Abstract
Previously we demonstrated that 17β-Estradiol (E2) induced rapid Ca2+ influx via L-type calcium channel activation, which was required for activation of Src/ERK/CREB/Bcl2 signaling cascade and subsequent induction of neuroprotective and neurotrophic responses in rat hippocampal and cortical neurons (Wu et al., 2005; Zhao et al., 2005). The current study determined the presence and specificity of membrane E2 binding sites and the functional consequence of E2 binding to membrane receptors in individual neurons. Using E2-BSA-FITC (fluorescein isothiocyanate) macromolecular complex, membrane E2 binding sites were observed in hippocampal neurons. Punctate FITC signal was observed on plasma membrane of soma and neuronal processes in E2-BSA-FITC binding neurons. No membrane binding was observed with BSA-FITC. Specificity of binding was demonstrated by competition with excess un-conjugated E2. An ER specific agonist, PPT, and an ER agonist, DPN, partially competed for E2-BSA-FITC binding. Imaging of intracellular Ca2+ ([Ca2+]i) in live neurons, revealed rapid Ca2+ responses in E2-BSA-FITC binding neurons within minutes that culminated in a greater [Ca2+]i rise and [Ca2+]i spikes at >20min. The same neurons in which E2-BSA-FITC induced a [Ca2+]i rise also exhibited activated pERK (extracellular signal-regulated kinase) that was translocated to the nucleus. Immunofluorescent analyses demonstrated that both excitatory and inhibitory neuronal markers labeled subpopulations of E2-BSA-FITC binding neurons. All E2-BSA-FITC binding neurons expressed L-type calcium channels. These results demonstrate, at a single cell level, that E2 membrane receptors mediate the rapid signaling cascades required for E2 neuroprotective and neurotrophic effects in hippocampal neurons. These results are discussed with respect to therapeutic targets of estrogen therapy in brain.
Keywords: Estrogen Receptor, Membrane Receptor, Calcium, PhosphoERK
1. Introduction
Increasing evidence indicates the presence of rat brain plasma membrane associated estrogen receptors (ERs). Membrane-associated estrogen receptors was first reported in 1977 using a ligand affinity-binding approach (Pietras and Szego, 1977). Later, Watson and colleagues detected ER proteins in the plasma membrane using ER specific antibodies (Pappas et al., 1995). In neuronal cells, both ERα and ERβ have been reported at extranuclear sites in hippocampus, dorsal raphe, striatum, red nucleus, and olfactory bulb (McEwen et al., 2001; Milner et al., 2001; Milner et al., 2005; Mitra et al., 2003; Zhang et al., 2002). Milner and colleagues were the first to provide ultrastructural evidence for ERα and β extranuclear localization (Milner et al., 2001; Milner et al., 2005). The cellular and subcellular localization of ERβ was similar to that of ERα, except that ERβ was more extensively found at extranuclear sites.
Multiple effects of estrogen are rapid and appear to not require direct interaction with an estrogen response element (ERE) (Cordey et al., 2003; Kuroki et al., 2000; Nethrapalli et al., 2001; Nilsen and Brinton, 2002; Nilsen and Diaz Brinton, 2003; Rudick and Woolley, 2000; Simoncini et al., 2000; Singh, 2001). Inhibition of rapid estrogen effects with antibodies against ERs in cells with intact membranes provides one piece of evidence that membrane-associated ERs are involved in these rapid estrogen effects (Marquez and Pietras, 2001). A second strategy is the use of membrane impermeable estrogen. Multiple laboratories have used this strategy with results indicating that estrogen activation of membrane sites of action results in regulation of calcium responses (Benten et al., 1998; Beyer and Raab, 1998; Chaban and Micevych, 2005; Watson et al., 2005), activation of mitogen-activated protein (MAP) kinases (Carrer et al., 2005; Chen et al., 2004), activation of PKC (Boyan et al., 2003) and other signaling pathways (Vasudevan et al., 2005). A third strategy has been to express membrane targeted ERs in ER negative cells. Results of these studies indicate that extracellular signal-regulated kinase (ERK), PI3K/AKT and cAMP signaling were activated by membrane ERs (Rai et al., 2005; Razandi et al., 2004). Together these data support the existence of membrane associated sites to which estrogen binds and which fulfill multiple criteria required for receptor status (Brinton, 1984; Cooper et al., 1978; Ross, 1990)
Here we investigated the expression of membrane associated ERs in hippocampal neurons and the functional consequence of activating these E2 binding sites. To conduct these analyses a fluorescent membrane impermeable E2-bovine serum albumin-fluorescein isothiocyanate (E2-BSA-FITC) macromolecular was used to label membrane ER (mER) coupled with simultaneous live cell calcium imaging. Subsequent to E2-BSA-FITC binding, hippocampal neurons were investigated for activation of pERK. Results of these analyses demonstrated specific binding of E2 to the plasma membrane of nearly a third of all cultured hippocampal cell bodies and their neurites. Activation of mER in live hippocampal neurons induced a rise in intracellular calcium and activation of pERK in the same neurons. Phenotypic characterization of E2-BSA-FITC labeled neurons revealed that 57% of mER expressing neurons were glutamatergic, 54% were inhibitory neurons and that all mER expressing neurons expressed L-type calcium channels.
2. Results
2.1 Membrane estrogen binding sites existed in rat primary hippocampal neurons
To investigate whether membrane 17β-estradiol (E2) binding sites mediated E2-induced rapid responses in rat primary hippocampal neurons, we first performed imaging studies using fluorescent membrane impermeable E2-bovine serum albumin-fluorescein isothiocyanate macromolecular complex (E2-BSA-FITC). Primary hippocampal neurons were incubated with 10μg/ml E2-BSA-FITC at 37°C for 30min and washed twice with 37°C PBS for 5min each prior to imaging. Figure 1A shows optical sections along the Z axis of an E2-BSA-FITC labeled neuron. We detected intensive FITC signals at the plasma membrane of soma as well as punctate staining at the neurites. Figure 1B shows representative DIC (left) and fluorescence (right) images of E2-BSA-FITC binding. Of the two neurons in the DIC image, one (on the right) was labeled with E2-BSA-FITC. Of the 913 primary hippocampal neurons analyzed from 3 independent E2-BSA-FITC labelings, 266 or 29.13% were specifically labeled with E2-BSA-FITC. The insert in Fig. 1B shows a magnified neuronal process. E2-BSA-FITC labeling exhibited a punctuate distribution along the neuritic plasma membrane. BSA-FITC did not bind to neurons under the same incubation condition (Fig. 1C) indicating the binding was mediated by E2 in the E2-BSA-FITC macromolecule.
Figure 1. E2-BSA-FITC labeled E2 membrane binding sites in cultured hippocampal neurons.
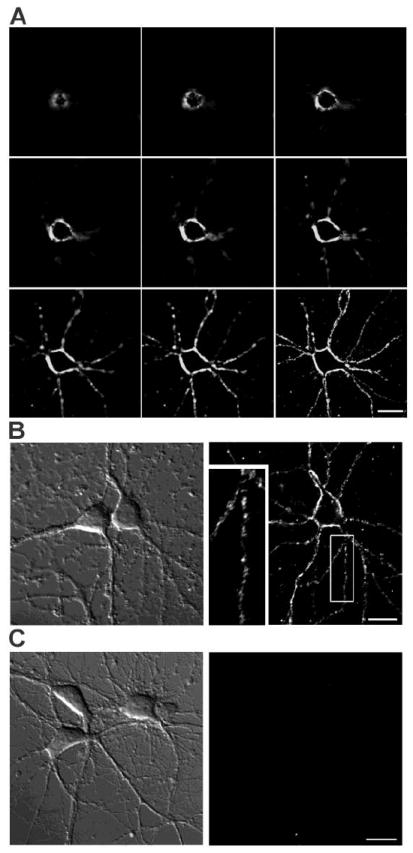
(A) Series of Z-axis optical sections of an E2-BSA-FITC labeled neuron. (B) Representative DIC (left) and fluorescence (right) images of E2-BSA-FITC labeled neurons. Of the 913 primary hippocampal neurons analyzed from 3 independent stainings, 266 or 29.13% were specifically labeled with E2-BSA-FITC after incubated with 10μg/ml E2-BSA-FITC at 37°C for 30min. Insert shows a magnified neuronal process. Note the punctate signal detected. (C) Representative DIC (left) and fluorescence (right) images of neurons after incubation with 10 g/m BSA-FITC at 37°C for 30min. Scale bar represents 20μm.
2.2 Estrogen receptor agonists competed for E2-BSA-FITC membrane binding
To further verify the specificity of E2-BSA-FITC binding, we performed a competition assay with excess non-labeled ER agonists. Cultured rat primary hippocampal neurons were preincubated with 4μg/ml (~15μM) E2 at 37°C for 30 min prior to adding E2-BSA-FITC for an additional 30min (Fig. 2, second row). Preincubation of free E2 completely blocked E2-BSA-FITC binding to hippocampal neurons. To determine whether ERα and ERβ could localize to the membrane, ER subtypes selective agonists were tested for their ability to block E2-BSA-FITC binding. Neurons were preincubated with 15μM ERα agonist, PPT (Fig. 2, third row), or 15μM ERβ agonist, DPN (Fig. 2, fourth row) at 37°C for 30min prior to E2-BSA-FITC exposure. Consistent with E2 binding to both ERα and ERβ, preincubation with E2 completely abolished E2-BSA-FITC membrane binding. Preincubation of PPT or DPN reduced FITC signal intensity compared to E2-BSA-FITC, but did not completely inhibit E2-BSA-FITC labeling (Fig. 2, arrowheads, third and fourth row). In both the PPT and DPN conditions, most but not all E2-BSA-FITC binding was inhibited indicating residual ERβ and ERα receptors respectively labeled by E2-BSA-FITC. The complete blockade of E2-BSA-FITC binding by the nonselective ERα and ERβ agonist E2 coupled with the diminution of E2-BSA-FITC binding by PPT and DPN suggests that both ERα and ERβ localize to the neuronal membrane. Images are representative of 180 neurons assessed in each condition across 3 independent experiments.
Figure 2. Selective estrogen receptor agonists competed with E2-BSA-FITC membrane binding.
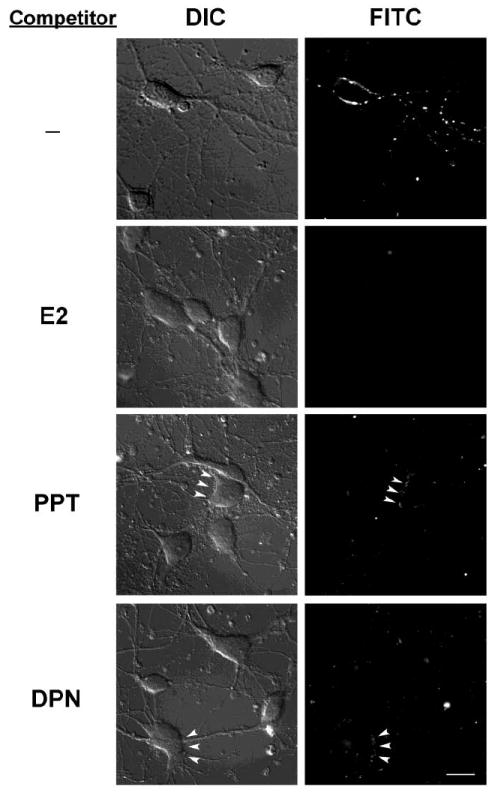
Representative DIC (left) and fluorescence (right) images of neurons pre-incubated with 4μg/ml free E2 (second row), 15μM estrogen receptor α selective agonist PPT (third row), or 15μM estrogen receptor β selective agonist DPN for 30min prior to E2-BSA-FITC was added for an additional 30min. Pre-incubation with E2 completely abolished E2-BSA-FITC membrane binding. Pre-incubation with PPT or DPN greatly reduced E2-BSA-FITC membrane signal. In both the PPT and DPN conditions, most but not all E2-BSA-FITC binding was inhibited indicating residual ERβ and ERα receptors respectively labeled by E2-BSA-FITC (arrowheads). Images are representative of total 180 neurons in each condition studied across 3 independent experiments.Sca lebar represents 20μm.
2.3 Membrane E2 binding induced calcium responses
Our previous findings indicated that E2 induced rapid calcium influx through L-type calcium channels which was required for the subsequent activation of Src/ERK/CREB/Bcl-2 signaling cascade (Wu et al., 2005). To identify the functional consequences induced by the membrane E2 binding, ratiometric intracellular imaging was conducted using the calcium sensitive dye Fura-2 in combination with E2-BSA-FITC binding. Primary hippocampal neurons cultured on a gridded coverslip were loaded with fura-2 ratiometric calcium sensitive dye for 30 min and then washed. Neurons were perfused with 10μg/ml E2-BSA-FITC during the recording followed by 2x wash with 37°C PBS to remove excess E2-BSA-FITC prior to recording the E2-BSA-FITC signals at the end of calcium imaging. Figure 3A shows a pseudo-colored image of the ratio of fluorescence intensity from 340 and 380 channels at the beginning of the recording to indicate the position of each neuron. Figure 3B shows the FITC signal recorded after calcium imaging to identify the neurons labeled with E2-BSA-FITC. In the first 10 min (Fig. 3C top), E2-BSA-FITC bound neurons (neuron #3, 4, 6 and 8) exhibited a slow rise of intracellular calcium ([Ca2+]i). In neurons # 3 and 4, the [Ca2+]i rise was apparent 1min after E2-BSA-FITC exposure while neurons 6 and 8 had a slower response onset which emerged after 5min of E2-BSA-FITC incubation (Fig 3C top). The insert in Fig. 3C shows the first three minutes in an expanded scale. Following 20 to 30 min E2-BSA-FITC exposure, spike-like [Ca2+]i responses emerged in E2-BSA-FITC labeled neurons (Fig. 3C bottom). Minimal [Ca2+]i responses were detected after 25min of E2-BSA-FITC incubation in neurons that did not bind E2-BSA-FITC. The rapid response time and differential response profiles between the early and late response was consistent in the 25 E2-BSA-FITC labeled neurons examined across 3 experiments. Our results indicate that membrane E2 binding was able to trigger [Ca2+]i responses in cultured hippocampal neurons as early as 1 min and 5 min. Two different [Ca2+]i response profiles were evident at the early and late stages of recording suggesting the possibility of different mechanisms involved in membrane E2-induced [Ca2+]i changes.
Figure 3. Membrane E2 binding induced rapid calcium responses.
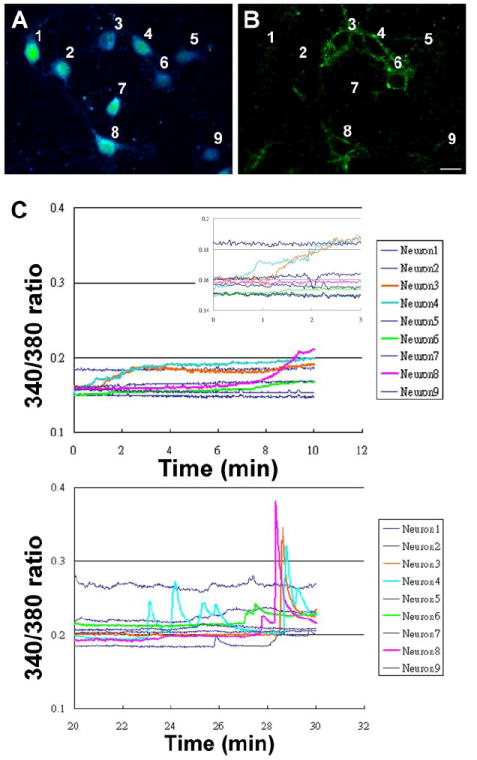
Cultured rat hippocampal neurons subjected to fura-2 calcium imaging while incubated with E2-BSA-FITC. (A) Pseudo color image showing 340/380 emission ratio prior to recording to indicate positions of each neuron. (B) FITC image after recording to locate E2-BSA-FITC bound neurons (#3, 4, 6, and 8). Figure 3 C shows the calcium responses in the first ten (top) and the 20th to 30th (bottom) minutes after E2-BSA-FITC was added. The insert in Fig. 3C shows the first three minutes in an expended scale. The rapid response time and differential response profiles between the early and late response was consistent in the 25 neurons examined across 3 experiments. Scale bar represents 20μm.
2.4 Membrane E2 binding activated ERK in single neurons
Earlier our group found that in rat hippocampal neurons, E2 activated extracellular signal-regulated kinase (ERK) and induced ERK translocation from cytoplasm to nucleus, which depended on E2 induced calcium influx (Nilsen and Brinton, 2002; Wu et al., 2005). To further investigate whether E2 induced calcium responses triggers downstream ERK activation via membrane ER, cultured neurons were incubated with 10μg/ml E2-BSA-FITC or BSA-FITC at 37°C for 20min and subsequently immunocytochemically labeled for phosphorylated ERK (pERK). E2-BSA-FITC labeled 47 out of 165 neurons from 3 independent experiments (28.5%) (Fig. 4A). Of those 47 E2-BSA-FITC bound neurons, quantitative computational threshold analysis indicated that 41 (87%) exhibited an elevated pERK signal at 20 min (Fig 4A). Of the 145 control neurons from 3 independent experiments treated with BSA-FITC, none exhibited a specific FITC signal or a pERK signal. In the representative images shown in Figure 4B, E2-BSA-FITC bound neuron (left column, arrow) exhibited strong pERK signal in nucleus, cytoplasm and neuronal processes. No pERK signal was detected in neurons that did not bind to E2-BSA-FITC. We also performed Western blots of pERK with neurons treated with free E2 or membrane-impermeable E2-BSA (Fig. 4C). Neurons were incubated with 10μg/ml E2-BSA or 10ng/ml E2 at 37°C for 20min. Total cell lysates were collected for pERK Western blot analysis. E2-BSA and free E2 activated ERK to the same level (E2 146.02±15.35, E2-BSA 154.96±8.32, * p<0.05 compared to vehicle control; no significant difference between E2 and E2-BSA).
Figure 4. Membrane E2 binding induced ERK activation.
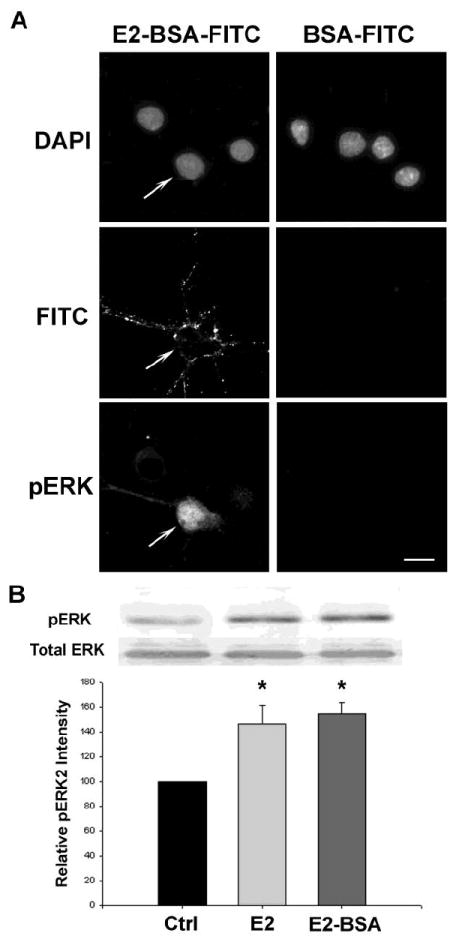
(A) Cultured rat hippocampal neurons were incubated with 10μg/ml E2-BSA-FITC or BSA-FITC and subjected to immunocytochemistry with phosphorylated ERK antibody. In total of 165 cells analyzed across 3 experiments, 47 of them were labeled with E2-BSA-FITC. ERK activity was found elevated in 43 neurons and 41 of them were labeled with E2-BSA-FITC. (B) In the representative image shown, the neuron bound to E2-BSA-FITC (arrow) exhibited high phosphorylated ERK signal in nucleus and throughout cytoplasm. No phosphorylated ERK signal was detected in neuron that did not bind to E2-BSA-FITC. BSA-FITC did not bind to neurons nor did it activate ERK. Scale bar represented 20μm. (C) Free E2 and membrane impermeable E2-BSA both increased ERK phosphorylation. Whole cell lysates were run on SDS-PAGE and probed with anti-pERK or total ERK antibodies. Representative Western blots and normalized quantification of band density were shown. *p<0.05 compared to control, n=3, Bars represent mean ± SEM.
2.5 E2-BSA-FITC co-labeled neurons with neuronal markers
To investigate the phenotype of neurons that bind membrane ER, we conducted immunocytochemical labeling for γ-amino butyric acid (GABA) to label inhibitory neurons and vesicular glutamate transporter 1 (vGluT1) to label excitatory neurons in neurons exhibiting E2-BSA-FITC binding. Based on our previous finding that E2-induced calcium influx through L-type channels was required to initiate the E2 neuroprotection pathway (Wu et al., 2005), we determined the colocalization of E2-BSA-FITC labeling and expression of L-type voltage gated calcium channel. Anti-GABA antibody stained cultured neurons primarily in cytoplasm and in nucleus consistent with the labeling reported in previous studies (Somogyi et al., 1985; Storm-Mathisen et al., 1983) (Fig 5. left). vGluT1 antibody labeling generated a punctate pattern along the plasma membrane and neuronal processes (Fig 5. middle) consistent with the labeling in previous study (Wojcik et al., 2004). L-type channel immunoreactivity was observed on plasma membrane and neuronal processes (Fig 5. bottom), which is consistent with the previous study (Hell et al., 1993). Microscopic fields for quantitative analysis were randomly selected and 15 fields from each of the 3 separate experiments were analyzed for immunoreactivity and E2-BSA-FITC binding (Table1). Of the 306 neurons analyzed for expression of GABA phenotype, 149 or 48.74 ± 1.89% were GABAergic. Of the 315 neurons analyzed for the vGluT1 glutamatergic phenotype, 142 or 44.60 ± 1.92% were positive. Of the 292 neurons analyzed for expression of L-type calcium channel, 252 or 86.77 ± 3.89 expressed L-type calcium channels. In neurons labeled with E2-BSA-FITC and GABA antibody, 47 or 54.19 ± 3.31 were co-labeled. In neurons labeled with E2-BSA-FITC and GluT1 antibody, 53 or 57.22 ± 2.00 were co-labeled. The percentage of GABAergic and glutamatergic neurons was not exclusive because the labelings were done independently. In neurons labeled with E2-BSA-FITC and antibody against L-type calcium channel, 85 or 33.73% were colabeled. A total of 266 of hippocampal neurons were labeled with E2-BSA-FITC (Table 1). Of these 185, or 69.54% of E2-BSA-FITC positive neurons were phenotypically identified as either GABA, vGluT1 or L-type calcium channel expressing neurons. The remaining 81 or 30.46% of the 266 mER expressing neurons remain to be phenotypically characterized. In summary, phenotypic characterization indicated that approximately 1/3 of hippocampal neurons expressed membrane ER as indicated by E2-BSA-FITC labeling. Of the phenotypically characterized hippocampal neurons, approximately 1/3 of GABAergic, glutamatergic and L-type calcium channel expressing hippocampal neurons were positive for a marker of membrane estrogen receptor, E2-BSA-FITC expression. Collectively, these results indicate that both excitatory and inhibitory neurons express membrane ER and that expression of L-type calcium channelscan colocalize with expression of membrane ER.
Figure 5. E2-BSA-FITC co-labeled with neuronal markers.
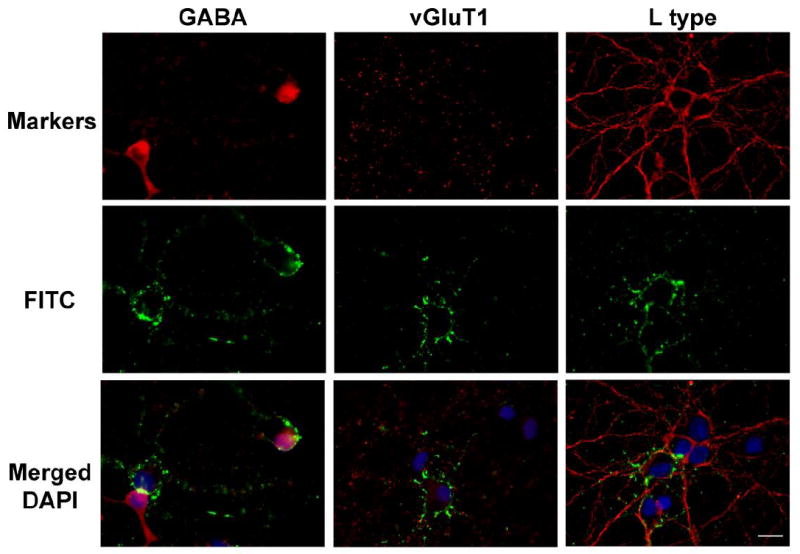
Cultured rat primary hippocampal neurons were labeled with E2-BSA-FITC then fixed and immunofluorescently labeled with antibodies against GABA (left column), vGluT1 (middle column), and L-type channel (right column) to phenotypically labeled E2-BSA-FITC labeled neurons. Immunofluorescent signals detected with each antibody are shown in the top row. E2-BSA-FITC signals are shown in the second row. Merged images of E2-BSA-FITC labeling (green), respective phenotypic labeling (red) and nuclear DAPI labeling (blue), are shown in the third row. GABA immunoreactivity was detected in 54% of E2-BSA-FITC positive neurons vGluT1 was detected in 57% of E2-BSA-FITC positive neurons. L-type channel was detected in 100%E2-BSA-FITC positive neurons. Scale bar = 20μm.
Table 1.
E2-BSA-FITC co-labeled with hippocampal neuron phenotypic markers.
| Phenotypic Markers | Total # neurons analyzed | Total # of neurons positive for phenotypic marker | % of total # of neurons positive for phenotypic marker | # of E2-BSA-FITC positive cells double labeled for phenotypic marker | % phenotype labeled E2-BSA-FITC |
|---|---|---|---|---|---|
| mER E2-BSA-FITC | 913 | 266 | 29% | NA | 29% |
| GABA | 306 | 149 | 49% | 47 | 32% |
| vGluT1 | 315 | 142 | 45% | 53 | 37% |
| L-type | 292 | 252 | 85% | 85 | 34% |
Hippocampal neurons were incubated with E2-BSA-FITC and subsequently labeled with specific antibodies for either GABA, the glutamate transporter vGluT1 or L-type calcium channel. A total of sixty randomly selected microscopic fields from three independent experiments were analyzed for each immunocytochemical phenotypic marker and data pooled across the experiments.
3. Discussion
The present study sought to determine the existence of membrane estrogen binding sites and the downstream signaling pathways induced by membrane estrogen receptors in individual hippocampal neurons. Results of this investigation indicated that 29% of cultured rat embryonic hippocampal neurons, including both excitatory and inhibitory neurons, expressed membrane binding sites for estrogen. When bound to estrogen, these membrane sites induced a rise in intracellular calcium and activated ERK. Both calcium and pERK are required signaling elements for activation of the estrogen-inducible neuroprotection pathway. Both GABAergic and glutamatergic neurons expressed membrane estrogen binding sites. The unifying feature of all neurons expressing the membrane ER is the expression of L-type calcium channels. This finding is particularly relevant given our earlier findings indicating that E2-induced neuroprotection and neurotrophism are dependent upon L-type calcium channel activation and influx of calcium (Wu et al., 2005; Zhao et al., 2005). The early and late calcium responses induced by E2-BSA-FITC were observed in both the GABA and glutamatergic phenotypes suggesting that the different calcium dynamics are mediated by signaling pathways activated by E2 and not by the phenotype of the neuron.
Using E2-BSA-FITC we were able to visualize neurons that express membrane binding sites for estrogen in a phenotypically heterogeneous primary neuron culture. Results of that analysis indicated that close to 30% of the total number of hippocampal neurons exhibited membrane estrogen binding sites. This is consistent with the proportion of E2 responsive neurons previously reported from our laboratory and others.For example, we found that E2 induced rapid neurotrophic effects in 30% of hippocampal neurons (Brinton, 1993). Later, Foy et al. (1999) found that estrogen enhanced NMDA receptor-mediated EPSPs and LTP in 36% of all cells tested in transverse rat hippocampal slices. Gu and Moss (1996) also reported that estrogen potentiated non-NMDA currents in 38% of cells tested in dissociated rat hippocampal neuron. Our group recently reported 29.6% of hippocampal neurons exhibited calcium responses to estrogen in calcium imaging studies (Wu et al., 2005). Beyer et al. (2003) used the same E2-BSA-FITC molecule to label midbrain primary neurons and detected a similar 20-30 % positive population. In a quantitative analysis of ERα positive neurons in the hippocampus, Woolley and colleagues observed approximately 15-60% ERα positive neurons in different regions of the hippocampus (Hart et al., 2001).
The membrane estrogen binding sites we found in this study fulfill some of the criteria for receptors (Brinton, 1984; Cooper et al., 1978; Ross, 1990). The specificity of the membrane receptor to estrogen was demonstrated by selective competition of estrogen and by non-labeling with BSA-FITC molecules. A receptor should also be a cellular macromolecule to which a drug binds to initiate its effects. In the present study, we demonstrated that binding of estrogen to the membrane receptor induced intracellular calcium responses and ERK activation. While the exact identity of the membrane estrogen receptor remains controversial, the majority of evidence supports that membrane ERs are encoded by the same genes as the nuclear ERs. This postulate is based on an array of evidence including: immunocytochemical analyses showing that membrane ERs found in different cell types are labeled by specific antibodies against ERα or ERβ at epitopes contained within the known ERs (Watson et al., 2002; Weiser et al., 2008); membrane localization of ERs can be found when ERα and β were transfected into ER negative cells (Razandi et al., 1999); and lack of endogenous membrane ERs in ERα and β double knockout mice (Razandi et al., 2004). Membrane ERs are likely to be splice variants of either or both ERα and β. In human, a 46KD ERα variant has been cloned from endothelial cells (Figtree et al., 2003). Both full length and this truncated ERα could be found on the cell membrane and mediated cell-surface binding of estrogen associated with acute activation of nitric oxide synthase. In rat brain, Price and colleagues identified several splice variants of ERβ (Price et al., 2000; Price et al., 2001) The variant, Erβ1δ4, lacks exon 4, which contains the nuclear translocation signal and part of ligand-binding domain (Price et al., 2000). Localization analyses revealed that Erβ1δ4 localized to the cytoplasm (Price et al., 2001). Our group identified three novel splice variants from rat cortical and hippocampal neurons (Milner et al., 2008; Wang and Brinton, 2003; Zhao and Brinton, 2005). One of these variants was derived form ERα, designated ERα-21aaN, the other splice variants were derived from ERβ, designated ERβ1Δ3 and ERβ2Δ4. ERα-21aaN and ERβ2Δ4 localized to the cytoplasm and plasma membrane (Wang and Brinton, 2003).
Several amino acids and protein modifications effect ER membrane targeting. Recent findings in endothelial cells have identified motifs in the ERα ligand-binding domain that are critical to membrane localization and function (Chambliss et al., 2005). Razandi and colleagues generated point mutations in ERα, which prevented the mutant from dimerization and membrane localization (Razandi et al., 2004). The authors transfected wild type and mutant constructs into CHO cells and detected monomers of both wild type and mutant constructs at the membrane before estrogen was added. After estrogen was added, wild type ERα formed homodimers in the membrane fraction and was able to mediate ERK, PI3K, and cAMP signaling while the mutant remained as a monomer and did not activate signaling pathways. The authors concluded that dimerization was important for ER to carry out rapid membrane functions. Another possible mechanism was that the mutated amino acids themselves, which were located in the ligand-binding domain, were essential for rapid estrogen signal transduction. Interactions between ER and caveolin protein also play an important role in ER membrane targeting (Acconcia et al., 2005; Razandi et al., 2003). In neurons, ER was co-purified with neuronal caveolae-like microdomain protein, flotillin (Toran-Allerand et al., 2002). Mutation of ERα palmitoylation site (cystine 447) disrupted interaction between ER and caveolins-1 in HeLa and HepG2 cell lines, prevented subsequence membrane targeting, and inhibited estrogen inducible ERK and PI3K activation (Acconcia et al., 2005).
Increasing evidence suggests that membrane ERs are involved in rapid estrogen signaling events. Recent studies have pointed to the potential importance of the integration of estrogen membrane and genomic functions (Levin, 2005). Using a “two-pulse” paradigm, Vasudevan et al. showed that these membrane signals regulated rapid signal events and subsequently regulated estrogen responsive element-mediated gene transcription (Vasudevan et al., 2005). Vasudevan and colleagues also found that calcium influx was essential to transduce estrogen signals consistent with our current and previous findings (Wu et al., 2005; Zhao and Brinton, 2005; Zhao et al., 2005). Our findings of membrane ER mediated calcium responses and ERK activation were consistent with previous reports. Recent studies demonstrated that estrogen activation of membrane sites of action in other systems and cell types results in regulation of calcium responses (Benten et al., 1998; Beyer and Raab, 1998; Chaban and Micevych, 2005; Watson et al., 2005). Several research groups reported that the estrogen activates ERK through membrane ER, which is consistent with our data in the current report. The membrane impermiable estrogen-BSA conjugates (Alexaki et al., 2006; Carrer et al., 2005; Chen et al., 2004) and the newly synthesized estrogen-dendrimer conjugates (Harrington et al., 2006) were used to demonstrate estrogen membrane effects on ERK activation in breast cancer cells, endothelial cells and neurons.
In conclusion, the present study visualized the existence and functional consequences of membrane estrogen receptors in rat primary hippocampal neuron culture. We found that 29% of cultured hippocampal neurons expressed membrane estrogen receptors. The receptors might be closely related to ERα and β as selective ER agonists were able to partially compete for binding. We were able to show that membrane E2 binding triggered an intracellular calcium rise that led to activation of pERK in individual hippocampal neurons. Both calcium and pERK were previously reported to be crucial components in estrogen neuroprotection and neurotrophism (Wu et al., 2005; Zhao et al., 2005). L-type calcium channels were found to be expressed in all neurons expressing membrane ER which is consistent with the obligatory role we previously reported for L-type calcium channel in estrogen signaling. In addition, the data indicate that membrane ER exists in both excitatory and inhibitory neurons. Further knowledge on the nature of membrane ERs and its signaling pathways can provide targets for development of brain selective estrogen receptor modulators for prevention of neurodegenerative disease and maintenance of cognitive function throughout the aging process.
4. Experimental Procedures
4.1 Chemicals
All culture materials were purchased from Invitrogen, Carlsbad, CA, USA. 17β-estradiol (E2) was purchased from Steraloids, Newport, RI, USA. E2-bovine serum albumin-fluorescein isothiocyanate macromolecular complex (E2-BSA-FITC) E2-BSA and BSA-FITC were purchased from Sigma-Aldrich, St. Louis, MO, USA. PPT and DPN were purchased from Tocris Bioscience, Ellisville, MO, USA. All other chemicals were purchased from ICN Biomedical, Costa Mesa, CA, USA unless noted otherwise.
4.2 Animals and neuronal culture
Use of animals was approved by the Institutional Animal Care and Use Committee in University of Southern California. All experiments conformed to the Animal Welfare Act, Guide to Use and Care of Laboratory Animals, and the US Government Principles of the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training guidelines on the ethical use of animals. In addition, the minimal numbers of required animals were used for these experiments and suffering was minimized. Pregnant Sprague Dawley rats were purchased from Harlan Sprague Dawley, Inc., Indianapolis, IN, USA. and housed under controlled conditions of temperature (22°C), humidity, and light (14 hour light: 10 hour dark); water and food were available ad libitum. Primary hippocampal culture was prepared as described (Brinton, 1993). Briefly, hippocampi were dissected from the brains of embryonic day 18 fetuses. Hippocampal tissues were dissociated by incubation with 0.02% trypsin in HBSS (5.4 mM KCl, 137 mM NaCl, 0.4 mM KH2PO4, 0.34 mM Na2HPO4·7H2O, 10mM glucose, and 10mM HEPES) for 5 min at 37°C and repeated passage through a series of fire-polished constricted Pasteur pipettes. For Ca2+ imaging or immunocytochemistry studies, 20,000 cells were plated onto each poly-D-lysine (10μg/ml) coated 22-mm coverslips or 0.1% polyethyenimine coated eight chambers plastic slides (Nalge Nunc International, Rochester, NY, USA). For Western blotting, 105cells/ml was seeded on 0.1% poly-ethylenimine coated 60mm plastic plates. Neurons were grown in Neurobasal Medium supplemented with 25μM glutamate, 500μM glutamine, 5 U/ml penicillin, 5μg/ml streptomycin, and B27 supplement at 37°C in a humidified 5% CO2 atmosphere. Experiments were performed after 10 days in culture.
4.3 E2-BSA-FITC binding and competition assay
E2-BSA-FITC stock solution were prepared in ddH2O and filtered with Microcon YM-30 filter (Millipore, Billerica, MA, USA) before use to remove free estrogen. Neurons cultured on coverslips were incubated with 10μg/ml E2-BSA-FITC for 30 min at 37°C and washed twice with 37 °C PBS before imaging. In competition experiments, neurons were preincubated with 4μg/ml free E2, 15μM PPT or 15μM DPN for 30 min at 37°C before E2-BSA-FITC was added for an additional 30min. Neurons were washed twice with 37°C PBS before imaging. Images were acquired using Slidebook Digital Imaging System (Intelligent Imaging Innovations, Inc, Denver, CO, USA) and were deconvolved with constrained iterative deconvolution method.
4.4 Calcium imaging
Intracellular calcium ([Ca2+]i) in hippocampal neurons was determined by ratiometric imaging of the Ca2+ sensitive fluorescent dye fura-2. Primary cultured hippocampal neurons were loaded with 2 μM fura-2 acetoxymethyl ester (Molecular Probes, Eugene, OR, USA) in HBSS buffer for 45min at 37°C then washed twice and incubated with HBSS buffer for 30min at 37°C to remove remaining fura-2 ester. Coverslips with loaded cells were mounted in a perfusion chamber on an inverted microscope (Axiovert 200M, Zeiss, Thornwood, NY, USA). We recorded fluorescence images of neurons loaded with fura-2 with excitation wavelength at 340nm and 380nm respectively with Slidebook Digital Imaging System. Images were recorded at 1 second intervals. Neurons were perfused with HBSS buffer at a flow rate of 1 ml/min during recording. Ratio of fluorescence intensity form 340 and 380 channels across time was analyzed with the Slidebook Digital Imaging System. Images were recorded for less than 10min to maintain a manageable file size.
4.5 Immunocytochemistry
Hippocampal neurons were washed twice with PBS and fixed with 4% paraformaldehyde (EMS, Hatfield, PA, USA) for 15min at RT. Fixed neurons were permeabilized with 0.5% Triton/PBS for 5min at RT. Neurons were incubated with primary antibodies (ERK1/2 [pTpY185/187], 1:500, Biosource, Camarillo, CA (Cowley et al., 1994); GABA, 1:1000, Sigma-Aldrich(Hoskison et al., 2007); L-type channel (α1C), 1:200, Alomone Lab, Jerusalem Israel (Liang et al., 2003); vGluT1, 1:1000, Sigma-Aldrich (Hendrickson et al., 2006)) overnight at 4°C then with secondary antibody for 1hr at RT (Cy3 labeled anti-rabbit or anti-mouse, 1:1000, Amersham Bioscience, Buckinghamshire, UK). Slides were mounted with mounting media with DAPI (Vector Laboratories, Burlingame, CA, USA) before sealed. Images were taken and quantified with Slidebook Digital Imaging System.
4.6 Western blot for ERK phosphorylation
E2 or E2-BSA was added to the cultures for 30min at 37°C. Cells were washed twice with 4°C PBS and lysed by incubation in lysis buffer (0.005% SDS, 0.1% IGEPAL in PBS) with protease and phosphatase inhibitors for 30 min at 4°C. Cell lysates were cleared by centrifugation at 12,000 rpm for 10 min and total proteins in supernatants were collected for following analysis. Total proteins were analyzed by SDS-PAGE probed with antibodies against active ERK (ERK1/2 [pTpY185/187], 1:750, Biosource (Wu et al., 2005)) and normalized with probes for the total ERK (1:2500, Santa Cruz Bio Tech, Santa Cruz, CA, USA). The membranes were incubated with horseradish peroxidase conjugated secondary antibodies (1:3,000, Vector Laboratories), and results were visualized with TMB peroxidase substrate kit (Vector Laboratories). Relative amounts of protein were quantified by optical density analysis using UnScan-It software (Silk Scientific, Orem, UT, USA). To avoid inter-assay variations, the values obtained were normalized with the value measured for the vehicle-treated control cultures in each experiment. Data are presented as the mean ±SEM from 3 independent experiments.
4.7 Statistics
Data were analyzed with Sigma Stat software (Systat Software, Inc., Point Richmond, CA, USA). Statistically significant differences between groups were determined by a one-way ANOVA followed by Student-Newman-Keuls multiple comparison analysis. All values are expressed as mean ±SEM with p<.05 considered as minimum confidence level.
Acknowledgments
This research was supported by the National Institute on Aging Grants 2R01AG032236 and 5P01AG026572 (to RDB) and the Kenneth T. and Eileen L. Norris Foundation (to RDB).
List of abbreviations used
- E2
17β-estradiol
- BSA
bovine serum albumin
- FITC
fluorescein isothiocyanate
- ERK
extracellular signal-regulated kinase
- GABA
γ-amino butyric acid
- MAP kinase
mitogen-activated protein kinase
- vGluT1
vesicular glutamate transporter 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–7. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, Castanas E. Activation of membrane estrogen receptors induce pro-survival kinases. J Steroid Biochem Mol Biol. 2006;98:97–110. doi: 10.1016/j.jsbmb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Benten WP, Lieberherr M, Giese G, Wunderlich F. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Lett. 1998;422:349–53. doi: 10.1016/s0014-5793(98)00039-8. [DOI] [PubMed] [Google Scholar]
- Beyer C, Raab H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. Eur J Neurosci. 1998;10:255–62. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- Beyer C, Pawlak J, Karolczak M. Membrane receptors for oestrogen in the brain. J Neurochem. 2003;87:545–50. doi: 10.1046/j.1471-4159.2003.02042.x. [DOI] [PubMed] [Google Scholar]
- Boyan BD, Sylvia VL, Frambach T, Lohmann CH, Dietl J, Dean DD, Schwartz Z. Estrogen-dependent rapid activation of protein kinase C in estrogen receptor-positive MCF-7 breast cancer cells and estrogen receptor-negative HCC38 cells is membrane-mediated and inhibited by tamoxifen. Endocrinology. 2003;144:1812–24. doi: 10.1210/en.2002-221018. [DOI] [PubMed] [Google Scholar]
- Brinton RD. 17 beta-estradiol induction of filopodial growth in cultured hippocampal neurons within minutes of exposure. Molecular and Cellular Neurosciences. 1993;4:36–46. doi: 10.1006/mcne.1993.1005. [DOI] [PubMed] [Google Scholar]
- Brinton RE. Psychobiology & Neuropharmacology. University of Arizona; Tucson: 1984. Neuropharmalogical Investigation of Vasopressin, A Putitive Memory Neural Peptide. [Google Scholar]
- Carrer HF, Cambiasso MJ, Gorosito S. Effects of estrogen on neuronal growth and differentiation. J Steroid Biochem Mol Biol. 2005;93:319–23. doi: 10.1016/j.jsbmb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca(2+) signaling in mouse dorsal root ganglion neurons. Journal of Neuroscience Research. 2005;81:31–7. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW. Dissecting the basis of nongenomic activation of endothelial nitric oxide synthase by estradiol: role of ERalpha domains with known nuclear functions. Mol Endocrinol. 2005;19:277–89. doi: 10.1210/me.2004-0008. [DOI] [PubMed] [Google Scholar]
- Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–25. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neurophamacology. Oxford University Press; New York, NY: 1978. [Google Scholar]
- Cordey M, Gundimeda U, Gopalakrishna R, Pike CJ. Estrogen activates protein kinase C in neurons: role in neuroprotection. J Neurochem. 2003;84:1340–8. doi: 10.1046/j.1471-4159.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–52. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor alpha 46-kDa isoform in human endothelial cells: relationship to acute activation of nitric oxide synthase. Circulation. 2003;107:120–6. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. Journal of Neurophysiology. 1999;81:925–9. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. Journal of Neuroscience. 1996;16:3620–9. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- Hart SA, Patton JD, Woolley CS. Quantitative analysis of ER alpha and GAD colocalization in the hippocampus of the adult female rat. Journal of Comparative Neurology. 2001;440:144–55. doi: 10.1002/cne.1376. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123:949–62. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Djajadi H, Erickson A, Possin D. Development of the human retina in the absence of ganglion cells. Exp Eye Res. 2006;83:920–31. doi: 10.1016/j.exer.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Hoskison MM, Yanagawa Y, Obata K, Shuttleworth CW. Calcium-dependent NMDA-induced dendritic injury and MAP2 loss in acute hippocampal slices. Neuroscience. 2007;145:66–79. doi: 10.1016/j.neuroscience.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. European Journal of Pharmacology. 2000;400:205–9. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Molecular Endocrinology. 2005;19:1951–9. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–60. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- Marquez DC, Pietras RJ. Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene. 2001;20:5420–30. doi: 10.1038/sj.onc.1204729. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98:7093–100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–71. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology. 2008;149:3306–12. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, Toran-Allerand CD. Estradiol (E2) elicits SRC phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology. 2001;142:5145–8. doi: 10.1210/endo.142.12.8546. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2842–7. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. Faseb J. 1995;9:404–10. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Lorenzon N, Handa RJ. Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain Research Molecular Brain Research. 2000;80:260–8. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A splice variant of estrogen receptor beta missing exon 3 displays altered subnuclear localization and capacity for transcriptional activation. Endocrinology. 2001;142:2039–49. doi: 10.1210/endo.142.5.8130. [DOI] [PubMed] [Google Scholar]
- Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19:1606–17. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Molecular Endocrinology. 1999;13:307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–46. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–65. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Ross EM. Pharmacodynamics: Mechanisms of Drug Action and the Relation. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Pergamon Press; Burlington, MA: 1990. pp. 35–56. [Google Scholar]
- Rudick CN, Woolley CS. Estradiol induces a phasic Fos response in the hippocampal CA1 and CA3 regions of adult female rats. Hippocampus. 2000;10:274–83. doi: 10.1002/1098-1063(2000)10:3<274::AID-HIPO8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–15. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Hodgson AJ, Chubb IW, Penke B, Erdei A. Antisera to gamma-aminobutyric acid. II. Immunocytochemical application to the central nervous system. J Histochem Cytochem. 1985;33:240–8. doi: 10.1177/33.3.2579123. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–20. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–96. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Brinton RD. Analysis of estrogen receptor splice variants responsible for neuroprotection. Abstract Viewer/Itinerary Planner. Society for Neuroscience. 2003 Online., Program No 504.16. [Google Scholar]
- Watson CS, Campbell CH, Gametchu B. The dynamic and elusive membrane estrogen receptor-alpha. Steroids. 2002;67:429–37. doi: 10.1016/s0039-128x(01)00172-6. [DOI] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC. Signaling from the membrane via membrane estrogen receptor-alpha: estrogens, xenoestrogens, and phytoestrogens. Steroids. 2005;70:364–71. doi: 10.1016/j.steroids.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57:309–20. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–63. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17beta-estradiol induced Ca(2+) influx via L-type calcium channels activates the SRC/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou de S, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Structure-based virtual screening for plant-based ERbeta-selective ligands as potential preventative therapy against age-related neurodegenerative diseases. J Med Chem. 2005;48:3463–6. doi: 10.1021/jm0490538. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]


