Preface
It is widely accepted that the development of carcinomas, the most common type of human cancer, is due to accumulation of somatic mutations in epithelial cells. The behavior of carcinomas is also influenced by the tumour microenvironment that includes extracellular matrix, blood vasculature, inflammatory cells and fibroblasts. Recent studies reveal that fibroblasts have a more profound influence on the development and progression of carcinomas than previously appreciated. These new findings also have therapeutic implications.
Work over the past two decades have delineated the genetic lesions that occur in epithelial cells leading to the initiation and progression of carcinomas, the most common form of human cancer1. The discoveries of genetic changes in somatic cancer cells have not only advanced our basic understanding of tumour formation but also significantly influenced the treatment of cancer with the use of new therapies targeted to specific pathways affected by genetic lesions (see Overview).
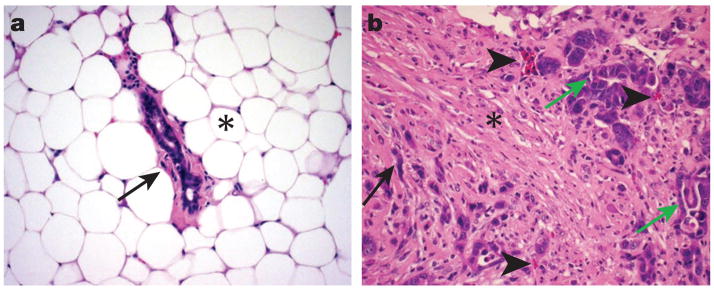
Carcinoma cells, like normal epithelial cells, live in a complex microenvironment that includes the extracellular matrix (ECM), diffusible growth factors and cytokines, and a variety of non-epithelial cell types, including those comprising the vasculature (endothelial cells, pericytes, smooth muscle cells), those that can respond to infection and injury (lymphocytes, macrophages, mast cells), and fibroblasts. It has long been recognized that carcinomas induce a modified stroma through expression of growth factors that promotes angiogenesis, altered ECM expression, accelerated fibroblast proliferation, and increased inflammatory cell recruitment2,3 (Figure 1).
Figure 1.
The stroma associated with normal mammary gland differs profoundly from stroma associated with a mammary carcinoma. (A) Note that the normal mammary gland has sparse connective tissue (arrow) surrounding the duct and abundant adipose tissue (*). (B) The carcinoma contains abundant connective likely as a result of growth factor production by the carcinogenic environment. Note the dense collagen bundles associated with fibroblasts (*) and the numerous small blood vessels and capillaries (arrow heads). The carcinoma cells form aberrant gland structures (green arrows) or grow in cords without gland formation (black arrow).
Blood vessels are a critical component of the tumour microenvironment. Without formation of new blood vessels, carcinomas cannot grow beyond a very small size or metastasize and reform in distant organs4. Tumour angiogenesis is due in part to secretion of endothelial growth factors by tumours, and indeed, a targeted therapy (Avastin) that blocks the action of one of these factors (VEGF) has recently been approved (see also Overview)5.
There is also a functional relationship between inflammation and cancer9. Cancers frequently arise in areas of chronic inflammation [see also review article by Beachy]. Examples include colon carcinoma associated with inflammatory bowel disease, stomach cancer in H. pylori infection, and hepatocellular carcinomas in hepatitis C infection. Inflammatory cells are also a key component of the microenvironment of carcinomas arising independent of chronic inflammation. Mechanisms whereby inflammatory cells influence cancer initiation and promotion likely involve secretion of cytokines, growth factors and chemokines by inflammatory cells that stimulate proliferation of epithelia as well as the generation of reactive oxygen species that can cause DNA damage9. [BM: Lucy, I swapped these two paragraphs around, thus references need to be renumbered]
The three-dimensional structure supporting epithelia through the ECM is critically important, and impaired interactions of epithelial cells with ECM can result in transformation of the epithelia6,7. The specialized ECM that separates the epithelial and endothelial cells from the stromal components is termed the basement membrane. Whereas stromal ECM proteins are produced by fibroblasts, the major structural proteins of the basement membrane including collagen VI, laminin, entactin, and heparan-sulphate proteoglycans are expressed by basal epithelia, myoepithelia, and fibroblasts in a tissue specific manner8. In turn, the unique composition of the basement membrane is thought to confer tissue specificity, epithelial polarity and functionality8.
Fibroblasts also play a well-recognized role in the carcinogenic process. They are responsible for synthesis, deposition and remodeling of much of the ECM in tumour stroma (Figure 1), and they are recognized as a source of paracrine growth factors that influence the growth of carcinoma cells. However, fibroblasts have largely been assumed to play a more passive role in cancer, responding to signals from the carcinoma cells. New data promote stromal fibroblasts from the mere role of “enablers” of cancer to the potential stature of “inducers” of certain carcinomas. In this review, we will use selected examples to illustrate the influence of the stromal fibroblasts in epithelial neoplasia.
Overview of stromal-epithelial interactions
The importance of stromal (or mesenchymal) - epithelial interactions in embryonic development and tumourigenesis is well established. The concept of a link between stromal cell maturation and adjacent epithelial proliferation was introduced over twenty years ago10, a view that has been supported by others11–16. This interaction is mediated by soluble paracrine signals and secreted ECM from developing mesenchyme that induce the adjacent epithelia to rapidly proliferate. As the epithelial cells differentiate, adjacent mesenchymal cells develop into differentiated stromal cells. These differentiated stromal cells generally express lower quantities of growth factors, and differentiated epithelia express cytokines for the maintenance of stromal differentiation, suggesting that a new balance of mesechymal-epithelial crosstalk is reached during tissue maturation. However, during tumourigenesis the prevailing model suggestsa process whereby pre-cancerous epithelial cells acquire multiple genetic mutations17 and the associated stroma becomes “activated”, commonly expressing myofibroblastic markers2,3. The characteristics of an activated carcinoma-associated fibroblast are not completely understood. However in our interpretation such cells express α-smooth muscle actin, ECM proteins, and growth factors that act in an autocrine and paracrine fashion to potentiate and support the survival of a tumour.
Emerging role of fibroblasts in epithelial cancer
Early evidence indicating an important role for stromal cells in cancer come from tissue culture experiments where epithelium from submandibular glands transformed by polyoma virus grow only in the presence of embryonic salivary gland mesenchyme, but not when the epithelium is cultured by itself29. The differences in structure of normal stroma and tumour stroma are well known2,3 (Figure 1) 3. Apart from histologic differences, tumour fibroblasts exhibit enhanced proliferation and migratory behavior in vitro30,31. The constituents of the extracellular matrix and the vascular architecture in tumour stroma also differ from that associated with normal epithelia4. These stromal events are in part mediated through autocrine growth factor singalling involving the factors discussed above32. In vitro co-culture and in vivo tissue recombination using xenograft systems demonstrate that tumour fibroblast-derived factors contribute to the transformation of immortalized epithelia33,34. In these studies, human fibroblasts from normal or tumour prostate were grown together with epithelial cells derived from SV40-T antigen immortalized benign prostate hyperplasia. Tumour fibroblasts, but not normal prostatic fibroblasts, stimulated epithelial proliferation and malignant transformation. However, tumour fibroblasts do not stimulate the growth of normal primary epithelial cells under identical conditions. This lead to the suggestion that cancer-associated fibroblasts express ECM proteins and growth factors that influence the insipient tumours cells and promote angiogenesis that is necessary to maintain epithelial transformation.
Further evidence for a a role of fibroblast in epithelial cancers comes from experiments in which irradiation of fibroblasts to cause sub-lethal DNA damage will induced genetic changes. When non-transformed mammary epithelial cells were transplanted into cleared fat pads of irradiated mice containing such irradiated fibroblasts, an elevated incidence of breast tumours was observed compared to the same epithelial cells inserted into non-irradiated fat pads35. Recently, tissue recombination of pancreatic cancer cells with irradiated pancreatic fibroblasts resulted in a more aggressive and invasive cancer, than when normal fibroblasts were used36. Together, these reports demonstrate that changes in fibroblasts can contribute to epithelial transformation and more invasive behavior. They also suggest that the mechanism of radiation-induced cancer may not only be the result of deleterious mutations in the epithelia, but also from alterations in stromal fibroblasts.
Interestingly, both proliferating fibroblasts and senescent human fibroblasts can promote the proliferation of pre-malignant and malignant epithelial cell proliferation in culture and the formation of tumours in mice37. This is likely due to the fact that senescent fibroblasts and cancer-associated fibroblasts express similar sets of paracrine growth factors that can contribute to cancer proliferation. In support of the role of paracrine growth factors in the senescent fibroblast mediated effects, Krtolica et al.37 found the proliferative effects on epithelial cells were observed even when senescent fibroblasts were only 10% of the fibroblast population. However, unlike the ability of cancer-associated fibroblasts to induce epithelial oncogenesis33, senescent fibroblasts are not able to convert non-transformed epithelia into carcinomas37.
In a recent study of gene expression profiles of each of the cell types present in normal breast tissue and breast carcinomas, changes in gene expression were found to occur in all cell types38. Of particular interest was the overexpression of the chemokines, CXCL14 and CXCL12, by myoepithelial cells and myofibroblasts, respectively. These two chemokines were then shown to bind to receptors on the epithelial cells enhancing their proliferation and invasion. This study provides an elegant example of paracrine effects of factors derived from stromal cells on carcinoma cells.
Fibroblast-derived growth factors
Multiple families of growth factors implicated as autocrine and paracrine mediators of stromal-epithelial interactions are involved in carcinoma initiation and progression. These include the fibroblast growth factor (FGF) family, the IGF family, the EGF family, hepatocyte growth factor (HGF), and the TGF-β family (Table 1). Most of these factors are predominantly stimulators of proliferation and can play a role in promoting the carcinogenic process. The TGF-β family is different. With initial demonstrations that TGF-β could act as a growth inhibitor of most epithelial cells18,19, it was speculated that this growth factor has a role in tumour suppression. Subsequent studies have indeed supported this hypothesis. For example, transgenic mouse studies demonstrated that increased TGF-β signalling can suppress tumour formation while loss or attenuation of the pathway enhances carcinogenesis20–23. In addition, inactivating mutations have been found in genes encoding components of the TGF-β signaling pathway in human tumours (see ref. 24 for a recent review). However, a loss of TGF-β sensitivity in carcinoma cells is frequently accompanied by increased expression of TGF-β by carcinoma cells. Other sources of TGF-β in the microenvironment include the inflammatory cells and stromal fibroblasts25. Furthermore, elevated levels of plasma TGF-β1 can be detected in patients with cancer and predicts early metastasis26–28. The presence of TGF-β in the tumour microenvironment has been suggested to promote tumour growth by enhancing stromal support and angiogenesis and by impairment of immune surveillance. In addition, while mutations in the TGF-β signaling pathway downstream of receptors may impair TGF-β-mediated growth inhibition, other pathway components may be retained, permitting TGF-β-mediated loss of adherens junctions, and increased motility, changes that would favour invasion and metastasis24. Thus TGF-β can exert both tumour suppressive and promoting functions.
Table 1.
The fibroblast derived soluble factors regulate epithelial growth, differentiation and apoptosis32,71–73.
| Soluble factors | Cells Expressed | Responding cells | Possible role |
|---|---|---|---|
| HGF and MSP | Fibroblasts | Epithelia | + Proliferation |
| + Transformation | |||
| + Morphogenic | |||
| IGF-1, IGF-2 | Fibroblast | Epithelia (breast) | − Apoptosis |
| + Proliferation | |||
| EGF and TGF-α | Epithelia and fibroblasts | Epithelia | + Proliferation |
| + Morphogenic | |||
| TGF-β1, TGF-β2, TGF-β3 | Epithelia and fibroblasts | Epithelia and fibroblasts | − Proliferation |
| +/− Apoptosis | |||
| + Morphogenic | |||
| FGF7/KGF | Fibroblast | Epithelia | + Proliferation |
| + Morphogenic | |||
| IL6, LIF, and oncostatin M | Fibroblast | Epithelia (colonic) | + Proliferation |
| + Transformation | |||
| FGF2 | Fibroblast | Epithelia | + Proliferation |
| + Transformation | |||
| FGF10 | Fibroblast | Epithelia | + Proliferation |
| NGF | Fibroblast | Epithelia | + Transformation |
| Stromal cell-derived factor 1alpha(CXCL12) | Fibroblast | Epithelia (glioblastoma) | + Proliferation |
| + Transformation | |||
| Wnt1, Wnt3 | Fibroblast | Epithelia | + Proliferation |
| + Transformation | |||
| MMP-1, MMP-7 | Fibroblast | through ECM and growth factor activation in the stroma affect epithelia | +/− Proliferation |
| +/− Apoptosis | |||
| + Morphogenic | |||
The abbreviations are HGF, hepatocyte growth factor; MSP, macrophage stimulating factor; IGF, insulin growth factor; EGF, epidermal growth factor; TGF-β, transforming growth factor; FGF, fibroblast growth factor; KGF, keratinocyte growth factor; IL6, interleukin 6, LIF, leukemia inhibitory factor; NGF, nerve growth factor.
Genetic modification of fibroblasts can induce epithelial cancer
Recent studies provide evidence for a major role for TGF-β singalling in fibroblasts in the initiation of carcinomas. In one report, mice were generated in which the the TGF-β type II receptor gene (Tgfbr2) was inactivated specifically in stromal fibroblasts using Cre-lox technology (Tgfbr2fspko mice)42. 100% of the mice exhibited prostatic intraepithelial neoplasia, a presumed forerunner of prostatic carcinoma, as well as invasive squamous cell carcinomas of the forestomach by six weeks of age42. Tgfbr2fspko fibroblasts overexpress HGF, and increases in the activating phosphorylation of the cognate HGF receptor, c-Met, were found in forestomach carcinoma cells. This suggests activation of paracrine hepatocyte growth factor (HGF) signalling as one possible mechanism for stimulation of epithelial proliferation. The Tgfbr2fspKO mouse model demonstrates that the TGF-beta signalling pathway known to suppress cell-cycle progression and tumour formation when acting on epithelial cells can also indirectly inhibit epithelial proliferation when acting in adjacent stromal fibroblasts in vivo. Consequently, loss of this pathway in fibroblasts results in increased epithelial proliferation and potentially may also promote invasive carcinoma in some tissues.
More recently in an orthotopic transplantation model, mammary fibroblasts engineered to ectopically express HGF or TGF-β1 alone or together induced mammary epithelia to develop ductal carcinoma in situ, adenocarcinoma, and poorly differentiated cancer, whereas transplantation of the same epithelial cell population with wild-type fibroblasts did not44. Unique to this study was the transplant of human (rather than mouse) mammary epithelia and fibroblasts into cleared mammary fat pads of immune deficient mice44. This provides another example where modification of stromal fibroblasts can influcence the malignant behavior of adjacent epithelia.
HGF and TGF-β in epithelial and stromal cross talk
The stromally-derived paracrine factors, HGF and TGF-β, have enjoyed the limelight in recent literature on epithelial-stromal crosstalk. Most cell types have the capacity to both express and respond to TGF-β25. in contrast, HGF is primarily expressed by fibroblasts, while the cognate receptor, c-Met, is primarily expressed by epithelia45. There are multiple reports to support the transforming ability of HGF46. As also discussed above, the role of TGF-beta is more complex and involves both tumour-suppressive and promoting roles.
The above mentioned report on tissue recombination of irradiated pancreatic fibroblasts with pancreatic cancer cells describe an elevated metastatic potential of the developing tumours36. This phenomenon was associated with increased c-Met activation in the carcinoma cells and TGF-β1 expression by the irradiated pancreatic fibroblasts. The fact that the authors did not observe a concomitant increase in HGF secretion by the fibroblasts suggests the possibility that the expression of an HGF-related ligand may be involved, such as MSP (macrophage stimulating protein) which can activate a heterodimeric receptor consisting of c-Met and the c-Met related molecule RON. Alternatively, the overexpression of c-Met, a common finding in many cancers, can be associated with ligand independent activation48 or increased sensitivity to physiological levels of HGF (note new reference 48A).
Apparent contradictions in our current understanding of the role of TGF-β in epithelial-stromal interactions have also emerged. How can overexpression of TGF-β with enhanced signalling and loss of TGF-β signalling through knockout of Tgfbr2 both result in enhanced tumourigenesis? The overexpression of TGF-β by fibroblasts in mammary and pancreas transplantation models of cancer formation35,36,44 can affect both epithelial cells and stromal fibroblasts. In contrast, the loss of TGF-β signalling in the Tgfbr2fspKO model affects TGF-β signalling only in fibroblasts. However, the enhanced HGF signalling in the two models developing mammary tumours is likely the result of epithelial responses to paracrine factors (since c-Met receptors are primarily found in epithelial cells48). It is of interest that conditional deletion of Tgfbr2 in the epithelia of the mammary gland and prostate have no detectable phenotypic alterations (unpublished observations).
The Tgfbr2fspko mouse model42 poses other important questions. If TGF-β is stimulatory of an activated stromal environment, how does the loss of TGF-β signalling in stromal fibroblasts still allow for an otherwise activated stromal phenotype? The fact that tumours in the Tgfbr2fspko mice are associated with an activated stroma suggests important roles for factors other than TGF-β in the development of an activated tumour microenvironment. As already discussed, a dual role for TGF-β as a tumour suppressor and promoter when acting on epithelial cells has emerged. However, in light of the above data42,44, the paradigm of the early and late roles of TGF-β singalling on tumourigenesis needs to be adapted to include the influences of both fibroblasts and epithelia in tumour susceptible tissues. In particular, both the direct effects of TGF-β on epithelia (direct via TGF-β receptors) and secondary effects (through the regulation of other growth factors) need to be considered.
While the role of TGF-β in epithelia from various tissues has long been accepted as growth inhibitory and morphogenic through multiple downstream singalling proteins39, the proliferative role of TGF-β in fibroblasts has been less clear as a result of the heterogeneity of fibroblasts (Box 1). Notably NIH3T3 and dermal fibroblasts in culture are growth stimulated when treated with TGF-β59. However, in the Tgfbr2fspko mice, the loss of TGF-β signalling in fibroblasts of the entire mouse 42, for the most part, had little effect on fibroblast abundance in most tissues examined (i.e. no evidence of stromal hyperplasia in the skin, lung, kidney, mammary gland, esophagus, liver, small intestine, or colon). This might indicate a tissue-selective role for TGF-β singalling in maintaining fibroblast homeostasis or the presence of redundant growth inhibitory signals from other factors that do not require the TGF-β type II receptor. There was however significant stromal hyperplasia in the prostate and forestomach of Tgfbr2fspko mice, the same organs that undergo epithelial transformation42. It is therefore important to keep in mind that multiple changes in several growth factor pathways as well as tissue-specific responses will ultimately determine the outcome of the complex epithelial-fibroblast interactions in tumours compared to that in the non-disease state (Box 1). In addition, our current knowledge on the role of many growth factors is primarily derived from studies of epithelial cells in culture. Undoubtedly, the ability to conditionally knock out growth factor signaling specifically in fibroblasts or epithelial cells will continue to advance our understanding of these networks of paracrine and autocrine signalling on epithelial proliferation and transformation that operate in vivo.
Box 1. Not all fibroblasts are created equal.
The figure illustrates stromal-epithelial interactions in normal and tumour tissues. Panel a illustrates wild-type stomach fibroblasts interacting with both the squamous epithelia (SE) of the forestomach and columnar glandular epithelia (GE) of the stomach body (below, green line indicates the area of epithelial transition). Panels b and c depicts the progression of squamous cell carcinoma (SC) in the forestomach as it infiltrates the glandular stomach epithelia (GE). The progression of the carcinoma is likely maintained through reciprocal signals to and from the fibroblasts (asterisk, panel b). Fibroblasts have the capacity to proliferate and/or take on an activated form that can be supportive and even initiate epithelial hyperplasia and eventual tumourigenesis. In turn, carcinoma-derived paracrine proliferative factors signal to the stroma. The cartoon in panel d depicts the non-carcinogenic balance epithelia and fibroblast maintain through dynamic interactions between the two compartments. A curious aspect of the Tgfbr2fspko model42 is that, apart from the prostate and forestomach, the other tissues in the mouse had no apparent signs of carcinogenic transformation. Functional differences between fibroblast from different organs my explain this.
In a reductionist approach to understanding epithelial-stromal interactions in normal tissues and in cancer, the fibroblast component is often generalized and obscure differences in fibroblasts from different tissues or differences within the same tissue. However, it is becoming clear that different fibroblast can have distinct functions. For example, during lung development, epithelial induction capacities are known to differ based on whether the mesenchyme is derived from the trachea or the tips of the growing lungs69. The origin of the mesenchymal fibroblasts apparently determines their sensitivity to sonic hedgehog (Shh) derived from the developing lung epithelia. Shh signals through the patched receptor homologue (Ptc) found in the adjacent mesenchyme to stimulate proliferation70. In turn, the specific mesenchyme supports the normal development and branching morphogenesis of the lung epithelia.
In mice, the fibroblasts associated with the squamous epithelium of the forestomach and esophagus appear phenotypically similar to the fibroblasts associated with the adjacent glandular epithelia of the stomach body. Although a similar proportion of fibroblasts in the esophagus, forestomach, and glandular stomach compartments were deficient for TGF-β singalling in Tgfbr2fspko mice, only the squamous epithelia of the forestomach responded to the oncogenic paracrine signals. It is possible that the loss of TGF-β signalling in stromal fibroblasts results in proliferative autocrine and paracrine signals to which the fibroblasts and squamous epithelia of the forestomach, uniquely responded with the formation of invasive carcinoma, but not other other epithelia of e.g. the esophagus which showed no hyperplastic or neoplastic response in the Tgfbr2fspko mice. Thus in this model, the paracrine signals required for malignancy of the glandular epithelia likely differ from that of the adjacent squamous epithelia.
Other paracrine factors
The mechanisms involved in the microenvironmental effects on carcinoma progression are an intense area of investigation (Table 1) and have yielded important insights in addition to the roles of TGF-β and HGF. Both the extracellular matrix and the matrix degrading enzyme family of matrix metalloproteinases (MMP) can promote epithelial transformation49. MMP levels and activities are often elevated in tumours. The activities of the large and diverse family of MMPs include pro-angiogenic and metastatic actions. They can also generate growth-regulating signals through the activation of growth factors, such as IGF (mediated through the cleavage of IGF binding proteins), FGFs (through cleavage of perlecan), TGF-β and TGF-α49,50. MMP-1 and MMP-7 are of fibroblastic origin and can induce increased susceptibility to mammary cancer when overexpressed in transgenic mice50. Additionally, MMP-2 and MMP-9 knockout mice have a reduced susceptibility for lung metastases following intravenous injection of carcinoma cells50,51.
In a more complex process, PDGF expressed by immortalized skin keratinocytes induces the expression of FGF7 (KGF) by fibroblasts52. FGF7 in turn has been shown to produce further epithelial proliferation and promote carcinogenesis. In the prostate, FGF7 and FGF10 are produced by fibroblasts and stimulate the proliferation of adjacent epithelia53,54. This is countered by the paracrine growth factor FGF9, expressed by prostate epithelia, and received by fibroblasts55. This is a clear example of alterations in paracrine growth factor pathways accompanying the carcinogenesis process; the precise role for these factors in this process is still being elucidated
In a recent study, expression of PDGF by melanoma cells was shown to increase pericyte (special vascular cells) recruitment and proliferation in a B16 tumour model56. The associated growth of the tumour was, however, not attributed to the increase in vasculature, but rather to pericyte-derived factors acting on the melanoma.
Another paracrine factor, Wnt1 (a known mammary epithelial oncogene), when expressed by fibroblasts initiates a morphologic transformation of neighboring C57MG mammary epithelial cells in co-culture experiments while no transformation of the Wnt1-expressing fibroblasts themselves was observed57. More recently, Derksen et al58 provided supporting data on the paracrine role of WNT family proteins enhancing the survival and growth of multiple myeloma cells in humans. The paracrine dynamics in all of these experiments is modulated by the presence or absence of contravening influences that are unevenly distributed in tissues.
The role of mutations in stromal cells
One of the more provocative implications of the recent publications by Bhowmick et al.42 and Kuperwasser et al.44 is that selected epithelial cells without apparent mutations can be induced to form carcinomas by association with genetically altered fibroblasts. In previous studies, the epithelial cells that were induced to be more carcinogenic by association with irradiated fibroblasts were already fully transformed into carcinomas36 or had p53 gene mutations35. In the studies involving recombination of human mammary epithelial cells with human fibroblasts modified to express HGF and/or TGF-β1 in cleared mammary fat pads of immune deficient mice44, it is possible of course that pre-existing mutations were present in the human mammary epithelial cells that formed carcinomas. This uncertainty is supported by the observation that only selected epithelial preparations gave carcinomas when recombined with HGF and/or TGF-β expressing fibroblasts. However, the pre-neoplastic and neoplastic lesions observed in Tgfbr2fspko mouse model42 suggest that premalignant and malignant epithelial tumours can develop due to mutations in fibroblasts preceding any subsequent tumourigenic changes in the epithelial cells. One possible explanation for this is that the rapid proliferation induced in epithelial cells by HGF (and likely other epithelial cell growth factors) secreted by the TβRII null fibroblasts leads to formation of genetic lesions (Figure 2). Another possible explanation which is not mutually exclusive with the previous one is the generation of oxygen free radicals or other endogenous mutagens, a process that could be accelerated by recruitment of inflammatory cells to involved sites60. The selective presence and timing of putative genetic alterations in some epithelial cells in the Tgfbr2fspKO model remains to be determined. The fact that only two tissue compartments evolved carcinomas in this model suggests that pre-existing mutations in epithelia may not be likely.
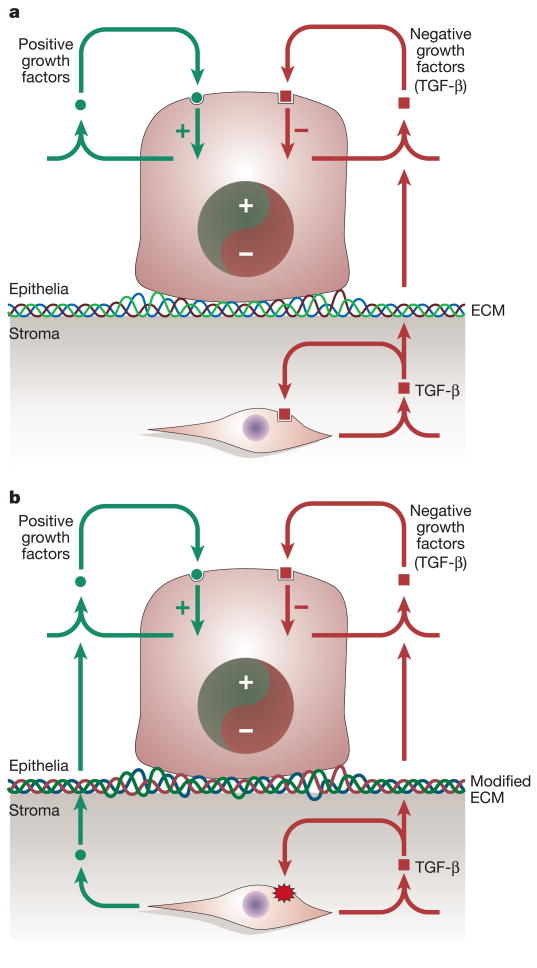
Figure 2. Epithelia can be reactive to a changing stromal environment.
(A) Homeostatic interactions between the epithelia and fibroblasts are maintained through positive and negative signals influencing the proliferation and differentiation of both the stroma and epithelia. (B) When singalling by a suppressive growth factor (TGF-β) to the stromal fibroblasts is lost (red starburst), it leads to elevated fibroblast proliferation. Resulting paracrine factors (e.g., HGF) and potential modifications in the ECM can stimulate the proliferation and transformation of epithelial cells in some tissues in vivo.
Earlier studies have demonstrated mutations in stromal fibroblasts that accompany carcinomas. Loss of heterozygosity (LOH) has been found with high frequency in stromal cells associated with human breast cancer61. This was determined by utilizing polymerase chain reaction (PCR) to examine microdissected tissues with 12 polymorphic DNA markers on chromosomes 2p, 3p, 11q, 16q and 17q known for a high frequency of LOH in human breast cancer. Further, mutations in two tumour suppressor genes, p53 and PTEN, were demonstrated in both mammary carcinoma cells and associated stromal fibroblasts62. However, the possibility that the stromal compartment contains fibroblasts derived from carcinoma cells that have undergone an epithelial to mesenchymal transition cannot be excluded63.
Additional evidence for a role of mutations in stromal cells in the development of epithelial hyperplasias; derives from studies of heritable juvenile polyposis syndrome, one of the hamartomatous polyposis syndromes that are characterized by an overgrowth of tissue native to the area in which they normally occur. In this condition, the loss of normal genetic function occurs predominantly in interstitial fibroblasts among colonic polyps64,65. It is of interest that the genes targeted in this syndrome encode bone morphogenetic protein (BMP) receptor 1A (BMPR1A), a member of the TGF-beta family of receptors, or SMAD4which plays a central role in signaling from both the BMP and TGF-beta receptors66.
These data along with those reported by Bhowmick et al.42 strongly support the hypothesis that TGF-β superfamily signaling in stromal fibroblasts frequently exerts a tumour-suppressive function on adjacent epithelia. This is of particular interest because TGF-β singalling pathways within epithelial cells are also tumour suppressive24. These findings suggest that normal cells in vivo restrict the malignant phenotype of neighboring cells, and that initiation and progression of carcinomas likely involves the overcoming of constraints from normal interstitial tissue through a combination of potential genetic, epigenetic, and stromal changes.
Implications for therapy
Because of the positive role TGF-β signalling in carcinomas such as an excess fibrosis, tumour progression and metastasis, the pharmaceutical industry has been developing inhibitors of TGF-β singalling pathways. Indeed, studies involving systemic inhibition of TGF-β singalling in adult animals has demonstrated no adverse effects and has decreased metastases from mammary carcinomas67. However, the more recent studes described above raise the question whether such inhibitors might potentially also promote carcinomas through the inhibition of TGF-β singalling in normal stroma, and therefore warrant caution in pursuing this approach
Another therapeutic target suggested by the data discussed in this review is HGF. As pointed out by Ohuchida et al,36 irradiation of stromal cells can cause activation of c-Met on carcinoma cells. This group further reported that specific antagonists of HGF could block the enhanced invasiveness of pancreatic carcinoma caused by irradiated fibroblasts. Overexpression and activation of c-Met is a common event in human cancer68, and a recent publication reported the development of a soluble c-Met receptor (decoy Met) that interferes with HGF binding to c-Met and c-Met homodimerization48. Local and systemic delivery of decoy Met significantly inhibited proliferation and metastasis in human tumour xenografts.
Conclusions and future directions
The studies discussed in this review suggest a more important role for stromal fibroblasts in carcinogenesis than was previously appreciated. Fibroblasts influence epithelial transformation by production of paracrine factors that impact both normal epithelia as well as carcinoma cells. In addition, there is now considerable evidence that mutations arising in stromal fibroblasts can precede carcinoma development. The tissue specificity of stromal-epithelial interactions likely accounts for a tissue and cell type specific role of the microenvironement in carcinoma development. Importantly, some of the data point to potential targets for therapy, specifically inhibition of the HGF-c-Met axis. This review has focused on the effect of various stroma-derived paracrine factors on epithelial cells. It is likely that these same factors also have significant effects on stromal cells, including fibroblasts, endothelial cells and inflammatory cells.
Valuable insights concerning the tissue microenvironment have been derived from co-culture and tissue recombination xenograph experiments, but such information may not be applicable to the in vivo situation because not all important environmental factors and cells are considered. The ability to overexpress specific factors or conditionally knockout specific genes in vivo in tissue fibroblasts and other cells of the normal stroma and tumour microenvironment will add greatly to our knowledge of the complex interactions involved in tissue homeostasis as well as those changes involved in the initiation and progression of cancer.
Acknowledgments
This work was supported by a DOD USAMRMC grant (to NAB), NIH grants (to HLM), and the Vanderbilt-Ingram Cancer Center Support Grant. We thank Jennifer Pietenpol and Simon Hayward for critical review of the manuscript and helpful advice.
References
- 1.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 2.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 3.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–9. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 5.Sandler AB, Johnson DH, Herbst RS. Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer. Clin Cancer Res. 2004;10:4258s–4262s. doi: 10.1158/1078-0432.CCR-040023. [DOI] [PubMed] [Google Scholar]
- 6.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–73. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunha GR, Reese BA, Sekkingstad M. Induction of nuclear androgen-binding sites in epithelium of the embryonic urinary bladder by mesenchyme of the urogenital sinus of embryonic mice. Endocrinology. 1980;107:1767–70. doi: 10.1210/endo-107-6-1767. [DOI] [PubMed] [Google Scholar]
- 11.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985;17:137–48. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- 13.Culig Z, et al. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Cunha GR, Donjacour AA. Mesenchymal-epithelial interactions in the growth and development of the prostate. Cancer Treat Res. 1989;46:159–75. doi: 10.1007/978-1-4613-1595-7_9. [DOI] [PubMed] [Google Scholar]
- 15.Hayward SW. Approaches to Modeling Stromal-Epithelial Interactions. J Urol. 2002;168:1165–1172. doi: 10.1016/S0022-5347(05)64620-4. [DOI] [PubMed] [Google Scholar]
- 16.Hayward SW, Cunha GR. The prostate: development and physiology. Radiol Clin North Am. 2000;38:1–14. doi: 10.1016/s0033-8389(05)70146-9. [DOI] [PubMed] [Google Scholar]
- 17.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 18.Tucker RF, Shipley GD, Moses HL, Holley RW. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984;226:705–7. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- 19.Moses HL, et al. Type β transforming growth factor is a growth stimulator and a growth inhibitor. Cancer Cells. 1985;3:65–71. [Google Scholar]
- 20.Pierce DF, Jr, et al. Proc Natl Acad Sci U S A. 1995;92:4254–8. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottinger EP, Jakubczak JL, Haines DC, Bagnall K, Wakefield LM. Transgenic mice overexpressing a dominant-negative mutant type II transforming growth factor beta receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7,12-dimethylbenz-[a]-anthracene. Cancer Res. 1997;57:5564–70. [PubMed] [Google Scholar]
- 22.Gorska AE, et al. Transgenic Mice Expressing a Dominant-Negative Mutant Type II TGF-β Receptor Have Impaired Mammary Development and Enhanced Mammary Tumor Formation. Am J Pathol In Press. 2003 doi: 10.1016/s0002-9440(10)63510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amendt C, Schirmacher P, Weber H, Blessing M. Expression of a dominant negative type II TGF-beta receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene. 1998;17:25–34. doi: 10.1038/sj.onc.1202161. [DOI] [PubMed] [Google Scholar]
- 24.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 25.Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10:303–60. [PubMed] [Google Scholar]
- 26.Tsushima H, et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258–62. [PubMed] [Google Scholar]
- 27.Shariat SF, et al. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856–64. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- 28.Shariat SF, et al. Preoperative plasma levels of transforming growth factor beta(1) strongly predict clinical outcome in patients with bladder carcinoma. Cancer. 2001;92:2985–92. doi: 10.1002/1097-0142(20011215)92:12<2985::aid-cncr10175>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Dawe CJ. In: Tissue interactions in carcinogenesis. Tarin D, editor. Academic Press; London: 1972. pp. 305–358. [Google Scholar]
- 30.Schor SL, Schor AM, Rushton G. Fibroblasts from cancer patients display a mixture of both foetal and adult-like phenotypic characteristics. J Cell Sci. 1988;90:401–407. doi: 10.1242/jcs.90.3.401. [DOI] [PubMed] [Google Scholar]
- 31.Schor SL, Schor AM, Rushton G, Smith L. Adult, foetal and transformed fibroblasts display different migratory phenotypes on collagen gels: evidence for an isoformic transition during foetal development. J Cell Sci. 1985;73:221–234. doi: 10.1242/jcs.73.1.221. [DOI] [PubMed] [Google Scholar]
- 32.Russell PJ, Bennett S, Stricker P. Growth factor involvement in progression of prostate cancer. Clin Chem. 1998;44:705–23. [PubMed] [Google Scholar]
- 33.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayward SW, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–42. [PubMed] [Google Scholar]
- 35.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–60. [PubMed] [Google Scholar]
- 36.Ohuchida K, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–22. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- 37.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–7. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allinen M, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 40.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–5. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 41.Strutz F, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 43.Iwano M, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuperwasser C, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res. 1997;57:3305–13. [PubMed] [Google Scholar]
- 46.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 47.Follenzi A, et al. Cross-talk between the proto-oncogenes Met and Ron. Oncogene. 2000;19:3041–9. doi: 10.1038/sj.onc.1203620. [DOI] [PubMed] [Google Scholar]
- 48.Michieli P, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 48A.Pennacchietti S, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 49.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–73. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 50.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 51.Itoh T, et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–51. [PubMed] [Google Scholar]
- 52.Brauchle M, Angermeyer K, Hubner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9:3199–204. [PubMed] [Google Scholar]
- 53.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6:2123–8. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]
- 54.Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chem. 1999;274:12827–34. doi: 10.1074/jbc.274.18.12827. [DOI] [PubMed] [Google Scholar]
- 55.Jin C, et al. Directionally specific paracrine communication mediated by epithelial FGF9 to stromal FGFR3 in two-compartment premalignant prostate tumors. Cancer Res. 2004;64:4555–62. doi: 10.1158/0008-5472.CAN-03-3752. [DOI] [PubMed] [Google Scholar]
- 56.Furuhashi M, et al. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004;64:2725–33. doi: 10.1158/0008-5472.can-03-1489. [DOI] [PubMed] [Google Scholar]
- 57.Jue SF, Bradley RS, Rudnicki JA, Varmus HE, Brown AM. The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol. 1992;12:321–8. doi: 10.1128/mcb.12.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derksen PW, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101:6122–7. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koskinen PJ, Sistonen L, Bravo R, Alitalo K. Immediate early gene responses of NIH 3T3 fibroblasts and NMuMG epithelial cells to TGF beta-1. Growth Factors. 1991;5:283–93. doi: 10.3109/08977199109000292. [DOI] [PubMed] [Google Scholar]
- 60.Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest. 2003;111:583–93. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moinfar F, et al. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–6. [PubMed] [Google Scholar]
- 62.Kurose K, et al. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–7. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 63.Xue C, Plieth D, Venkov C, Xu C, Neilson EG. The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 2003;63:3386–94. [PubMed] [Google Scholar]
- 64.Jacoby RF, et al. A juvenile polyposis tumor suppressor locus at 10q22 is deleted from nonepithelial cells in the lamina propria. Gastroenterology. 1997;112:1398–403. doi: 10.1016/s0016-5085(97)70156-2. [DOI] [PubMed] [Google Scholar]
- 65.Wirtzfeld DA, Petrelli NJ, Rodriguez-Bigas MA. Hamartomatous polyposis syndromes: molecular genetics, neoplastic risk, and surveillance recommendations. Ann Surg Oncol. 2001;8:319–27. doi: 10.1007/s10434-001-0319-7. [DOI] [PubMed] [Google Scholar]
- 66.Waite KA, Eng C. From developmental disorder to heritable cancer: it’s all in the BMP/TGF-beta family. Nat Rev Genet. 2003;4:763–73. doi: 10.1038/nrg1178. [DOI] [PubMed] [Google Scholar]
- 67.Yang YA, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–15. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 69.Wessells NK. Mammalian lung development: interactions in formation and morphogenesis of tracheal buds. J Exp Zool. 1970;175:455–66. doi: 10.1002/jez.1401750405. [DOI] [PubMed] [Google Scholar]
- 70.Bellusci S, et al. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 71.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 72.Barbero S, et al. Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003;63:1969–74. [PubMed] [Google Scholar]
- 73.Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–54. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]