Abstract
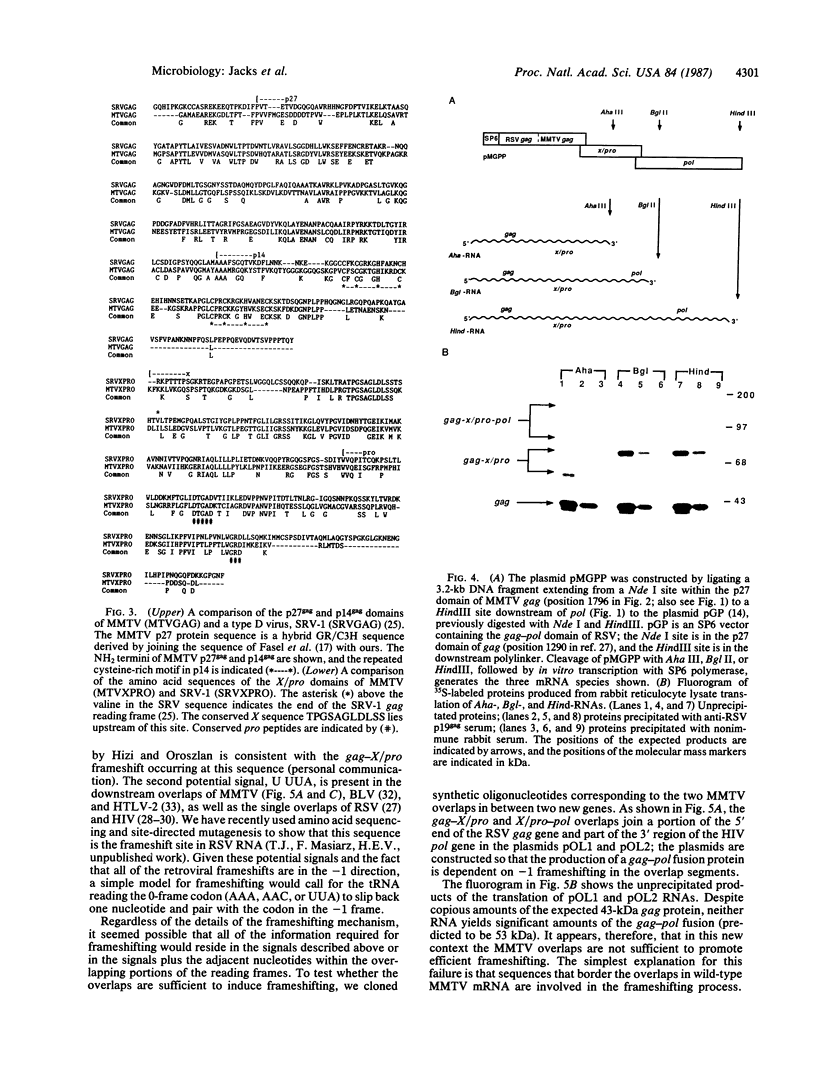
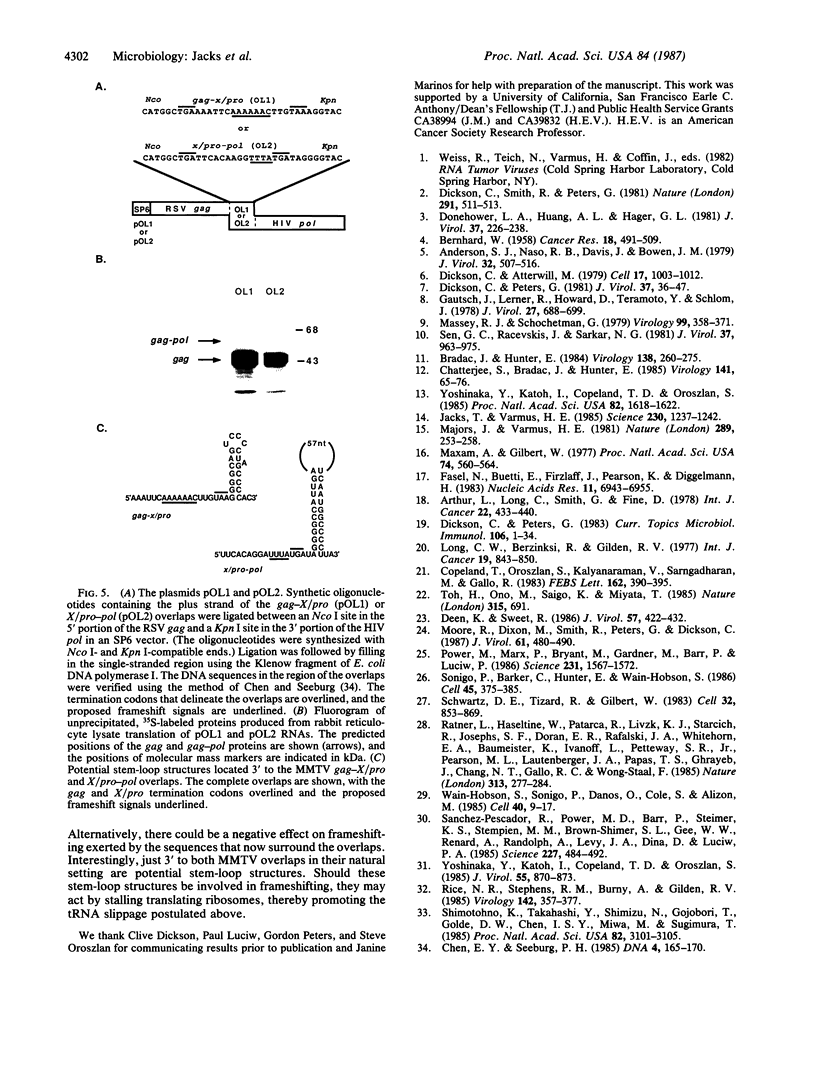
The primary translation products of retroviral pol genes are polyproteins initiated in an upstream gene (gag). To investigate the manner in which the gag-initiated polyproteins of the mouse mammary tumor virus are produced, we determined the nucleotide sequence of a 1.8-kilobase DNA fragment that spans the region between gag and pol in the C3H strain of mouse mammary tumor virus. The sequence reveals three overlapping open reading frames: the first encodes products of gag (p27gag and p14gag); the second encodes a protein domain of unknown function (termed X) that is highly related to a similarly positioned sequence in simian type D retroviruses and the viral protease (pro); and the third encodes the reverse transcriptase. The reading frames are organized to permit uninterrupted readthrough from gag to pol if ribosomal frameshifts occur in the -1 direction within each of the two overlapping regions, one of which is 16 nucleotides in length and the other 13 nucleotides. Cell-free translation of RNA containing these overlap regions shows that fusion of the reading frames by ribosomal frameshifting occurs efficiently: about one-fourth of the ribosomes traversing the gag-X/pro overlap and one-tenth traversing the X/pro-pol overlap shift frames, generating gag-related polyproteins in ratios similar to those observed in vivo. Synthetic oligonucleotides containing either of the overlap regions inserted into novel contexts do not induce frameshifting; hence the overlapping portions of the reading frames are not sufficient to induce a frameshift event, and a larger sequence context or secondary structure may be implicated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Naso R. B., Davis J., Bowen J. M. Polyprotein precursors to mouse mammary tumor virus proteins. J Virol. 1979 Nov;32(2):507–516. doi: 10.1128/jvi.32.2.507-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur L. O., Long C. W., Smith G. H., Fine D. L. Immunological characterization of the low-molecular-weight DNA binding protein of mouse mammary tumor virus. Int J Cancer. 1978 Oct 15;22(4):433–440. doi: 10.1002/ijc.2910220411. [DOI] [PubMed] [Google Scholar]
- BERNHARD W. Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 1958 Jun;18(5):491–509. [PubMed] [Google Scholar]
- Bradac J., Hunter E. Polypeptides of Mason-Pfizer monkey virus. I. Synthesis and processing of the gag-gene products. Virology. 1984 Oct 30;138(2):260–275. doi: 10.1016/0042-6822(84)90350-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Bradac J., Hunter E. A rapid screening procedure for the isolation of nonconditional replication mutants of Mason-Pfizer monkey virus: identification of a mutant defective in pol. Virology. 1985 Feb;141(1):65–76. doi: 10.1016/0042-6822(85)90183-7. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Oroszlan S., Kalyanaraman V. S., Sarngadharan M. G., Gallo R. C. Complete amino acid sequence of human T-cell leukemia virus structural protein p15. FEBS Lett. 1983 Oct 17;162(2):390–395. doi: 10.1016/0014-5793(83)80793-5. [DOI] [PubMed] [Google Scholar]
- Deen K. C., Sweet R. W. Murine mammary tumor virus pol-related sequences in human DNA: characterization and sequence comparison with the complete murine mammary tumor virus pol gene. J Virol. 1986 Feb;57(2):422–432. doi: 10.1128/jvi.57.2.422-432.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Composition, arrangement and cleavage of the mouse mammary tumor virus polyprotein precursor Pr77gag and p110gag. Cell. 1979 Aug;17(4):1003–1012. doi: 10.1016/0092-8674(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Protein-coding potential of mouse mammary tumor virus genome RNA as examined by in vitro translation. J Virol. 1981 Jan;37(1):36–47. doi: 10.1128/jvi.37.1.36-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Proteins encoded by mouse mammary tumour virus. Curr Top Microbiol Immunol. 1983;106:1–34. doi: 10.1007/978-3-642-69357-1_1. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Peters G. In vitro synthesis of polypeptides encoded by the long terminal repeat region of mouse mammary tumour virus DNA. Nature. 1981 Jun 11;291(5815):511–513. doi: 10.1038/291511a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Buetti E., Firzlaff J., Pearson K., Diggelmann H. Nucleotide sequence of the 5' noncoding region and part of the gag gene of mouse mammary tumor virus; identification of the 5' splicing site for subgenomic mRNAs. Nucleic Acids Res. 1983 Oct 25;11(20):6943–6955. doi: 10.1093/nar/11.20.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Lerner R., Howard D., Teramoto Y. A., Schlom J. Strain-specific markers for the major structural proteins of highly oncogenic murine mammary tumor viruses by tryptic peptide analyses. J Virol. 1978 Sep;27(3):688–699. doi: 10.1128/jvi.27.3.688-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Varmus H. E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985 Dec 13;230(4731):1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Long C. W., Berzinski T. R., Gilden R. V. Immunologic studies of the low molecular weight DNA binding protein of murine oncornaviruses. Int J Cancer. 1977 Jun 15;19(6):843–850. doi: 10.1002/ijc.2910190616. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Gene order of mouse mammary tumor virus precusor polyproteins and their interaction leading to the formation of a virus. Virology. 1979 Dec;99(2):358–371. doi: 10.1016/0042-6822(79)90015-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Stephens R. M., Burny A., Gilden R. V. The gag and pol genes of bovine leukemia virus: nucleotide sequence and analysis. Virology. 1985 Apr 30;142(2):357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Racevskis J., Sarkar N. H. Synthesis of murine mammary tumor viral proteins in vitro. J Virol. 1981 Mar;37(3):963–975. doi: 10.1128/jvi.37.3.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D. W., Chen I. S., Miwa M., Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Translational readthrough of an amber termination codon during synthesis of feline leukemia virus protease. J Virol. 1985 Sep;55(3):870–873. doi: 10.1128/jvi.55.3.870-873.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]