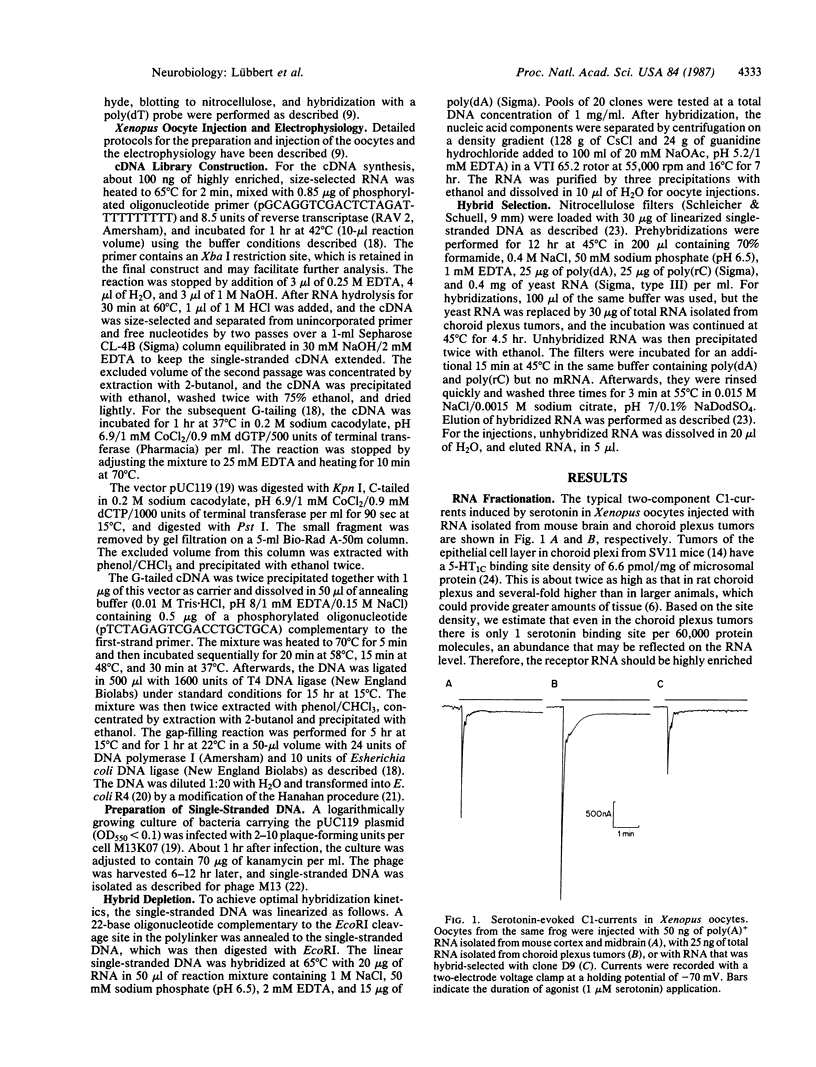
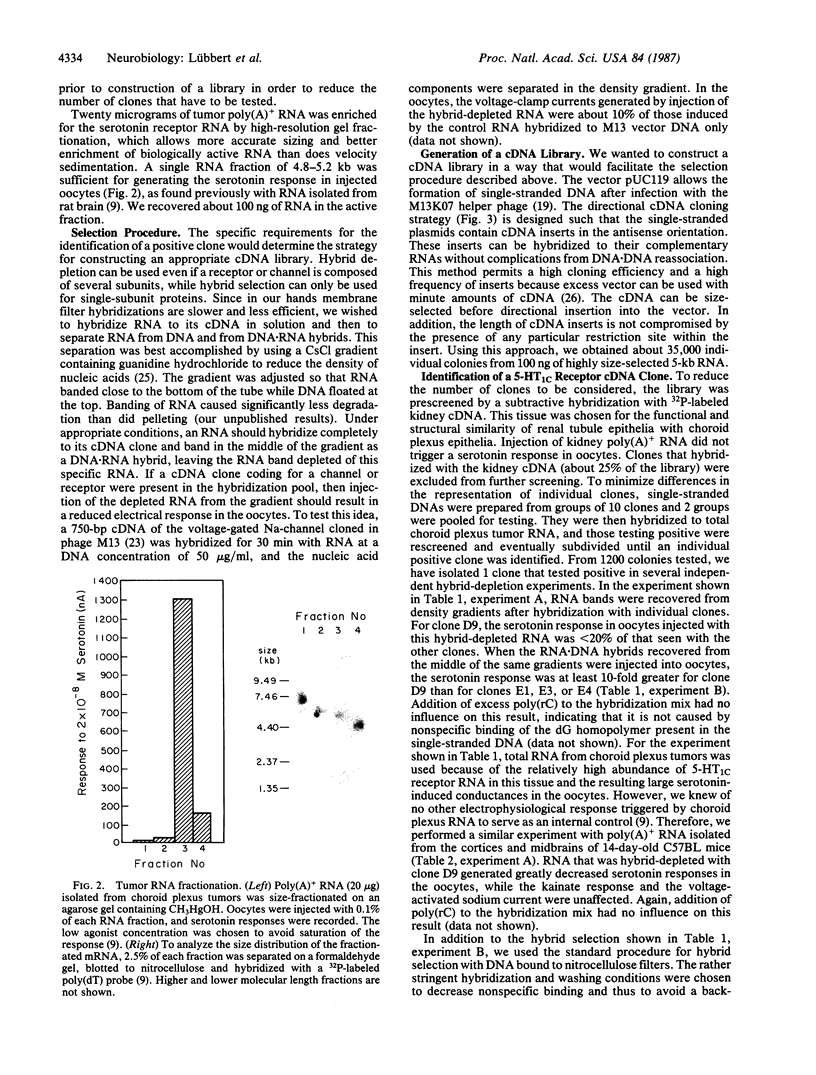
Abstract
We describe a strategy for the cloning of neurotransmitter-receptor and ion-channel cDNAs that is based on electrophysiological assays of mRNA-injected Xenopus oocytes. This procedure circumvents the purification of these membrane proteins, which is hindered by their low abundance and their hydrophobic nature. It involves methods for RNA fractionation by high-resolution gel electrophoresis, directional cDNA cloning in a single-stranded vector, and screening of the cDNA library by voltage-clamp measurements of currents induced by serotonin in mRNA-injected oocytes. The applicability of our approach is demonstrated by the isolation of a serotonin receptor cDNA clone from a mouse choroid plexus papilloma. The clone was identified by hybrid-depletion and hybrid-selection procedures. The receptor expressed in oocytes injected with hybrid-selected RNA is fully functional, indicating that it is composed of a single subunit encoded by a 5-kilobase RNA. The pharmacology of the hybrid-selected receptor confirms that we have successfully cloned a serotonin 5-HT1C receptor cDNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Messing A., van Dyke T., Levine A. J., Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984 Jun;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Erlitz F. L. Use of primer-restriction-end adapters in a novel cDNA cloning strategy. Gene. 1985;34(2-3):305–314. doi: 10.1016/0378-1119(85)90139-8. [DOI] [PubMed] [Google Scholar]
- Dascal N., Ifune C., Hopkins R., Snutch T. P., Lübbert H., Davidson N., Simon M. I., Lester H. A. Involvement of a GTP-binding protein in mediation of serotonin and acetylcholine responses in Xenopus oocytes injected with rat brain messenger RNA. Brain Res. 1986 Dec;387(3):201–209. doi: 10.1016/0169-328x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. Guanidinium-CsCl density gradients for isopycnic analysis of nucleic acids. Science. 1975 Nov 7;190(4214):584–586. doi: 10.1126/science.1188358. [DOI] [PubMed] [Google Scholar]
- Goldin A. L., Snutch T., Lübbert H., Dowsett A., Marshall J., Auld V., Downey W., Fritz L. C., Lester H. A., Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kowalski J., Smith J. H., Ng N., Denhardt D. T. Vectors for the direct selection of cDNA clones corresponding to mammalian cell mRNA of low abundance. Gene. 1985;35(1-2):45–54. doi: 10.1016/0378-1119(85)90156-8. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Snutch T. P., Dascal N., Lester H. A., Davidson N. Rat brain 5-HT1C receptors are encoded by a 5-6 kbase mRNA size class and are functionally expressed in injected Xenopus oocytes. J Neurosci. 1987 Apr;7(4):1159–1165. doi: 10.1523/JNEUROSCI.07-04-01159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemiss D. N., Fozard J. R. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol. 1983 May 20;90(1):151–153. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Pazos A., Hoyer D., Palacios J. M. The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. Eur J Pharmacol. 1984 Nov 27;106(3):539–546. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- Pedigo N. W., Yamamura H. I., Nelson D. L. Discrimination of multiple [3H]5-hydroxytryptamine binding sites by the neuroleptic spiperone in rat brain. J Neurochem. 1981 Jan;36(1):220–226. doi: 10.1111/j.1471-4159.1981.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Multiple serotonin receptors: differential binding of [3H]5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979 Nov;16(3):687–699. [PubMed] [Google Scholar]
- Saha B. K., Strelow S., Schlessinger D. Electrophoretic elution of nucleic acids from acrylamide and agarose gels. J Biochem Biophys Methods. 1983 Jul;7(4):277–284. doi: 10.1016/0165-022x(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Van Dyke T., Finlay C., Levine A. J. A comparison of several lines of transgenic mice containing the SV40 early genes. Cold Spring Harb Symp Quant Biol. 1985;50:671–678. doi: 10.1101/sqb.1985.050.01.082. [DOI] [PubMed] [Google Scholar]
- Yagaloff K. A., Hartig P. R. 125I-lysergic acid diethylamide binds to a novel serotonergic site on rat choroid plexus epithelial cells. J Neurosci. 1985 Dec;5(12):3178–3183. doi: 10.1523/JNEUROSCI.05-12-03178.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagaloff K. A., Lozano G., van Dyke T., Levine A. J., Hartig P. R. Serotonin 5-HT1C receptors are expressed at high density on choroid plexus tumors from transgenic mice. Brain Res. 1986 Oct 22;385(2):389–394. doi: 10.1016/0006-8993(86)91089-9. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Boeke J. D. The filamentous phage (Ff) as vectors for recombinant DNA--a review. Gene. 1982 Jul-Aug;19(1):1–10. doi: 10.1016/0378-1119(82)90183-4. [DOI] [PubMed] [Google Scholar]