either mAb P4 (German cases) or mAb F89/160.1.5 in combination with mAb 2G11 (Norwegian cases), but if considered necessary immunohistochemistry was repeated with further antibodies as stated above. The intensity of PrPSc deposits in the PET blots was evaluated on a scale of 0.5 to 4 (0 = no PrPSc deposits visible; 0.5 = very little indefinable deposits; 1 = very little distinct PrPSc deposits; 1.5 = little distinct PrPSc deposits; 2 = moderate PrPSc deposits, all deposition forms well distinguishable; 2.5 = moderate to pronounced PrPSc deposits, all deposition forms well distinguishable; 3 = pronounced PrPSc deposits, deposition forms partly interfere with each other; 3.5 = pronounced PrPSc deposits, deposition forms interfere with each other; 4: maximal PrPSc deposits, deposition forms interfere with each other). The value system of the scale itself was established and agreed on by two independent persons that routinely evaluate PET blots.
Western blot analysis
Ten percent tissue homogenates (wt/vol) were either prepared in PBS containing 0.5% desoxycholic acid sodium salt (DOC) using glass grinding tubes and pestles or 20% homogenates were obtained by the standard sampling procedure of the TeSeE Western Blot Kit (Bio-Rad, Hercules, CA, USA).
Twenty percent homogenates were processed using the TeSeE sheep/goat Western Blot Kit according to the manufacturer's instructions. The antibody P4 was added at a dilution of 1:1000 to the primary antibody of the kit. Ten percent homogenates were subjected to a different protocol using homemade 15% acrylamid gels, a 0.45 μm nitrocellulose (NC) membrane (Bio-Rad) for semi-dry blotting and mAbP4 (1:2000). The membrane was treated with 4 M GdnSCN and blocked with 0.2% casein in PBS including 1% Tween for 30 min respectively before the primary antibody was applied overnight at 4 °C. An HRP-conjugated goat anti-mouse antibody (Dako, Carpintera, CA, USA) and Super Signal Femto West Maximum Sensitivity Substrate (Perbio, Erembodegem, Belgium) were used to visualize the result on x-ray film. The molecular size of PrPSc was compared only within one system.
Results
Western blot
In all sheep that had been classified as atypical/Nor98 scrapie cases, the characteristic small fragment of 11-12 kDa [9] was present in CNS tissue samples after proteinase K digestion. Hereof the typical triplet pattern of 18-30 kDa in all classical scrapie sheep was clearly distinguishable. We usually used CNS tissue for Western blotting to determine the molecular profile. Only in one sheep with classical scrapie PrPSc were amounts in the brainstem so minimal that lymphatic tissue was needed to perform a valid Western blot. To ensure that Western blot PrPSc patterns of different tissues were comparable in one sheep, lymphatic tissues of further sheep with classical scrapie (from this study) were examined as well.
PET blot and immunohistochemistry
Disease-associated prion protein could be identified in the CNS of all scrapie sheep with the PET blot and there was no PrPSc detectable in the tissues of the negative control group. As previously described, immunohistochemical methods were able to confirm the presence of PrPSc deposits in all sheep except for one atypical/Nor98 case, despite using of a panel of antibodies [23].
Immunolabeling with the PET blot method allowed the identification of a number of deposition forms of PrPSc in the CNS and all were confirmed by immunohistochemical methods.
As described before [23] PrPSc was detectable in the LRS tissue of all classical scrapie sheep where it was present, but in none of the atypical/Nor98 scrapie animals with available LRS tissue could PrPSc be found (for availability of lymphatic tissue see Table 1). Figure 1a/b shows PET blot and immunohistochemical staining of the PrPSc aggregates in the follicle of a tonsil derived from a classical scrapie case.
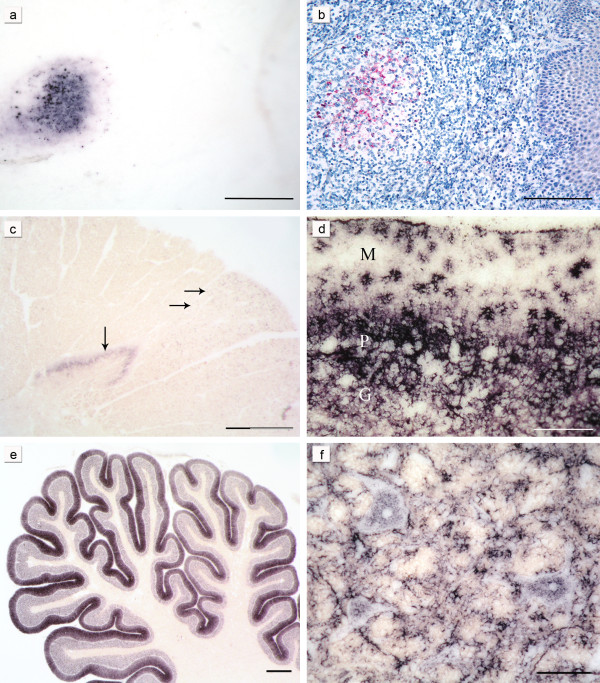
Figure 1.
Characteristic PrPSc deposition patterns in classical and atypical/Nor98 scrapie: The same lymph follicle in the tonsil of a sheep with classical scrapie is shown stained either with the PET blot method (a) or conventional immunohistochemistry (b) (both mAb P4, bars = 250 μm). In the cervical spinal cord segment of a sheep with atypical/Nor98 scrapie (c) stained with the PET blot method (mAb P4, bar = 800 μm) synaptic PrPSc aggregates are present in the substantia gelatinosa (vertical arrow) and granular PrPSc in the corticospinal tract (horizontal arrows). In the cerebellar cortex of a classical scrapie sheep stained with the PET blot (d) complex PrPSc deposits are visible. Glia-associated PrPSc deposits take a stellate form in the molecular layer (mAb P4, bar = 150 μm; M = molecular layer, P = layer of Purkinje cells, G = granular cell layer). In the majority of the atypical/Nor98 scrapie sheep the cerebellar cortices show a more intense staining of the molecular layer than the granular layer (e) (PET blot, mAb P4; bar 600 μm). Intraneuronal, perineuronal and glia-associated PrPSc aggregates (f) in the reticular formation of a classical scrapie sheep (PET blot, mAb P4; bar = 50 μm). Tissue sections derived from sheep with the genotypes VRQ/ARH (a,b), AHQ/AHQ (c,e) and ARQ/ARQ (d,f).
Deposition forms
Intra- and perineuronal PrPSc aggregates were found with the PET blot solely in classical scrapie (Figure 1f), as were subpial, subenpendymal, and perivascular deposits. Extra neuronal PrPSc aggregates in the brains of sheep affected by classical scrapie often had a ramified appearance and were found in grey and white matter structures (Figure 1d and 1f). They were addressed as glia-associated PrPSc aggregates and found to be relatively conspicuous in the cerebellar molecular layer where they took a stellate form [36] (Figure 1d).
In contrast, PrPSc aggregates found in the white matter of atypical/Nor98 scrapie sheep were always well-defined granules that varied a bit in size and were occasionally arranged like pearls on a string. The latter deposition form could also be observed in classical cases, but here also linear PrPSc was sometimes present. PrPSc deposits in the grey matter of atypical/Nor98 scrapie cases generally showed a fine granular pattern, also termed "synaptic/reticular" in human TSEs rather than "fine granular" [12,37] (see Figure 1c). In some atypical/Nor98 scrapie cases, larger plaque-like aggregates could be seen in the substantia nigra, basal ganglia, thalamic nuclei and white matter. However, a differentiation between real plaques (amyloid) and plaque-like deposits is not possible with immunohistochemical detection methods or with the PET blot method as demonstrated before [38].
A discrimination between globular and punctuate deposits in the white matter of atypical/Nor98 cases [11,21] was irreproducible with the PET blot, which is why the term "granular" for the PrPSc deposits that were present in the white matter was chosen. Punctuate PrPSc deposits, comprising smaller aggregates than granular PrPSc deposits but more defined than the reticular PrPSc aggregates, were detected in the grey matter of classical scrapie sheep.
Small deviations in the composition of the complex deposition pattern could not be related to genotypes in the sheep examined.
Distribution of PrPSc in the CNS
Sequential appearance of PrPSc distribution in the CNS of classical scrapie sheep
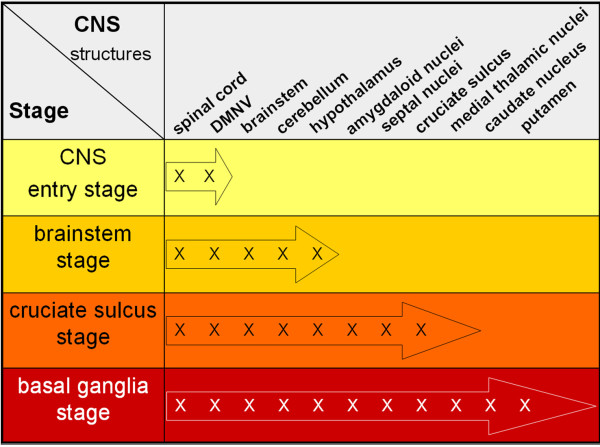
To determine the sequential appearance of PrPSc in the CNS, all field cases of classical scrapie were subjected to a thorough examination regarding the anatomical structures affected by PrPSc deposition. In the following, all cases were arranged according to the amount of PrPSc they had accumulated in total, and the occurrence of PrPSc in a panel of 127 neuroanatomical loci was compared between the cases. From this evaluation arose a classification of the classical scrapie cases into four stages of PrPSc spread in the CNS (see Figures 2, 3, 4 and 5). Criteria for these turned out to be certain neuroanatomical structures whose involvement marked a stage, meaning that the respective structure accumulated PrPSc aggregates (with a minimal score of 1) in all animals belonging to this stage and the following stage/stages. They are described in detail below and visualized in Figures 3 and 4.
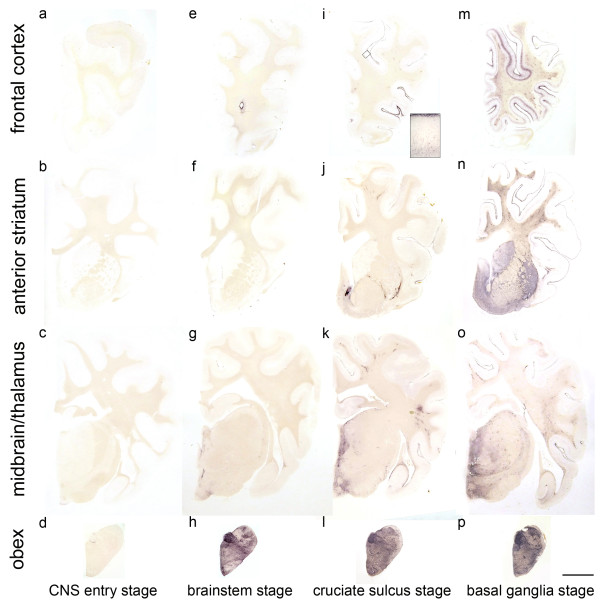
Figure 2.
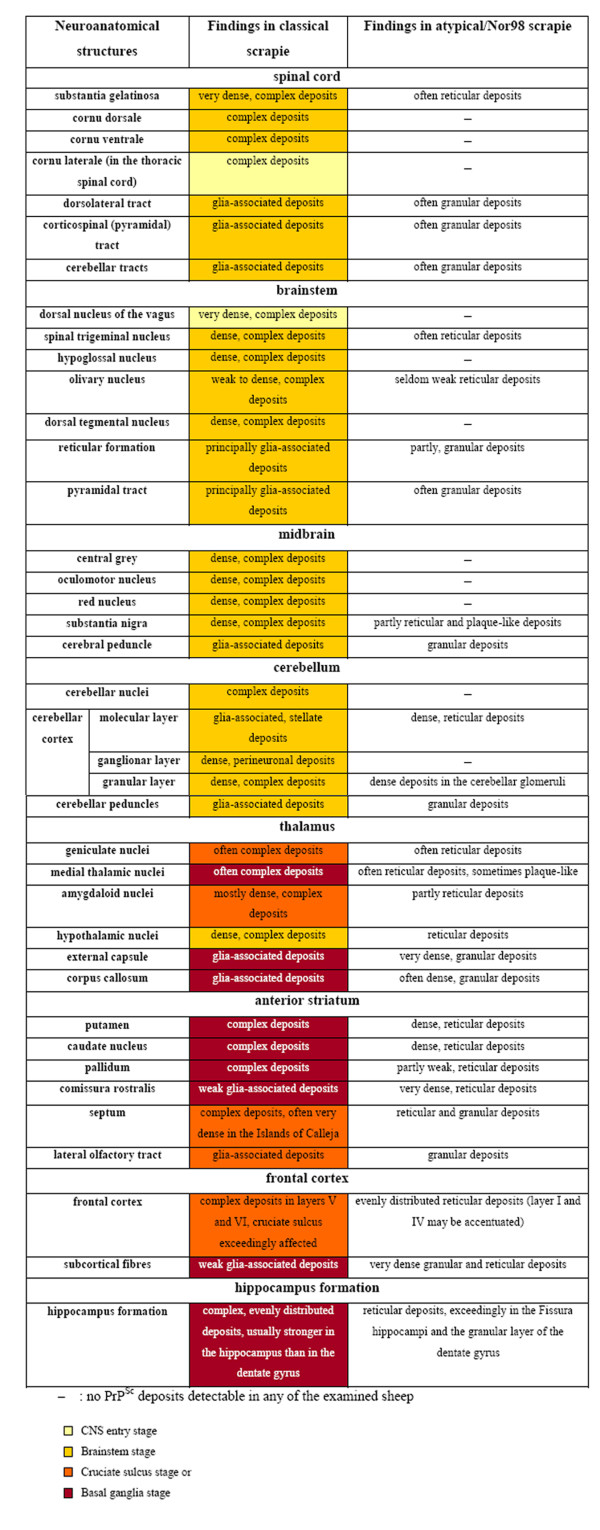
Classification of the PrPSc spread during disease development in classical scrapie.The examined classical scrapie cases classified into four stages of PrP spread according to certain affected neuroanatomical sites (PET blots, mAb P4). In the CNS entry stage (a - d) only discrete PrPSc deposits are visible in the obex region, while in the brainstem stage (e - h) PrPSc aggregates are clearly visible in the brainstem and start to appear in more rostral structures. Once PrPSc deposits can be found in the deep cortical layers of the frontal cortex (i), the cruciate sulcus stage (i - l) is reached. In the basal ganglia stage, intense deposits in basal ganglia and thalamic nuclei can be found (m - p). Brain sections shown for the first, third and fourth stage derived from sheep with the genotype ARQ/ARQ while the sheep whose brain sections are depicted in the brainstem stage carried the genotype ARH/VRQ (bar = 5 mm).
Figure 3.
Progression of classical scrapie in the brain shown for certain affected neuroanatomical sites. The colour code agrees with the one in Figure 5.
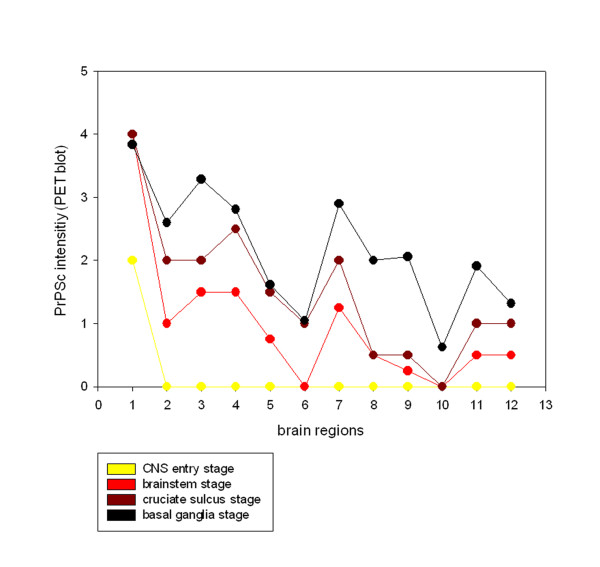
Figure 4.
Accumulation of PrPSc in different brain regions during disease progression: The four stages of the examined classical scrapie cases are depicted in four overlying graphs that illustrate how PrPSc aggregates (PET blot method, mAb P4) are increasingly accumulated in the brains from caudal (left) to rostral (right). Evaluation of PrPSc intensity was performed on a scale from 0.5 - 4 (see material and methods) and shown for the following brain areas: 1 dorsal motor nucleus of the vagus nerve (DMNV), 2 inferior olive, 3 dorsal tegmental nucleus, 4 cerebellar molecular layer, 5 cerebellar granular layer, 6 cerebral peduncle, 7 central grey (mesencephalon), 8 caudate nucleus, 9 ventral pallidum, 10 rostral commissure, 11 cruciate sulcus, 12 frontal white matter.
Figure 5.
Form and appearance of PrPSc deposits in classical and atypical/Nor98 scrapie sheep presented for representative CNS regions. As a sequential development of PrPSc distribution could not be observed upon analysis of the present atypical/Nor98 scrapie cases, no coding colours were used for the results of this scrapie type in contrast to classical scrapie sheep. PrPSc deposits are detectable in the respective location in classical scrapie sheep belonging to the stages of spread indicated in the bottom of the figure using the same colour code as in Figure 3.
CNS entry stage: One sheep showed only few discrete PrPSc deposits in the brain that were restricted to the dorsal motor nucleus of the vagus nerve (DMNV), the solitary tract nucleus and the spinal trigeminal tract in the brainstem. Further PrPSc aggregates could be detected in the substantia intermedialis lateralis and centralis of the thoracic spinal cord. This first stage, where PrPSc is detectable only in these CNS areas, can be considered the "CNS entry stage" in accordance with studies of other authors who have monitored the ascension of PrPSc from the intestines to the CNS [29,30].
Brainstem stage: In the second stage, all segments of the spinal cord and all nuclei of the obex region accumulate PrPSc which also disseminates to the more rostral parts of the medulla; this may therefore be called "brainstem stage". In the caudal medulla the cellulae marginales and substantia gelatinosa of the spinal trigeminal tract nucleus show a very intense staining. The mesencephalon and thalamus display discrete PrPSc deposits which are generally found to be subpial and/or perivascular while the mamillary body, habenular nuclei and the hypothalamic nuclei accumulate substantial amounts of PrPSc. The cerebellar nuclei accumulate PrPSc if the rostral medulla is largely involved and focal deposits of PrPSc are visible in the cerebellar cortex.
Cruciate sulcus stage: During the next stage, the mesencephalon, amygdaloid nuclei, septal nuclei, optic tract, cerebral peduncle, hippocampus formation, frontal cortex and subcortical white matter are increasingly affected. Regarding the frontal cortex, it is notably the sulcus cruciatus - and in a number of cases only this part of the cortex - that accumulates PrPSc in its deeper cortical layers (see Figure 2i). This stage is therefore designated "cruciate sulcus stage". PrPSc deposits in the cerebellar cortex are not yet evenly distributed.
Basal ganglia stage: In the final stage, PrPSc deposits can be seen also in the medial thalamic nuclei (mediodorsal, ventrolateral, ventral posterior and anterior group), the corpora geniculata and the basal ganglia. A positive staining for PrPSc in the latter determines a classical case in our definition for the "basal ganglia stage". The white matter also displays remarkable amounts of PrPSc, which are strongly linked to perivascular distribution.
All stages are depicted in Figures 2, 3 and 4 and the stage at which PrPSc reaches a respective neuroanatomical site is indicated in Figure 5 using a colour code. In the sheep examined in this study we could not find any influence of the different genotypes on the neuroanatomical distribution of PrPSc aggregates.
Comparison of PrPSc deposition patterns in classical and atypical/Nor98 scrapie
PrPSc deposits in atypical/Nor98 scrapie cases were examined and evaluated in the same way as with the classical field cases.
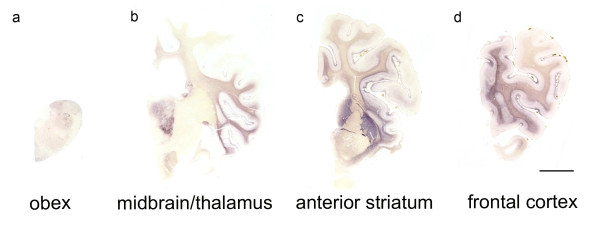
In contrast to the classical scrapie cases, differentiating distribution/spread stages of PrPSc in the CNS was not feasible with the atypical/Nor98 scrapie cases. In Figure 6, the same brain sections that illustrate the different stages of classical scrapie in Figure 2 are depicted for a case of atypical/Nor98 scrapie. In all atypical/Nor98 scrapie sheep, where brainstem material was available (n = 15) apart from one (see below), PrPSc aggregates were detectable in the rhombencephalon and mesencephalon. Regularly affected neuroanatomical structures were the spinal trigeminal nucleus, reticular formation, pyramid, pontine fibres, substantia nigra and cerebral peduncle. In the spinal cord the corticospinal tract and substantia gelatinosa accumulated PrPSc in most cases (Figure 1c). Certain grey matter structures such as the DMNV, hypoglossal nucleus, dorsal tegmental nucleus, oculomotor nucleus, red nucleus and central grey of the mesencephalon, never displayed any PrPSc in the examined atypical/Nor98 scrapie cases. These listed neuroanatomical sites, however, accumulated large amounts of PrPSc in the respective stage of PrPSc distribution in the CNS of classical scrapie sheep as explained above (Figures 2, 3, 4 and 5). There were no PrPSc aggregates detectable in the cerebellar nuclei of the examined atypical/Nor98 scrapie cases, in contrast to the classical scrapie cases as described above. The synaptic or reticular PrPSc staining pattern in the cerebellar cortex of atypical/Nor98 scrapie sheep was in most cases more intense in the molecular than in the granular layer (Figure 1e). Intra- and extracellular complex PrPSc aggregates in the cerebellar cortex of classical scrapie sheep were predominantly present in the granular layer and surrounding the Purkinje cells; the molecular layer displayed mainly glia-associated PrPSc deposits that took a stellate form (Figure 1d). The cerebellar peduncles and white matter of the cerebellum itself showed PrPSc aggregates for both scrapie types. In the diencephalon of most atypical/Nor98 scrapie sheep, the corpora geniculata, medial thalamic nuclei and reticular nucleus accumulated PrPSc aggregates. In all atypical/Nor98 cases where the anterior striatum could be examined (n = 14), PrPSc deposits were also present in the caudate nucleus and putamen. The white matter of diencephalon and telencephalon showed PrPSc deposits in both types of sheep scrapie. In atypical/Nor98 scrapie, these were mainly confined to the subcortical fibres and certain white matter tracts, e.g. the corpus callosum or the commissura rostralis (Figure 7d, arrow), while the distribution in classical scrapie was more disseminated.
Figure 6.
PrPSc distribution in the brain of atypical/Nor98 scrapie: Brain sections of an atypical/Nor98 scrapie case stained with the PET blot (mAb P4) have a different PrPSc distribution than the ones of classical scrapie cases as shown in Figure 2 (bar = 5 mm). Brain section derived from a sheep with the genotype ARQ/AHQ.
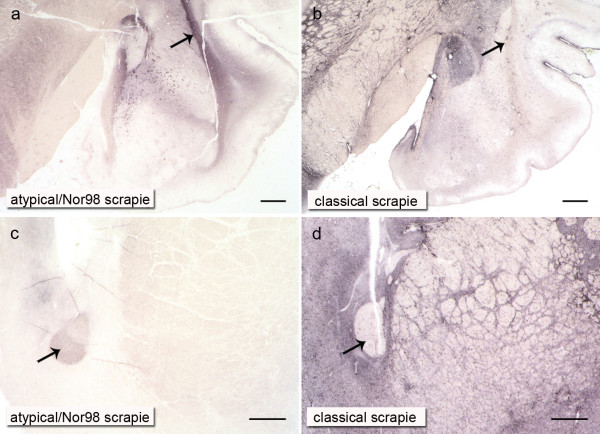
Figure 7.
Differences in the neuroanatomical distribution of PrPSc deposit in atypical/Nor98 and classical sheep scrapie: In atypical/Nor98 scrapie, white matter structures like the external capsule or rostral commissure contain substantially more PrPSc than the subcortical nuclei or basal ganglia respectively (a and c), whereas this is the reverse in classical scrapie (b and d). The external capsule (a and b) and the rostral commissure (c and d) are marked with arrows (mAb P4, bars = 1 mm). Tissue derived from sheep with the genotypes ARQ/AHQ (a), ARQ/ARQ (b and d) and AHQ/AFRQ (c).
There was one case in which PrPSc deposits were detectable with the PET blot only in the supratentorial (cerebral) brain structures and to a very small degree in the cerebellar cortex. The brainstem, including midbrain and spinal cord, were completely spared in this case, which was eventually considered to represent an early stage of atypical/Nor98 scrapie [23].
In Figure 7 the contrasts in PrPSc intensity existing in the grey and white matter between the two types of scrapie are demonstrated in a case of atypical/Nor98 scrapie and a classical scrapie case of the "basal ganglia stage": in classical scrapie it is the centromedial amygdaloid nuclei (Figure 7b) as well as the septal nuclei and basal ganglia (Figure 7d) that show substantially more PrPSc than the external capsule (Figure 7b, arrow) and the rostral commissure (Figure 7d, arrow). In atypical/Nor98 scrapie, this principle turns out to be exactly the opposite, with the external capsule (Figure 7a, arrow) and the rostral commissure (Figure 7c, arrow) accumulating rather intense PrPSc deposits in contrast to the adjacent grey matter.
The lateral olfactory tract displayed PrPSc aggregates in both scrapie types with the respective PrPSc deposition patterns described above. Yet, the Islands of Calleja - clusters of neuronal granular cells in the olfactory tubercle - showed dense PrPSc deposits solely in classical scrapie cases and were completely devoid of PrPSc in atypical/Nor98 scrapie sheep. Regarding the hippocampus formation in classical scrapie cases, there was usually a more intense staining of the hippocampus and the fissura hippocampi compared to the dentate gyrus. In contrast to the atypical/Nor98 scrapie cases, there was no obvious accentuation of any layers. Atypical/Nor98 scrapie sheep showed a rather intense PrPSc staining of the granular layer of the dentate gyrus, the fissura hippocampi and the interconnective fibres between hippocampus and alveus (similar to the subcortical white matter) in comparison to the adjacent layers. The pyramid layer of the hippocampus appeared to be completely devoid of PrPSc deposits. The intensity of PrPSc staining in a single case was usually in agreement with the intensity of PrPSc deposits that could be found in the cerebral cortex of both scrapie types. As mentioned above, the complex PrPSc aggregates in classical scrapie were mainly confined to the deeper cortical layers (laminae V and VI) while reticular/synaptic PrPSc deposits in the cortices of atypical/Nor98 scrapie sheep were distributed more evenly, although an accentuation of laminae I and IV could be noted in some cases. Like in classical scrapie, differences regarding the distribution of PrPSc deposition could not be related to genotypes.
Discussion
In this study 24 cases of classical and 25 cases of atypical/Nor98 scrapie cases were examined with the PET blot method, focusing on the similarities and differences in the distribution of PrPSc deposits that were detectable with this method. Recently the PET blot has been shown to provide a sensitive and specific detection of PrPSc in both types of sheep scrapie in the same manner as had been previously shown for human, bovine and rodent neuronal and non-neuronal tissues [30,35,38-41]. The high sensitivity of this method allows PrPSc deposits to be detected even in FFI patients where conventional immunohistochemistry fails to detect them, and contrasts with Western blotting, which requires up to 1 g of tissue equivalent [42]. The PET blot provides, apart from its sensitivity and specificity, a good overview of where to find PrPSc in a brain section (Figure 2), as no counterstaining is necessary. The fine resolution of the immunolabeling gives a good impression of the structures that accumulate PrPSc (Figure 1f), but the general delineation of the single cell is better with immunohistochemistry, which is why these two methods complement each other in a sensible way.
Neuroinvasion and spread of PrPSc in the ovine brain
In this study, we also give a more detailed account of how the disease-associated PrP aggregates seem to spread in the CNS tissue of sheep infected with classical scrapie. The sequential detection of PrPSc aggregates in the CNS of classical scrapie sheep implies that a cell-to-cell spread takes place from the entry sites in the spinal cord and obex to the cerebellum, diencephalon and frontal cortex via the rostral brainstem. From these entry sites we conclude that the vagus nerve for the DMNV and sympathetic fibres for the spinal cord are the structures that transport the infectious agent to the CNS. This is very similar to the results obtained in hamsters after oral inoculation with the 263K scrapie strain [43]. The cerebellum may also receive PrPSc via the cerebellar tracts of the spinal cord. Noticeable perivascular PrPSc deposition in the brains of scrapie-affected sheep also raises the possibility that the infectious agent reaches the brain via the haematogenous route [44]. The distribution of brain metastases in humans reflects a haematogenous entry into the brain as it is proportional to the cerebral blood flow per area. From this one can conclude that a general PrPSc uptake from the blood would cause quite a different cerebral distribution pattern of PrPSc deposits than we observed [45]. There are three other possible explanations for the perivascular accumulation of PrPSc aggregates in classical scrapie. Cells of glial origin, e.g. microglia, might use the blood vessels as a structural lead for their movement and carry PrPSc molecules with them, possibly also distributing them among the astrocytes forming the blood brain barrier (BBB). This would be a vascular spread in the broader sense. As a second possibility, microglia cells that have incorporated PrPSc move to the blood vessels in order to dispose of the aggregates and this leads to a perivascular deposition of the aggregates. Another way for PrPSc to reach blood vessels could be that they spread via sympathetic nerve fibres of the Plexus nervorum perivascularis. Haematogenous neuroinvasion has also been discussed with regard to the circumventricular organs (CVOs) due to the fact that these are usually affected in scrapie-infected sheep and that they are not protected by the BBB [46]. The possibility that the CVOs might be in contact with PrPSc from the blood during the pathogenesis of the disease cannot be excluded, but our results argue against a major involvement of the CVOs in neuroinvasion. In a very early case the DMNV was affected, but the area postrema and further CVOs were devoid of PrPSc (Figure 2d). This agrees again with the results obtained for the oral infection of hamsters with scrapie [30].
In contrast to the classical scrapie cases, a sequential development of PrPSc distribution cannot be seen upon analysis of the present atypical/Nor98 scrapie cases. PrPSc distribution in one sheep of presumably early disease phase suggests that the aggregation of PrPSc has its origin in the di- or telencephalon. A spontaneous genesis of misfolded PrP could arise in the cerebral cortex. On the other hand, an ascending spread of the infectious agent that bypasses the brainstem and enters the CNS via sensible nerve fibres should be taken into consideration, e.g. proprioceptive fibres [47] or the spinothalamic tract. This would lead to further spreading of PrPSc from the thalamic nuclei to the cerebellar and cerebral cortex and from these to the brainstem and spinal cord, e.g. via the corticospinal tract.
Where does the spread of Nor98- PrPSc start in the brain?
It has been speculated by Nentwig et al. [48] that the PrPSc deposits and histopathologic lesions in atypical/Nor98 scrapie possibly evolve from the cerebrum to the cerebellum and the brainstem, but according to their examination of six sheep brains, this concept would not explain the PrPSc distribution in one sheep where they found PrPSc mainly in the cerebellum. However, immunohistochemistry - as used by these authors - is sometimes not able to detect the fine reticular deposits, e.g. seen in the cortex of Creutzfeldt-Jakob disease type 1, especially in the rare VV1 subtype [49]. The sensitive PET blot method, in contrast, is able to visualize these reticular deposits [38]. PrPSc deposits in one case described by Nentwig et al. could have therefore simply been missed in the cortex by immunohistochemistry. If this proved to be correct, according to the argumentation of Nentwig et al., PrPSc deposition and histopathologic lesions could indeed evolve from the cerebrum into the cerebellum and the brainstem. The 15 whole brains of atypical/Nor98 scrapie sheep examined by Moore et al. [21] should accordingly represent more or less the final stage of disease, as PrPSc can be generally found in all parts of the brain, including the brainstem. In other reports on the occurrence of atypical/Nor98 scrapie, cases have been described in which no PrPSc was detectable by immunohistochemistry in the obex region at all, but in the cerebellum and cerebrum [50-54]. If the misfolding of PrPc in atypical/Nor98 scrapie does really start in the cerebrum it is obvious why early stages are not present in the worldwide pool of preserved atypical/Nor98 brains, as only the sampling of brainstem and cerebellum is compulsory in small ruminants according to EU regulations. Thus the question of whether PrPSc accumulation might start sporadically in the cerebrum - and if so, at one or more sites at the same time? - cannot be resolved by this or any other current study using field cases of atypical/Nor98 scrapie. This situation is comparable to the one with CJD type 1, where a spontaneous misfolding of PrPc in the cerebral cortex and a caudal spread from there is assumed, but not proven [55]. The incidence of atypical/Nor98 in sheep is higher than that of CJD in humans [17]. A case control study of atypical/Nor98 scrapie has shown that animal movement does not seem to be a factor for the transmission of atypical/Nor98 scrapie between flocks; thus if sheep were to acquire this prion disease from their environment, its contagiousness would indeed be very low [56]. It has been speculated that this might be due to the relatively low protease stability, which could also explain the lack of intracellular PrPSc deposits [33].
There are certainly small differences between the PrPSc distribution detected by Moore et al. [21] in their described atypical/Nor98 scrapie cases and the ones revealed here by the PET blot method. For instance, in the present atypical/Nor98 scrapie material, PrPSc was never detectable in the cerebellar nuclei. Also, the affected parts of the hippocampus appear to be different. This might be due to differences in the treatment of tissue, the methods and/or differences in the antibodies used (mAb2G11 versus mAbP4). As previously reported, perineuronal staining has also been detected for the substantia nigra in some atypical/Nor98 scrapie sheep using immunohistochemistry [11], whereas in our study only plaque-like PrPSc deposits could be seen in this neuroanatomical structure. Similarly, neuronal deposits could be found in many affected sites of classical scrapie, but in contrast to previous publications [22], neither PET blot nor immunohistochemistry revealed PrPSc in the Purkinje cells of the cerebellum. It is known that especially intraneuronal immunoreactivity needs to be interpreted with caution [10]. However, the congruence between previous reports on PrPSc deposition patterns and the present results is obvious.
Conclusion
In summary, this study gives a basic description of PrPSc deposition patterns in classical as compared to atypical/Nor98 scrapie cases using the sensitive and specific PET blot method. We were able show a sequential appearance of PrPSc aggregates in the CNS of sheep with classical scrapie, but not in atypical/Nor98 scrapie. The four emerging stages of spread in classical scrapie were defined by the accumulation of PrPSc in certain neuroanatomical structures. These structures accumulated PrPSc aggregates in all animals belonging to this stage and the following stage/stages. Further conclusions drawn from this study regarding atypical/Nor98 scrapie might help in future to elucidate its origin and potentially related prion disease types like Creutzfeldt-Jakob disease type 1.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WMW carried out the PET blot studies, participated in immunohistochemistry, Western blot, tissue acquisition and the design of the study and drafted the manuscript. SLB participated in immunohistochemistry, Western blot, tissue acquisition and design of the study and co-edited the manuscript. AW, WEW, BeB and BjB participated in tissue acquisition and diagnosing the cases. WJSS conceived the study, participated in its design and coordination and co-edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Wiebke M Wemheuer, Email: wiebke.wemheuer@med.uni-goettingen.de.
Sylvie L Benestad, Email: sylvie.benestad@vetinst.no.
Arne Wrede, Email: awrede@med.uni-goettingen.de.
Wilhelm E Wemheuer, Email: wwemheu1@gwdg.de.
Bertram Brenig, Email: bbrenig@gwdg.de.
Bjørn Bratberg, Email: bjorn.bratberg@vetinst.no.
Walter J Schulz-Schaeffer, Email: wjschulz@med.uni-goettingen.de.
Acknowledgements
We would like to thank Tatjana Pfander, Nadine Rupprecht and Kerstin Brekerbohm for their skilful technical assistance. The work was supported by the VolkswagenStiftung (grants ZN 1294 and ZN 2168 to W.J.S.S).
References
- McGowan JP. Scrapie in sheep. Scottish J Agric. 1922;5:365–375. [Google Scholar]
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Oesch B, Westaway D, Walchli M, McKinley MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE, Prusiner SB, Weissmann C. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendheim PE, Brown HR, Rudelli RD, Scala LJ, Goller NL, Wen GY, Kascsak RJ, Cashman NR, Bolton DC. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology. 1992;42:149–156. doi: 10.1212/wnl.42.1.149. [DOI] [PubMed] [Google Scholar]
- Bruce ME, Boyle A, Cousens S, McConnell I, Foster J, Goldmann W, Fraser H. Strain characterization of natural sheep scrapie and comparison with BSE. J Gen Virol. 2002;83:695–704. doi: 10.1099/0022-1317-83-3-695. [DOI] [PubMed] [Google Scholar]
- Hope J, Wood SC, Birkett CR, Chong A, Bruce ME, Cairns D, Goldmann W, Hunter N, Bostock CJ. Molecular analysis of ovine prion protein identifies similarities between BSE and an experimental isolate of natural scrapie, CH1641. J Gen Virol. 1999;80:1–4. doi: 10.1099/0022-1317-80-1-1. [DOI] [PubMed] [Google Scholar]
- Stack MJ, Chaplin MJ, Clark J. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol. 2002;104:279–286. doi: 10.1007/s00401-002-0556-2. [DOI] [PubMed] [Google Scholar]
- Benestad SL, Sarradin P, Thu B, Schönheit J, Tranulis MA, Bratberg B. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec. 2003;153:202–208. doi: 10.1136/vr.153.7.202. [DOI] [PubMed] [Google Scholar]
- EFSA. Opinion of the Scientific Panel on Biological Hazards on classification of atypical Transmissible Spongiform Encephalopathy (TSE) cases in Small Ruminants. The EFSA Journal. 2005;276:1–30. [Google Scholar]
- Benestad SL, Arsac JN, Goldmann W, Nöremark M. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res. 2008;39:19. doi: 10.1051/vetres:2007056. [DOI] [PubMed] [Google Scholar]
- Wemheuer WM, Benestad SL, Wrede A, Schulze-Sturm U, Wemheuer WE, Hahmann U, Gawinecka J, Schütz E, Zerr I, Brenig B, Bratberg B, Andreoletti O, Schulz-Schaeffer WJ. Similarities between forms of sheep scrapie and Creutzfeldt-Jakob disease are encoded by distinct prion types. Am J Pathol. 2009;175:2566–2573. doi: 10.2353/ajpath.2009.090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S, Nugier J, Morel N, Boutal H, Créminon C, Benestad SL, Andréoletti O, Lantier F, Bilheude JM, Feyssaguet M, Biacabe AG, Baron T, Grassi J. Rapid typing of transmissible spongiform encephalopathy strains with differential ELISA. Emerg Infect Dis. 2008;14:608–616. doi: 10.3201/eid1404.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt PB, Muileman IH, Schreuder BE, Bos-de Ruijter J, Gielkens AL, Smits MA. Identification of five allelic variants of the sheep PrP gene and their association with natural scrapie. J Gen Virol. 1995;76:509–517. doi: 10.1099/0022-1317-76-3-509. [DOI] [PubMed] [Google Scholar]
- Hunter N. PrP genetics in sheep and the applications for scrapie and BSE. Trends Microbiol. 1997;5:331–334. doi: 10.1016/S0966-842X(97)01081-0. [DOI] [PubMed] [Google Scholar]
- Lühken G, Buschmann A, Groschup MH, Erhardt G. Prion protein allele A136 H154Q171 is associated with high susceptibility to scrapie in purebred and crossbred German Merinoland sheep. Arch Virol. 2004;149:1571–1580. doi: 10.1007/s00705-004-0303-1. [DOI] [PubMed] [Google Scholar]
- Lühken G, Buschmann A, Brandt H, Eiden M, Groschup MH, Erhardt G. Epidemiological and genetical differences between classical and atypical scrapie cases. Vet Res. 2007;38:65–80. doi: 10.1051/vetres:2006046. [DOI] [PubMed] [Google Scholar]
- Moum T, Olsaker I, Hopp P, Moldal T, Valheim M, Moum T, Benestad SL. Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J Gen Virol. 2005;86:231–235. doi: 10.1099/vir.0.80437-0. [DOI] [PubMed] [Google Scholar]
- González L, Martin S, Begara-McGorum I, Hunter N, Houston F, Simmons M, Jeffrey M. Effects of agent strain and host genotype on PrP accumulation in the brain of sheep naturally and experimentally affected with scrapie. J Comp Pathol. 2002;126:17–29. doi: 10.1053/jcpa.2001.0516. [DOI] [PubMed] [Google Scholar]
- Miller JM, Jenny AL, Taylor WD, Marsh RF, Rubenstein R, Race RE. Immunohistochemical detection of prion protein in sheep with scrapie. J Vet Diagn Invest. 1993;5:309–316. doi: 10.1177/104063879300500301. [DOI] [PubMed] [Google Scholar]
- Moore SJ, Simmons M, Chaplin M, Spiropoulos J. Neuroanatomical distribution of abnormal prion protein in naturally occurring atypical scrapie cases in Great Britain. Acta Neuropathol. 2008;116:547–559. doi: 10.1007/s00401-008-0433-8. [DOI] [PubMed] [Google Scholar]
- van Keulen LJ, Schreuder BE, Meloen RH, Poelen-van den Berg M, Mooij-Harkes G, Vromans ME, Langeveld JP. Immunohistochemical detection and localization of prion protein in brain tissue of sheep with natural scrapie. Vet Pathol. 1995;32:299–308. doi: 10.1177/030098589503200312. [DOI] [PubMed] [Google Scholar]
- Wemheuer WM, Benestad SL, Wrede A, Wemheuer WE, Brenig B, Bratberg B, Schulz-Schaeffer WJ. Detection of classical and atypical/Nor98 scrapie by the paraffin-embedded tissue blot method. Vet Rec. 2009;164:677–681. doi: 10.1136/vr.164.22.677. [DOI] [PubMed] [Google Scholar]
- Andréoletti O, Simon S, Lacroux C, Morel N, Tabouret G, Chabert A, Lugan S, Corbière F, Ferre P, Foucras G, Laude H, Eychenne F, Grassi J, Schelcher F. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat Med. 2004;10:591–593. doi: 10.1038/nm1055. [DOI] [PubMed] [Google Scholar]
- Lacroux C, Corbière F, Tabouret G, Lugan S, Costes P, Mathey J, Delmas JM, Weisbecker JL, Foucras G, Cassard H, Elsen JM, Schelcher F, Andreoletti O. Dynamics and genetics of PrPSc placental accumulation in sheep. J Gen Virol. 2007;88:1056–1061. doi: 10.1099/vir.0.82218-0. [DOI] [PubMed] [Google Scholar]
- Ligios C, Sigurdson CJ, Santucciu C, Carcassola G, Manco G, Basagni M, Maestrale C, Cancedda MG, Madau L, Aguzzi A. PrPSc in mammary glands of sheep affected by scrapie and mastitis. Nat Med. 2005;11:1137–1138. doi: 10.1038/nm1105-1137. [DOI] [PubMed] [Google Scholar]
- Thomzig A, Schulz-Schaeffer W, Wrede A, Wemheuer W, Brenig B, Kratzel C, Lemmer K, Beekes M. Accumulation of pathological prion protein PrPSc in the skin of animals with experimental and natural scrapie. PLoS Pathog. 2007;3:e66. doi: 10.1371/journal.ppat.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersdal C, Ulvund MJ, Espenes A, Benestad SL, Sarradin P, Landsverk T. Mapping PrPSc propagation in experimental and natural scrapie in sheep with different PrP genotypes. Vet Pathol. 2005;42:258–274. doi: 10.1354/vp.42-3-258. [DOI] [PubMed] [Google Scholar]
- van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Pathogenesis of natural scrapie in sheep. Arch Virol Suppl. 2000. pp. 57–71. [DOI] [PubMed]
- McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol. 2001;75:9320–9327. doi: 10.1128/JVI.75.19.9320-9327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Keulen LJ, Vromans ME, Dolstra CH, Bossers A, Van Zijderveld FG. Pathogenesis of bovine spongiform encephalopathy in sheep. Arch Virol. 2008;153:445–453. doi: 10.1007/s00705-007-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder SJ, Dexter GE, Heasman L, Warner R, Moore SJ. Accumulation and dissemination of prion protein in experimental sheep scrapie in the natural host. BMC Vet Res. 2009;5:9. doi: 10.1186/1746-6148-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MM, Konold T, Simmons HA, Spencer YI, Lockey R, Spiropoulos J, Everitt S, Clifford D. Experimental transmission of atypical scrapie to sheep. BMC Vet Res. 2007;3:20. doi: 10.1186/1746-6148-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz E, Scharfenstein M, Brenig B. Genotyping of ovine prion protein gene (PRNP) variants by PCR with melting curve analysis. Clin Chem. 2006;52:1426–1429. doi: 10.1373/clinchem.2006.069666. [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ, Fatzer R, Vandevelde M, Kretzschmar HA. Detection of PrP(Sc) in subclinical BSE with the paraffin-embedded tissue (PET) blot. Arch Virol Suppl. 2000. pp. 173–180. [DOI] [PubMed]
- González L, Martin S, Jeffrey M. Distinct profiles of PrP(d) immunoreactivity in the brain of scrapie- and BSE-infected sheep: implications for differential cell targeting and PrP processing. J Gen Virol. 2003;84:1339–1350. doi: 10.1099/vir.0.18800-0. [DOI] [PubMed] [Google Scholar]
- Kitamoto T, Tateishi J. Human prion diseases with variant prion protein. Philos Trans R Soc Lond B Biol Sci. 1994;343:391–398. doi: 10.1098/rstb.1994.0034. [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ, Tschöke S, Kranefuss N, Dröse W, Hause-Reitner D, Giese A, Groschup MH, Kretzschmar HA. The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am J Pathol. 2000;156:51–56. doi: 10.1016/S0002-9440(10)64705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezmi S, Bencsik A, Baron T. PET-blot Analysis Contributes to BSE Strain Recognition in C57Bl/6 Mice. J Histochem Cytochem. 2006;54:1087–1094. doi: 10.1369/jhc.5A6892.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden AH, Ritchie DL, Head MW, Ironside JW. Detection and localization of PrPSc in the skeletal muscle of patients with variant, iatrogenic, and sporadic forms of Creutzfeldt-Jakob disease. Am J Pathol. 2006;168:927–935. doi: 10.2353/ajpath.2006.050788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomzig A, Kratzel C, Lenz G, Krüger D, Beekes M. Widespread PrPSc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 2003;4:530–533. doi: 10.1038/sj.embor.embor827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder AT, Mednick AS, Brown P, Spire JP, Van Cauter E, Wollmann RL, Cervenakova L, Goldfarb LG, Garay A, Ovsiew F, Gajdusek DC, Roos RP. Clinical and genetic studies of fatal familial insomnia. Neurology. 1995;45:1068–1075. doi: 10.1212/wnl.45.6.1068. [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer W, McBride PA, Beekes M, Kretzschmar HA. Spread of PrPSc in orally infected animals during incubation time of prion disease. XIV International Conference of Neuropathology. 2000. p. 663.
- Jeffrey M, Goodsir CM, Holliman A, Higgins RJ, Bruce ME, McBride PA, Fraser JR. Determination of the frequency and distribution of vascular and parenchymal amyloid with polyclonal and N-terminal-specific PrP antibodies in scrapie-affected sheep and mice. Vet Rec. 1998;142:534–537. doi: 10.1136/vr.142.20.534. [DOI] [PubMed] [Google Scholar]
- Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- Sisó S, Jeffrey M, González L. Neuroinvasion in sheep transmissible spongiform encephalopathies: the role of the haematogenous route. Neuropathol Appl Neurobiol. 2009;35:232–246. doi: 10.1111/j.1365-2990.2008.00978.x. [DOI] [PubMed] [Google Scholar]
- Rüb U, Schultz C, Del TK, Gierga K, Reifenberger G, de Vos RA, Seifried C, Braak H, Auburger G. Anatomically based guidelines for systematic investigation of the central somatosensory system and their application to a spinocerebellar ataxia type 2 (SCA2) patient. Neuropathol Appl Neurobiol. 2003;29:418–433. doi: 10.1046/j.1365-2990.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- Nentwig A, Oevermann A, Heim D, Botteron C, Zellweger K, Drogemuller C, Zurbriggen A, Seuberlich T. Diversity in Neuroanatomical Distribution of Abnormal Prion Protein in Atypical Scrapie. PLoS Pathog. 2007;3:e82. doi: 10.1371/journal.ppat.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, Poser S, Rojiani A, Streichemberger N, Julien J, Vital C, Ghetti B, Gambetti P, Kretzschmar H. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46:224–233. doi: 10.1002/1531-8249(199908)46:2<224::AID-ANA12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Buschmann A, Biacabe AG, Ziegler U, Bencsik A, Madec JY, Erhardt G, Lühken G, Baron T, Groschup MH. Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests. J Virol Methods. 2004;117:27–36. doi: 10.1016/j.jviromet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Buschmann A, Lühken G, Schultz J, Erhardt G, Groschup MH. Neuronal accumulation of abnormal prion protein in sheep carrying a scrapie-resistant genotype (PrPARR/ARR) J Gen Virol. 2004;85:2727–2733. doi: 10.1099/vir.0.79997-0. [DOI] [PubMed] [Google Scholar]
- Gavier-Widen D, Nöremark M, Benestad S, Simmons M, Renstrom L, Bratberg B, Elvander M, af Segerstad CH. Recognition of the Nor98 variant of scrapie in the Swedish sheep population. J Vet Diagn Invest. 2004;16:562–567. doi: 10.1177/104063870401600611. [DOI] [PubMed] [Google Scholar]
- Onnasch H, Gunn HM, Bradshaw BJ, Benestad SL, Bassett HF. Two Irish cases of scrapie resembling Nor98. Vet Rec. 2004;155:636–637. doi: 10.1136/vr.155.20.636. [DOI] [PubMed] [Google Scholar]
- Orge L, Galo A, Machado C, Lima C, Ochoa C, Silva J, Ramos M, Simas JP. Identification of putative atypical scrapie in sheep in Portugal. J Gen Virol. 2004;85:3487–3491. doi: 10.1099/vir.0.80246-0. [DOI] [PubMed] [Google Scholar]
- Heye N, Cervos-Navarro J. Focal involvement and lateralization in Creutzfeldt-Jakob disease: correlation of clinical, electroencephalographic and neuropathological findings. Eur Neurol. 1992;32:289–292. doi: 10.1159/000116844. [DOI] [PubMed] [Google Scholar]
- Hopp P, Omer MK, Heier BT. A case-control study of scrapie Nor98 in Norwegian sheep flocks. J Gen Virol. 2006;87:3729–3736. doi: 10.1099/vir.0.81951-0. [DOI] [PubMed] [Google Scholar]