Abstract
Background
There is a paucity of population-based studies on Staphylococcus aureus bacteremia (SAB) in the United States. We determined the incidence and trends of SAB in Olmsted County, Minnesota, over an 8-year period.
Methods
A retrospective, population-based, cohort study was done to evaluate the initial episodes of SAB occurring in adult residents of Olmsted County, Minnesota, from January 1, 1998 through December 31, 2005 using the microbiology databases at Mayo Clinic and Olmsted Medical Center.
Results
Among 247 evaluable adult patients with SAB, who were included in the incidence calculation, 57.9% were males and the median age was 72.1 years (range 19.5 - 98.5). Bacteremic episodes were classified according to acquisition site: 23.5% were nosocomial (NA-SAB); 58.7% were healthcare-associated (HCA-SAB); and 23.8% were community-acquired (CA-SAB). MRSA constituted 31.6% of the cases. No community-acquired MRSA bacteremia was noted. The age-adjusted incidence rate of SAB was 28.3/100,000 person-years for females, and 53.5/100,000 person-years for males, with an age- and gender-adjusted rate of 38.2/100,000 person-years. The age- and gender-adjusted incidence of NA-SAB, HCA-SAB, and CA-SAB was 9.0, 22.6 and 6.6/100,000 person-years, respectively. The age- and gender-adjusted incidence of MSSA was 25.4/100,000 and of MRSA was 12.4/100,000 person-years. Overall, the incidence rate increased with age, but not over calendar year. The exception was MRSA-B, which increased at a rate of 19.8% (± 5.5%) per year during the study period.
Conclusions
The incidence of SAB in adults remained stable in Olmsted County, Minnesota, from 1998 to 2005, but the proportion due to MRSA has significantly increased over the 8-year period.
Keywords: Incidence, Staphylococcus aureus, bacteremia, population-based, Olmsted County
Introduction
Over the past three decades, increases in both frequency of Staphylococcus aureus bacteremia (SAB) and resistance to antimicrobial agents have enhanced interest in this major pathogen [1]. Rates of SAB from 1980 to 1989 reported by the National Nosocomial Infections Surveillance System (NNIS) increased by 283% in non-teaching hospitals and 176% in large teaching hospitals [2]. From 1995 to 2002, data from 49 US hospitals participating in the Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) project reported that S. aureus was the second most common bloodstream isolate, accounting for 20% of hospital-acquired bacteremias [3]. Staphylococus aureus was the second most common blood stream isolate in Olmsted County population from 2003 to 2005 [4], and the leading cause of bacteremia is persons aged 65 years or older [5].
Knowledge of incidence is crucial in determining the burden of disease in a population and is used to allocate healthcare resources for prevention and management. Only Denmark and the United Kingdom have nationwide surveillance systems for SAB [6, 7]. In addition, investigators in Australia, New Zealand, Finland, Canada and Sweden have published population-based incidence rates for their respective localities [8-13].
There are only limited data from population-based studies in the US regarding the incidence of SAB. A population-based US study was done in 4 metropolitan areas in Connecticut over ten years ago and demonstrated an incidence of community-onset SAB of 17/100,000 person-years [14]. Uslan et al [4] reported an incidence rate of 32/100,000 person-years in Olmsted County, MN from 2003 to 2005. No temporal trends in incidence, however, were reported in these two cross-sectional US population-based studies. We, therefore, conducted a retrospective population-based cohort study to describe the epidemiology of SAB in Olmsted County, Minnesota, over an 8-year period (1998-2005). Incidence and trends of SAB over time are described by age, gender and site of infection acquisition.
Methods
Setting and population
Olmsted County's population is served by a largely unified medical care system that has accumulated comprehensive clinical records since 1966 [15]. Olmsted County (2000 population 124 277) lies 90 miles southeast of Minneapolis/St. Paul. Approximately 70% of the county population resides within the city limits of Rochester, Minnesota, the centrally located County seat. The population is mainly white and is largely middle class with approximately 82% of adults having graduated from high school. The characteristics of Olmsted County population are similar to those of US non-Hispanic whites with the exception of a higher proportion of the working adults employed in the healthcare industry.
The Rochester Epidemiology Project (REP) is a medical record linkage system that indexes medical records from all individuals seen by a healthcare provider and residing in Olmsted County. A single dossier exists for each patient, into which medical diagnoses, surgical interventions and other key information from medical records are regularly abstracted and entered into computerized indexes using the International Classification of Diseases, ninth edition, clinical modifications [13-15]. The REP provides access to all inpatient, outpatient, emergency department and nursing home records of Olmsted County residents, regardless of provider, allowing for accurate population-based incidence studies [15].
Case ascertainment
Cases of SAB were identified using the microbiology databases at Mayo Clinic Rochester (MCR) and Olmsted Medical Center (OMC), the only two microbiology labs in Olmsted County. All Staphylococcus aureus isolates cultured from blood were deemed to be clinically significant. SAB was defined as the isolation of Staphylococcus aureus from blood, alone or with coagulase-negative staphylococci (CNS), the latter having been isolated from only one set of blood cultures, in an adult resident (age ≥18) of Olmsted County between 1 Jan 1998 and 31 December 2005. Polymicrobial bacteremias (S. aureus with another pathogen), blood cultures obtained at autopsy, non-residency in Olmsted County at the time of bacteremia, and refusal to be included in medical research according to the state of Minnesota law were exclusion criteria. SAB was classified as nosocomial, healthcare-associated or community-acquired as defined by Freidman et al [16] as follows: Nosocomial bloodstream infection was defined by a positive blood culture obtained from patients who had been hospitalized for 48 hours or longer. If a patient was transferred from another hospital, the duration of inpatient stay was calculated from the date of the first hospital admission.
Health care–associated bloodstream infection was defined by a positive blood culture obtained from a patient at the time of hospital admission or within 48 hours of admission if the patient fulfilled any of the following criteria:
Received intravenous therapy at home; received wound care or specialized nursing care through a health care agency, family, or friends; or had self-administered intravenous medical therapy in the 30 days before the bloodstream infection. Patients whose only home therapy was oxygen use were excluded.
Attended a hospital or hemodialysis clinic or received intravenous chemotherapy in the 30 days before the bloodstream infection.
Was hospitalized in an acute care hospital for 2 or more days in the 90 days before the bloodstream infection.
Resided in a nursing home or long-term care facility.
Community-acquired bloodstream infection was defined by a positive blood culture obtained at the time of hospital admission or within the 48 hours after hospital admission for patients who did not fit the criteria for a health care–associated infection.
Because the REP includes data from two institutions, multiple culture systems for detecting SAB were used during the time course of the study. These systems included the Septi-Chek manual bottles (PML Microbiologics, Wilsonville, OR), BACTEC 9240 automated system (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD) and the BACTEC 9050 automated blood culture system (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD). The BACTEC systems used both aerobic and anaerobic vials, and incubation time has been 5 days since 1998. Two sets of blood cultures were routinely obtained and performed using standard microbiology techniques according to the Clinical Laboratory Standards Institute. Both laboratories are certified by the College of American Pathologists.
The medical records of all cases of SAB were manually reviewed by the primary investigator (W.I.A) to confirm diagnosis and residency status (using the REP resources). Cases judged problematic were reviewed with the senior investigator (L.M.B). Only the first episode of SAB per person during the study period was included in the incidence calculations.
Data analysis
For calculating incidence, the entire adult population of Olmsted County was considered to be at risk for infection. The denominator age- and gender-specific person-years were derived from decennial census figures. Age was divided into 4 groups (18-39, 40-59, 60-79, and 80+), while the study period was divided into four 2-year intervals (1998-1999, 2000-2001, 2002-2003, and 2004-2005). For years following the most recent decennial census in 2000, a growth rate of 1.9% was applied to project the population numbers. Rates were age- and gender-adjusted to the population of white adults in the United States in 2000. Multivariable Poisson regression was used to test for temporal trends in incidence, separately, among the following groups: overall SAB, SAB by each of the settings of acquisition (nosocomial, healthcare-associated, or community-acquired), MRSA, and MSSA while adjusting for age and gender. Both calendar year groupings and age groupings were treated as continuous variables in the multivariable Poisson regression model whereas, gender was treated as a binary variable. For all analyses, a p-value of <0.05 was considered statistically significant.
Results
Olmsted County SAB Cohort
During the 8-year study period, 287 adult residents of Olmsted County had at least one episode of SAB. Forty patients were excluded: 3 (1%) were diagnosed at autopsy, 16 (5.6%) refused to allow their records to be used in research, and 21 (7.3%) were polymicrobial. Six SAB episodes accompanied by coagulase-negative staphylococcus in one set of blood cultures were included in the analysis. Thus, 247 patients were included in the incidence calculations.
Table 1 summarizes the demographic and clinical characteristics of the 247 SAB cases. The median (range) age of cases was 72.1 (19.5 – 98.5) years and 57.9% were male. Eighty-five percent were white non-Hispanic reflecting the general demographic characteristics of southeastern Minnesota. Of the 247 incident SAB episodes, 78 (31.6%) were MRSA and 165 (66.8%) were MSSA, and 4 (1.6%) were unknown. Fifty-eight isolates (23.5%) were classified as nosocomial (NA-SAB), 145 (58.7%) as healthcare-associated (HCA-SAB) and 44 (17.8%) as community-acquired (CA-SAB).
Table 1. Demographic and clinical characteristics of SAB cases in Olmsted County, MN, 1998-2005.
| Characteristic | n (%) |
|---|---|
| Age, median, years | 72.1 |
| Male | 143 (57.9) |
| Race | |
| White non Hispanic | 211 (85.4) |
| Black non Hispanic | 5 (2.0) |
| Asian/Pacific islander | 6 (2.4) |
| American Indian/Alaskan native | 1 (0.4) |
| Hispanic | 4 (1.6) |
| Other | 2 (0.8) |
| Unknown | 18 (7.3) |
| Type of SAB | |
| MRSA | 78 (31.6) |
| MSSA | 165 (66.8) |
| Unknown | 4 (1.6) |
| Infection acquisition setting | |
| Nosocomial | 58 (23.5) |
| Healthcare-associated | 145 (58.7) |
| Community-acquired | 44 (17.8) |
| Comorbidities | |
| Diabetes mellitus | 90 (36.4) |
| Congestive heart failure | 74 (30.0) |
| End stage renal disease | 46 (18.6) |
| Malignancy | 39 (15.8) |
| HIV infection | 0 (0.0) |
| Transplantation | 10 (4.0) |
| Chronic obstructive pulmonary disease | 45 (18.2) |
| Cirrhosis | 15 (6.0) |
| Stroke/paraplegia | 31 (12.6) |
| Injection drug use | 4 (1.6) |
| Source/complication of bacteremia | |
| Line-associated/endovascular* | 70 (28.3) |
| Respiratory | 30 (12.1) |
| Skin/soft tissue | 43 (17.4) |
| Intra-abdominal | 4 (1.6) |
| Central nervous system | 1 (0.4) |
| Bone/joint | 28 (11.3) |
| Other | 18 (7.3) |
| Unknown | 53 (21.5) |
SAB: S. aureus bacteremia.
Including 18 endocarditis cases.
For the period 1998-2005, the age- and gender-adjusted incidence of SAB was 38.2 per 100,000 person-years (95% CI: 33.4 - 43.0) (Table 2). The age-adjusted incidence among males and females was 53.5/100,000 (95% CI: 44.5 – 62.5) and 28.3/100,000 (95% CI: 22.8 – 33.8) person-years, respectively. Rates increased with age for both males and females (Figure 1).
Table 2. Incidence rates of SAB in Olmsted County, MN, by time period.
| 1998-1999 No. (rate/105) |
2000-2001 No. (rate/105) |
2002-2003 No. (rate/105) |
2004-2005 No. (rate/105) |
All years No.(rate/105) |
|
|---|---|---|---|---|---|
| Total | 46 (26.2) | 63 (34.7) | 68 (36.1) | 70 (35.9) | 247 (33.4) |
| Gender | |||||
| Male | 25 (29.8) | 36 (41.5) | 41 (45.5) | 41 (43.9) | 143 (40.4) |
| Female | 21 (22.9) | 27 (28.6) | 27 (27.5) | 29 (28.5) | 104 (26.9) |
| Age | |||||
| 18-39 | 2 (2.6) | 6 (7.6) | 7 (8.6) | 2 (2.4) | 17 (5.3) |
| 40-59 | 13 (20.7) | 15 (22.5) | 14 (20.3) | 17 (23.7) | 59 (21.8) |
| 60-79 | 20 (74.3) | 23 (82.1) | 31 (106.7) | 30 (99.6) | 104 (91.1) |
| >80 | 11 (143.1) | 19 (235.6) | 16 (191.2) | 21 (242.2) | 67 (204.3) |
| Adjusted a | 30.2 (21.4-39.0) | 39.4 (29.6-49.1) | 41.2 (31.3- 51.0) | 41.3 (31.6-51.0) | 38.2 (33.4-43.0) |
SAB: S. aureus bacteremia.
Rates were directly adjusted for age and gender to 2000 US adult white population (with 95% CI).
Figure 1. Incidence of SAB in Olmsted County, MN, 1998-2005 by age and gender.
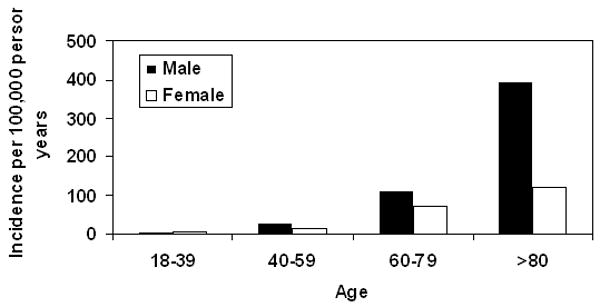
SAB: S. aureus bacteremia.
Temporal Trends, 1998-2005
Age- and gender-specific incidence rates for periods 1998-1999, 2000-2001, 2002-2003, and 2004-2005 are compared in Table 2. Although the incidence rates appear to be increasing slightly over time, there was no statistically significant linear trend over the 8-year study period (p=0.13; adjusting for age and gender). Gender-specific incidence rates are illustrated in Figure 2.
Figure 2. Age-adjusted* incidence of SAB in Olmsted County, MN, 1998-2005.
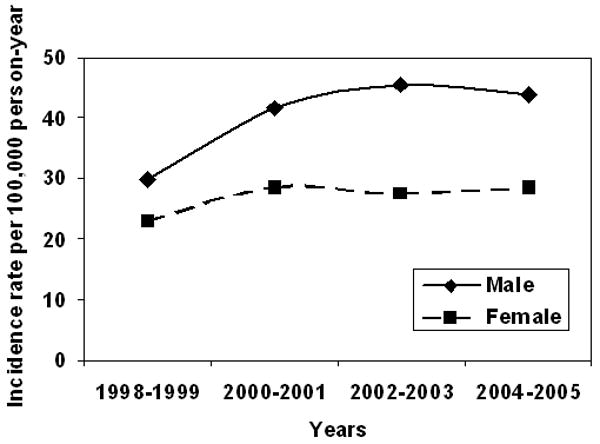
*Adjusted to US 2000 white adult population.
SAB: S. aureus bacteremia.
The age- and gender-adjusted incidence rates by setting are shown in Table 3; setting is categorized as nosocomial SAB (NA-SAB), healthcare-associated SAB (HCA-SAB) and community-acquired SAB (CA-SAB). For the period 1998-2005, the age- and gender-adjusted incidence of NA-SAB, HCA-SAB, and CA-SAB were 9.0 (95% CI: 6.7 – 11.3), 22.6 (95% CI: 18.9 – 26.3), and 6.6/100,000 (95% CI: 4.6 – 8.5) person-years, respectively. For each of the settings of infection acquisition, there was not a statistically significant linear increase in the incidence rate over the 8-year study period (NA-SAB, p=0.28; HCA-SAB, p=0.08; CA-SAB, p=0.41; adjusting for age and gender) (Figure 3). The incidence was generally higher in males versus females across the study period for each of the settings of acquisition. The median age was higher in HCA-SAB and NA-SAB than in CA-SAB (74.9 and 71.0 vs. 59.7 years, respectively). Incidence generally increased with older age for each setting.
Table 3. Incidence rates of SAB in Olmsted County, MN, by infection site of acquisition.
| 1998-1999 No (rate/105) |
2000-2001 No (rate/105) |
2002-2003 No (rate/105) |
2004-2005 No (rate/105) |
All years No. (rate/105) |
|
|---|---|---|---|---|---|
| NA-SAB | |||||
| Total | 12 (6.8) | 10 (5.5) | 19 (10.1) | 17 (8.7) | 58 (7.8) |
| Gender | |||||
| Male | 4 (4.8) | 7 (8.1) | 14 (15.5) | 9 (9.6) | 34 (9.6) |
| Female | 8 (8.7) | 3 (3.2) | 5 (5.1) | 8 (7.9) | 24 (6.2) |
| Age | |||||
| 18-39 | 0 (0.0) | 2 (2.5) | 3 (3.7) | 1 (1.2) | 6 (1.9) |
| 40-59 | 2 (3.2) | 1 (1.5) | 4 (5.8) | 5 (7.0) | 12 (4.4) |
| 60-79 | 7 (26.0) | 5 (17.8) | 8 (27.5) | 8 (26.6) | 28 (24.5) |
| >80 | 3 (39.0) | 2 (24.8) | 4 (47.8) | 3 (34.6) | 12 (36.6) |
| Adjusted a | 8.1 (3.5 - 12.7) | 6.2 (2.3 - 10.1) | 11.4 (6.2 - 16.6) | 10.0 (5.2 - 14.8) | 9.0 (6.7 - 11.3) |
| HCA-SAB | |||||
| Total | 24 (13.7) | 39 (21.5) | 37 (19.7) | 45 (23.1) | 145 (19.6) |
| Gender | |||||
| Male | 16 (19.1) | 19 (21.9) | 18 (20.0) | 25 (26.8) | 78 (22.0) |
| Female | 8 (8.7) | 20 (21.1) | 19 (19.4) | 20 (19.7) | 67 (17.4) |
| Age | |||||
| 18-39 | 0 (0.0) | 4 (5.1) | 3 (3.7) | 0 (0.0) | 7 (2.2) |
| 40-59 | 7 (11.2) | 7 (10.5) | 7 (10.1) | 8 (11.2) | 29 (10.7) |
| 60-79 | 10 (37.2) | 15 (53.5) | 16 (55.0) | 20 (66.4) | 61 (53.5) |
| >80 | 7 (91.1) | 13 (161.2) | 11 (131.5) | 17 (196.1) | 48 (146.4) |
| Adjusted a | 16.0 (9.6 - 22.4) | 24.6 (16.8 -32.4) | 22.4 (15.2 -29.7) | 26.9 (19.0 -34.8) | 22.6 (18.9 -26.3) |
| CA-SAB | |||||
| Total | 10 (5.7) | 14 (7.7) | 12 (6.4) | 8 (4.1) | 44 (5.9) |
| Gender | |||||
| Male | 5 (6.0) | 10 (11.5) | 9 (10.0) | 7 (7.5) | 31 (8.8) |
| Female | 5 (5.5) | 4 (4.2) | 3 (3.1) | 1 (1.0) | 13 (3.4) |
| Age | |||||
| 18-39 | 2 (2.6) | 0 (0.0) | 1 (1.2) | 1 (1.2) | 4 (1.2) |
| 40-59 | 4 (6.4) | 7 (10.5) | 3 (4.3) | 4 (5.6) | 18 (6.7) |
| 60-79 | 3 (11.1) | 3 (10.7) | 7 (24.1) | 2 (6.6) | 15 (13.1) |
| >80 | 1 (13.0) | 4 (49.6) | 1 (12.0) | 1 (11.5) | 7 (21.3) |
| Adjusted a | 6.1 (2.3 - 9.9) | 8.6 (4.0 - 13.1) | 7.3 (3.2 - 11.5) | 4.4 (1.3 - 7.5) | 6.6 (4.6 - 8.5) |
SAB: S. aureus bacteremia. NA-SAB: nosocomial SAB. HCA-SAB: healthcare-associated SAB. CA-SAB: community-acquired SAB.
Rates were directly adjusted for age and gender to 2000 US adult white population (with 95% CI).
Figure 3. Adjusted* incidence of SAB in Olmsted County, MN, 1998-2005 by setting.
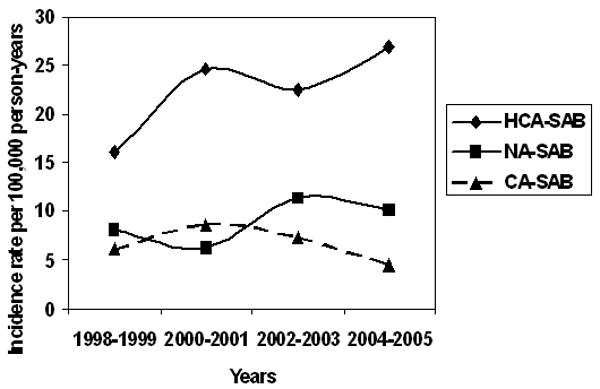
* Age- and gender-adjusted to US 2000 white adult population.
SAB: S. aureus bacteremia.
HCA-SAB Healthcare-associated S. aureus bacteremia.
NA-SAB: Nosocomial S. aureus bacteremia.
CA-SAB: Community-acquired S. aureus bacteremia.
The age- and gender-adjusted incidence rates by type of bacteremia are shown in Table 4; type of bacteremia was classified as MRSA-B or MSSA-B. The age- and gender-adjusted incidence of MRSA-B and MSSA-B were 12.4 (95% CI: 9.7 – 15.2) and 25.4 (95% CI: 21.5 – 29.3) per 100,000 person-years, respectively. There was a significant increasing linear trend in incidence of MRSA-B with calendar year across the study period (Figure 4) (p<0.01; adjusting for age and gender) largely explained by an increasing trend in healthcare-associated MRSA-B from 3.5 (95 % CI: 0.4 – 6.5) to 14 (95% CI: 8.4 – 19.6) per 100,000 person-years (p=0.002; adjusting for age and gender). Adjusted-incidence of nosocomial MRSA-B was 3.0 (95% CI: 1.6 – 4.3) per 100,000 person-years. The numbers were too small to conduct a trend analysis of nosocomial MRSA-B. After adjustment for age and gender, the incidence of MSSA-B did not significantly change over the 8-year study period (Figure 4)(p=0.50). For MSSA and MRSA, incidence was generally higher with increasing age and for males. Median age was slightly higher for MRSA-B than for MSSA-B (70.4 vs. 65.7 years). MRSA-B represented 59/145 (40.7%) of HCA-SAB and 19/58 (32.8%) of NA-SAB. No cases of community-acquired MRSA-B occurred during the study period.
Table 4. Incidence rates of SAB in Olmsted County, MN; MRSA and MSSA.
| 1998-1999 No (rate/105) |
2000-2001 No (rate/105) |
2002-2003 No (rate/105) |
2004-2005 No (rate/105) |
All years No (rate/105) |
|
|---|---|---|---|---|---|
| MRSA-B | |||||
| Total | 8 (4.6) | 17 (9.4) | 24 (12.7) | 31 (15.9) | 80 (10.8) |
| Gender | |||||
| Male | 6 (7.2) | 8 (9.2) | 15 (16.6) | 16 (17.1) | 45 (12.7) |
| Female | 2 (2.2) | 9 (9.5) | 9 (9.2) | 15 (14.8) | 35 (9.1) |
| Age | |||||
| 18-39 | 0 (0.0) | 1 (1.3) | 2 (2.4) | 0 (0.0) | 3 (0.9) |
| 40-59 | 1 (1.6) | 3 (4.5) | 5 (7.2) | 9 (12.6) | 18 (6.7) |
| 60-79 | 3 (11.1) | 8 (28.6) | 10 (34.4) | 13 (43.2) | 34 (29.8) |
| >80 | 4 (52.0) | 5 (62.0) | 7 (83.7) | 9 (103.8) | 25 (76.2) |
| Adjusteda | 5.5 (1.7 - 9.3) | 10.7 (5.6 - 15.9) | 14.5 (8.7 - 20.3) | 18.2 (11.7 - 24.6) | 12.4 (9.7 - 15.2) |
| MSSA-B | |||||
| Total | 38 (21.7) | 45 (24.8) | 45 (23.9) | 37 (19.0) | 165 (22.3) |
| Gender | |||||
| Male | 19 (22.7) | 28 (32.2) | 27 (30.0) | 24 (25.7) | 98 (27.7) |
| Female | 19 (20.8) | 17 (18.0) | 18 (18.3) | 13 (12.8) | 67 (17.4) |
| Age | |||||
| 18-39 | 2 (2.6) | 4 (5.1) | 6 (7.3) | 2 (2.4) | 14 (4.3) |
| 40-59 | 12 (19.1) | 12 (18.0) | 10 (14.5) | 8 (11.2) | 42 (15.6) |
| 60-79 | 17 (63.2) | 15 (53.5) | 20 (68.8) | 16 (53.1) | 68 (59.6) |
| >80 | 7 (91.1) | 14 (173.6) | 9 (107.6) | 11 (126.9) | 41 (125.0) |
| Adjusteda | 24.7 (16.8 -32.6) | 28.1 (19.8 - 36.4) | 27.1 (19.1 - 35.0) | 21.9 (14.8 - 28.9) | 25.4 (21.5 - 29.3) |
SAB: S. aureus bacteremia. MRSA-B: Methicillin-resitant S. aureus bacteremia. MSSA-B: Methicillin-susceptible S. aureus bacteremia.
Rates were directly adjusted for age and gender to 2000 US adult white population (with 95% CI).
Note that 2 subjects had both MRSA-B and MSSA events during the study period; for both subjects, each episode was considered an incidence event in the 2 respective bacteremia analyses.
Figure 4. Adjusted* incidence of MRSA-B and MSSA-B in Olmsted County, MN, 1998-2005.
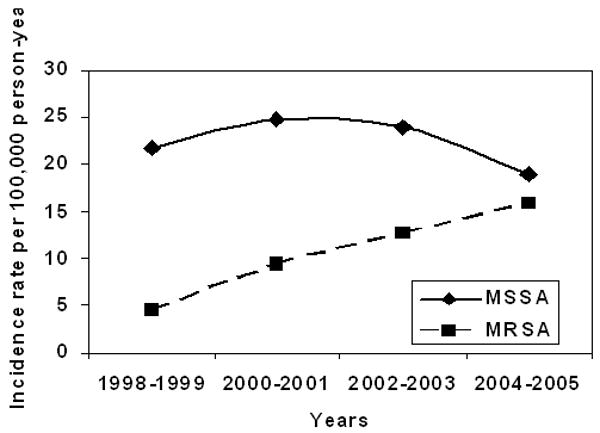
* Age and gender- adjusted to US 2000 white adult population.
MSSA-B: Methicillin-susceptible S. aureus bacteremia.
MRSA-B: Methicillin-resistant S. aureus bacteremia.
Discussion
We present a population-based investigation in the US that describes trends in the incidence of SAB over a contemporary 8-year period. The age- and gender-adjusted incidence of overall SAB in adults of Olmsted County MN was 38.2/100,000 person-years. The incidence of SAB remained stable from 1998-2005. Rates for nosocomial, healthcare-associated and community-acquired SAB did not change significantly over the study period but there was a trend of increasing healthcare-associated SAB rate, overall, and decreasing community-acquired SAB among females only.
It is difficult to compare the incidence in our survey to that of community-onset SAB (CO-SAB) in Connecticut because of differences in methodology employed. Morin et al [14] reported the incidence of CO-SAB in 4 metropolitan areas in Connecticut in 1998 was 17/100,000 person-years. Community-onset cases represented 48% of total SAB in that study. Long-term care facilities (LTCF) were considered hospitals in the Morin study, thus, 55 cases (13.7%) of SAB in LTCF were excluded from incidence calculation of CO-SAB.
Population-based incidence data on SAB in the literature are summarized in Table 5. Rates of SAB increased in Finland from 1995 to 2001 [10] from 11 to 17/100,000 person-years, but MRSA constituted <1% of the cases. In Denmark, SAB rates have increased from 1981 to 2000 [22] but has stabilized from 2002 to 2006 with a slight increase in MRSA rate from <1% to 1.5% [6]. Denmark is well-recognized for its aggressive control measures to detect and contain cases of MRSA and has successfully decreased the percentage of MRSA-B cases from 10-30% of SAB in the late 1960s to <1% in the 1980s [23]. The incidence of SAB did not change significantly from 2000 to 2006 in Canada, but rates of MRSA bacteremia significantly increased and were attributed to an increase in nosocomial and healthcare-associated SAB [12]. In England, in the first 3 years of compulsory reporting (2001-2004) of SAB cases, there was an increase in total SAB but the 4th year (2004-2005) showed a 4.5% decrease. MRSA rates remained high at 38.9% [24]. The incidence of SAB in Olmsted County is one of the highest reported in the literature; therefore, prevention measures, in particular vaccine development, are critical and deserve further investigation [25, 26].
Table 5. Incidence rates of SAB reported in the literature.
| Country | Year | Population | SAB/105/y | % MRSA |
|---|---|---|---|---|
| Australia [6]a | 1998-2002 | 19,500,000 | 35.0 | 27 |
| Australia – Victoria [17]b | 1990-1999 | 4,502,000 | 23.0 | 28 |
| Canada – Calgary [9] | 1999-2000 | 1,200,000 | 17.9 | 3 |
| Canada – Calgary [10]c | 2000-2006 | 1,200,000 | 19.7 | 11 |
| Denmark – Northern Jutland [18] | 1996-1998 | 493,000 | 31.0 | ND |
| Denmark [4]d | 2006 | 5,164,678 | 25.6 | 1.4 |
| Finland [8] | 1995-2001 | 5,200,000 | 14.0 | <1 |
| Republic of Ireland [19]e | 1998 | 3,700,000 | 24.5 | 31 |
| New Zealand [7] | 1996-1997 | 1,400,000 | 41.0 | 5 |
| New Zealand [20] | 1998-2006 | 478,000 | 21.5 | 0.4 |
| Sweden – Skaraborg [11] | 2003-2005 | 255,109 | 27.6 | ND |
| UK – England [5]f | 2002 | 49,200,000 | 36.8 | 39 |
| UK – Northern Ireland [5]g | 2002 | 1,697,000 | 23.4 | 38 |
| UK – Wales [5]g | 2002 | 2,920,000 | 21.9 | 47 |
| US – Connecticut [12]h | 1998 | 1,124,337 | 35.4 | ND |
| US – Olmsted County, MN [13] | 2003-2005 | 124,277 | 32.0 | ND |
| US – Olmsted County, MN i | 1998-2005 | 124,277 | 38.2 | 32 |
SAB: S. aureus bacteremia.
SAB rates in 17 hospitals were used to estimate incidence for Australia.
Annual incidence of MSSA was 17.1/100,000 and for MRSA was 4.8/100,000. Methicillin susceptibility was not stated for 1% of SAB cases.
Annual incidence for MSSA was 17.5/100,000 and for MRSA 2.2/100,000.
Surveillance system in Denmark since 1957.
Retrospective data by questionnaire sent to microbiology laboratories with 90% of them in Republic of Ireland participating.
Compulsory reporting system for SAB started in England in April 2001.
Voluntary reporting system used and likely underreporting.
Incidence for community-onset SAB was 17/100,000. This constituted 48% of total SAB. MRSA was 15% of community-onset cases.
Current study. Incidence age- and gender-adjusted for US 2000 white adult population.
MRSA-B significantly increased at a rate of 19.8% (± 5.5%) per year in our study. Age- and gender-adjusted incidence of MRSA-B increased from 5.5 to 18.2/100,000 person-years over the study period mainly due to increasing healthcare-associated SAB and is not explained by community-acquired MRSA-B, as this was not seen. Klevens [27] reported an incidence of invasive MRSA infections of 31.8/100,000 person-years in 9 CDC Emerging Infections Program metropolitan sites in 2005. Bacteremias constituted 75.2% of invasive cases in that study. Metropolitan areas have a higher representation of blacks - who have a higher incidence of MRSA-B than whites [27] - and have a higher prevalence of injection drug users than in Olmsted County. These differences probably account, in part, for the lower MRSA-B rates observed in our population.
Similar to findings by Laupland et al (12) in Calgary, Canada, the increasing rate of MRSA-B demonstrated in our investigation has been offset by a decreasing rate of MSSA-B in the final two years of the study period. Subsequent population-based surveillance in Olmsted County will determine if the overall burden of SAB will increase with the emergence of MRSA. In a study by Burton DC et al [28], although the overall proportion of S. aureus central line-associated blood stream infections (BSI) due to MRSA increased 25.8% from 1997 to 2007 in US intensive care units reporting data to the CDC, the overall MRSA central line-associated BSI incidence decreased over the same period and reflected a decrease in S. aureus central line-associated BSI.
Our study has several limitations. Our calculated incidence of SAB in Olmsted County is probably underestimated because of the exclusion of 5.6% with SAB who refused review of their medical records. Furthermore, Olmsted County residents who had SAB while outside the county, or patients with SAB who did not have blood cultures done, or received appropriate antibiotics before blood cultures were obtained, could have been missed. These, however, are not likely to constitute a large proportion of SAB cases. Prior studies have correlated an increase in incidence of SAB to an increase in the annual number of blood culture sets processed [10]. We did not obtain data on the number of blood cultures processed in Olmsted County during the study period, but our SAB incidence was stable over the 8-year period. We did not perform pulsed-field gel electrophoresis (PFGE) typing of the MRSA-B isolates as they were not available, however it would be interesting to know how many of the nosocomial or healthcare-associated MRSA-B are due to the USA300 type. Active sentinel site surveillance at 12 hospital laboratories in Minnesota from 2000 to 2005 showed that USA300 replaced USA400 as the predominant type of CA-MRSA [29]. Olmsted County Medical Center laboratory was one of the 12 sentinel sites. Last, the applicability of our study findings is limited to white non-Hispanic midwestern adult US population given the limited diversity in racial and ethnic backgrounds of Olmsted County population [15]. Low dialysis rates in our cohort and possibly higher socioeconomic status could not be adjusted for. This study's methodology is strong due to the use of the Rochester Epidemiology Project tools and resources for case ascertainment and data collection on a population level. Population-based studies are optimal for incidence calculations because of limited selection (referral) bias [30].
In summary, the incidence of SAB has not significantly changed in Olmsted County between 1998 and 2005. In contrast, the proportion due to MRSA has significantly increased over the 8-year period. MRSA-B occurred exclusively in the hospital and healthcare-associated setting.
Acknowledgments
The authors thank Dr Imad M. Tleyjeh for his input in the initial design of the study.
This publication was made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR) of the NIH.
The authors also received funding from the Baddour Family Fund and the Department of Medicine, College of Medicine, Mayo Clinic, Rochester, MN.
Footnotes
The authors report no potential conflicts of interest.
References
- 1.Petti CA, Fowler VG., Jr Staphylococcus aureus bacteremia and endocarditis. Infect Dis Clin N Am. 2002;16:413–435. doi: 10.1016/s0891-5520(01)00003-4. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee SN, Emori TG, Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. Am J Med. 1991;91(suppl 3B):86S–9S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 3.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–9. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 5.Crane SJ, Uslan DZ, Baddour LM. Bloodstream infections in a geriatric cohort: a population-based study. Am J Med. 2007;120:1078–1083. doi: 10.1016/j.amjmed.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 6.Danish Staphylococcus aureus bacteremia group. Annual report on Staphylococcus aureus bacteraemia cases 2006. Statens Serum Institut.; Copenhagen: [May 10, 2009]. Available from http://www.ssi.dk/sw3425.asp. [Google Scholar]
- 7.Staphylococcus aureus bacteraemia: England, Wales, and Northern Ireland, January to December 2002. [May 10, 2009];CDR Wkly. 2003 March 20;13 Available from http://www.hpa.org.uk/cdr/archives/2003/cdr1203.pdf.
- 8.Collingnon P, Nimmo GR, Gottlieb T, Gosbell IB, Australian Group on Antimicrobial Resistance Staphylococcus aureus bacteremia, Australia. Emer Infect Dis. 2005;11:554–61. doi: 10.3201/eid1104.040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J. 2001;31:97–103. [PubMed] [Google Scholar]
- 10.Lyytikäinen O, Ruotsalainen E, Järvinen A, Valtonen V, Ruutu P. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995-2001. Eur J Clin Microbiol Infect Dis. 2005;24:399–404. doi: 10.1007/s10096-005-1345-3. [DOI] [PubMed] [Google Scholar]
- 11.Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and risk factors for invasive Staphylococcus aureus infections. J Infect Dis. 2003;18:1452–9. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- 12.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J Infect Dis. 2008;198:336–43. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsson G, Dashti S, Wahlberg T, Andersson R. The epidemiology of and risk factors for invasive Staphylococcus aureus infections in western Sweden. Scand J Infect Dis. 2007;39:6–13. doi: 10.1080/00365540600810026. [DOI] [PubMed] [Google Scholar]
- 14.Morin CA, Hadler JL. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J Infect Dis. 2001;184:1029–34. doi: 10.1086/323459. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology. 1999;52:1214–20. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 17.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 18.Veitch M. Staphylococcus aureus bacteraemia in Victoria, 1990-1999. Communicable Diseases Network of Australia Conference; Canberra, Australia. 2001. [May 10, 2009]. p. 18. abstract 23. Available from http://www.health.gov.au/internet/main/Publishing.nsf/Content/cda-cdna-cdconf.htm/$FILE/cdc01abs.pdf. [Google Scholar]
- 19.Schønheyder HC. Two thousand seven hundred and thirty nine episodes of bacteremia in the county of Northern Jutland 1996-1998. Presentation of a regional clinical database. Ugeskr Laeger. 2000;162:2886–91. [PubMed] [Google Scholar]
- 20.Mc Donald P, Mitchell E, Johnson H, et al. MRSA bacteremia: north/south study of MRSA in Ireland 1999. J Hosp Infect. 2002;52:288–291. doi: 10.1053/jhin.2002.1274. [DOI] [PubMed] [Google Scholar]
- 21.Huggan PJ, Wells JE, Browne M, et al. Population-based epidemiology of Staphylococcus aureus bloodstream infection in Canterbury, New Zealand. Intern Med J. 2009 doi: 10.1111/j.1445-5994.2009.01910.x. Accepted Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Benfield T, Espersen F, Frimodt-Møller N, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–63. doi: 10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 23.Phagetypes and resistance-patterns in Staphylococcus aureus bacteraemia cases compared with all reported Staphylococcus aureus cases. Staphylococcus Laboratory, Statens Serum Institut; 1999. [May 10, 2009]. Available from http://www.ssi.dk/graphics/en/surveillance/Aar99-comparison.pdf. [Google Scholar]
- 24.The fourth year of regional and national analyses of the Department of Health's mandatory Staphylococcus aureus surveillance scheme in England: April 2001 – March 2005. [May 10, 2009];CDR Wkly. 2005 June 23;15 Available from http://www.hpa.org.uk/cdr/archives/2005/cdr2505.pdf.
- 25.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Eng J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 26.Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am. 2009;23:153–71. doi: 10.1016/j.idc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 28.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units,1997-2007. JAMA. 2009;301:727–36. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 29.Como-Sabetti K, Harriman KH, Buck JM, Glennen A, Boxrud DJ, Lynfield R. Community-associated methicillin-resistant Staphylococcus aureus: trends in case and isolate characteristics from six years of prospective surveillance. Public Health Rep. 2009;124:427–35. doi: 10.1177/003335490912400312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steckelberg JM, Melton LJ, 3rd, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88:582–8. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]


