Abstract
CD4+ T cell depletion is a fundamental component of HIV infection and AIDS pathogenesis, and is not always reversed following anti-retroviral therapy (ART). Here the SIV-infected Rhesus macaque model was utilized to assess recombinant simian IL-7 in its glycosylated form (rsIL-7gly) to enhance regeneration of CD4+ T cells, particularly the crucial central memory (CM) compartment, post ART. We assessed the impact of rsIL-7gly administration as single injections and as a cluster of three doses. Irrespective of the dosing strategy utilized, the rsIL-7gly administration transiently increased proliferation of both CM and naïve cells, in both CD4+ and CD8+ subsets without increasing SIV levels in the blood. Administration of rsIL-7gly at intervals of 4-6 weeks maximized the proliferative response to therapy, but resulted in only transient increases in peripheral blood T cell counts. However, more frequent rsIL-7gly ‘clustered’ dosing (weekly × 3, with 2 weeks rest and then repeat) induced only an initial proliferative burst by CD4+ T cells, this dosing strategy resulted in sustained increases in peripheral blood CD4+ T cell counts. The clustered rsIL-7gly treatment regimen was shown to increase the half-life of a bromodeoxy-uridine (BrDU) label among memory T cells in the blood when compared to macaques treated with ART alone, consistent with enhanced cell survival. These results indicate that dosing intervals have a major impact on the response to rsIL-7gly in SIV+ ART treated RM, and that optimum dosing strategies may be ones that induce CD4+ T cell proliferation initially and provide increased CD4+ T cell survival.
Introduction
The SIV/macaque model is closely analogous to HIV infection in humans including high levels of viral replication, elevated levels of immune activation (1-4), rapid depletion of CD4+ cells at mucosal sites (5-8) and gradual depletion of peripheral blood CD4+ T cells (7, 8). Current anti-retroviral therapies are sufficient to reduce the rate of viral replication, thus slowing the loss of HIV susceptible CD4+ T cells and facilitating regeneration (9). However, 5-20% of patients exhibit a discordant outcome to highly active anti-retroviral therapy (HAART) resulting in an effective suppression of viral replication but only minimal CD4+ T-cell recovery (10-12). These discordant patients are most likely to benefit from therapy options designed to compliment the existing HAART regimens to restore the host's immune system. Interleukin-7 (IL-7) is a candidate immune therapeutic that could be used in conjunction with HAART to improve CD4+ T cell recovery in this patient population (13-16).
CD4+ T cells can be subdivided into naïve (N), or antigen inexperienced, and memory, or antigen experienced, cells (17). The memory population can be further subdivided into lymphoid tissue-based central memory (CM) cells, effector-site targeted transitional effector memory (TrM) and fully differentiated effector memory (EM) cells (18, 19). The latter 2 populations express high levels of CCR5 and are the primary targets of HIV and SIV, with the CM population serving as the major source of effector memory generation (20). The macaque model has been critical to understanding how the loss of each cell population contributes to AIDS progression (20-23). In particular it has been appreciated that while overt immunosuppression is associated with destruction of the CD4+ EM compartment, the failure of this compartment can be traced to homeostatic failure of the CM population and inadequate TrM and EM production (24-26). Therefore, an immune therapy targeted to assist in the recovery of the CD4+ T cells in blood as well as effector sites would be useful to complement current anti-retroviral drugs for treatment of some patients.
Cytokines involved in T-cell homeostasis and regulation such as Interleukin-2 (IL-2), Interleukin-15 (IL-15) and Interleukin-7 (IL-7) have been proposed as immune therapeutics for HAART treated HIV+ patients (13, 15, 16, 27). The receptor for these cytokines share a common gamma chain paired with a unique high affinity alpha chain receptor (28-30). IL-2 has been the most thoroughly studied candidate thus far, and initially exhibited some promising results although it was associated with a number of negative side effects (31-39). However, two recent large clinical trials, SILCAAT and ESPRIT, were unable to demonstrate any clear clinical benefit for IL-2 therapy iin HIV-infected patients (31). IL-15 has been less well studied and while an effect on proliferation of CD4+ and CD8+ EM T cells has been observed (40-43), it also increases the susceptibility of CD4+ cell subsets to SIV infection (44, 45) confounding its use as an immune therapy. The potential for IL-7 as an immune therapeutic lies in its non-redundant role for maintaining T cell levels, both in increasing proliferation and preventing apoptosis in naïve and central memory (CM) CD4+ and CD8+ T cells (14, 22, 46-51). Previous studies in the macaque model have shown that in both treatment naïve and ART treated macaques, IL-7 has little discernable impact on plasma viremia (14, 52-54). These studies have also contributed a great deal to documenting the expansion of therapeutically relevant cell populations including the CD4+ naïve and CM subsets (14, 52-56). In non-human primate models, IL-7 can impact both the CD4+ and CD8+ T cell compartments (14, 52-57). Indeed, the effects of IL-7 were not limited to the peripheral blood and included both lymph nodes as well as other organs (e.g. spleen, lung, kidney and gut) (55, 56, 58). Most of these studies however used nonglycosylated rmIL-7 (14, 52, 54, 55-57) and in some instances, the animals developed neutralizing antibodies (52, 54, 57). Recently, IL-7 administration to both cancer and HIV+ patients demonstrated similar increases in CD4+ T cells indicating that IL-7 therapy could increase naïve, CM, TrM and EM CD4+ T cells (59-62). The goals of these studies were three-fold: 1) To assess the ability of IL-7 administered to SIV+ macaques undergoing ART to increase CD4+ T cell levels; 2) To determine the importance of CD4+ T cell proliferation for any observed IL-7 induced CD4+ T cell increases; 3) To investigate the ability of different IL-7 regimens to achieve a CD4+ T cell recovery. These data provide evidence that IL-7 can be an effective immune therapy in the SIV+ macaques that are on effective anti-retroviral therapy. In addition, these data provide insights into factors that contribute to a successful outcome following IL-7 treatment, including the identification of an optimum dosage and schedule for IL-7 to increase CD4+ T cell levels.
Materials and Methods
Animals and Viruses
A total of 17 Indian rhesus macaques (macaques) were housed at the Oregon National Primate Research Center in accordance with standards of the Center's Animal Care and Use Committee and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Macaques enrolled in this study were between five and seven years of age and were deemed free of Cercopithicine herpesvirus 1, D type simian retrovirus, simian T-lymphotropic virus type 1, and SIV infection. Of those 17 macaques, 8 remained SIV negative and were used as healthy controls. 9 animals were infected with SIVmac239 intravenously using 5ng equivalents of SIV p27 (1.0 ×105 infectious centers). During chronic infection (>100 days post infection) these macaques were treated with anti-retroviral therapy (ART) consisting of PMPA and FTC. ART treatment was undertaken for a minimum of 37 days prior to rsIL-7gly administration to allow CD4+ T cell counts and viral load to stabilize. The number of baseline CD4+ and CD8+ T cells per microliter of blood prior to the administration of rsIL-7gly are presented such that the fold change information is more meaningful (Sup. Table 1). Macaques enrolled in sequential studies were given a minimum of a ten-week interval between protocols to allow the animals to return to a basal state. The following SIV-infected macaques were enrolled in the dosing interval study RM2-01 (ONPRC number: 23185), RM2-02 (23772), RM2-03 (23186), RM2-04 (23208), RM2-05 (22657), and RM2-06 (23788) (Table I). These animals were treated daily with PMPA (30mg/kg) and FTC (50mg/kg). Two rsIL-7gly doses were administered one week apart (Table I). A third rsIL-7gly dose was given to 2 macaques at either 2, 4, or 6 weeks after the last rsIL-7gly dose (Table I). RM2-01, RM2-03, and RM2-04 were then used to test the efficacy of a single rsIL-7gly dose at 6-week dosing intervals (three total doses) while still being stably treated with PMPA (30mg/kg) and FTC (50mg/kg) for the remainder of the study period. To test the efficacy of clustering rsIL-7gly doses, two macaques RM2-05 and RM2-07 (23201), received three doses of rsIL-7gly administered at weekly intervals followed by a three week “wash-out” during which no rsIL-7gly was administered the series was repeated three times (Table I). ART treated controls for this series of experiments were: RM2-02, RM2-06, RM2-08 (23092) and RM2-09 (23892). Both groups of animals (ART and ART+rsIL-7gly) were given 4 weeks of daily ART (PMPA (30mg/kg) and FTC (50mg/kg)) that was reduced to a maintenance dose (20 mg/kg PMPA; 20 mg/kg FTC). None of the macaques enrolled in these studies reached disease states that were not clinically manageable as defined by the presence of AIDS-defining opportunistic infections, wasting syndrome unresponsive to therapy, or non-Hodgkin lymphoma.
TABLE I. rsIL-7gly dosing regimens administered.
Rhesus macques were divided into 4 different dosing schemes over the course of these studies. Table 1 shows the number of animals, the number of rsIL-7gly doses each animal received during the study and the interval between rsIL-7gly doses. Some macaques were used in multiple studies and in each case the interval between studies was greater than 10 weeks.
| Study Title | RM# | Total # of rsIL-7gly doses | Interval between doses | Days of rsIL-7gly Administration |
|---|---|---|---|---|
| rsIL-7gly treatment in SIVneg RMs | 8 uninfected RMs | 2 | 2 doses of rsIL-7gly at a one week interval | 0,7 |
| rsIL-7gly 2, 4 or 6 week Intervals | RM2-01 | 3 | 2 doses of rsIL-7gly at one week interval (N=6); followed by one dose at 2, 4 or 6 week intervals (N=2) | 0,7 21or 35 or 49 |
| RM2-02 | ||||
| RM2-03 | ||||
| RM2-04 | ||||
| RM2-05 | ||||
| RM2-06 | ||||
| rsIL-7gly 6-week intervals | RM2-01 | 3 | One rsIL-7gly dose every 6 weeks | 0,42,84 |
| RM2-03 | ||||
| RM2-04 | ||||
| Clustered rsIL-7gly Doses | RM2-05 | 9 | 3 doses of rsIL-7gly at one week intervals followed by two weeks without; repeated three times | 0,7,14, 35,42,49, 70,77,84 |
| RM2-07 | ||||
| ART controls | RM2-02 | 0 | n/a | n/a |
| RM2-06 | ||||
| RM2-08 | ||||
| RM2-09 |
Interleukin-7 Treatment
Animals were treated with glycosylated recombinant macaque Interleukin-7 (rsIL-7gly) produced in Chinese hamster ovary (CHO) cells and glycosylated at a minimum of 3/5 possible sites (2/4 N-linked and 1/1 O-linked sites) (Cytheris). rsIL-7gly (30ug/kg) was administered subcutaneously in three dosing regimens with macaques receiving one, two or three weekly doses (Table I). All macaques, except macaques serving as ART controls during the clustered dosing regimen, were treated with rsIL-7gly during the chronic phase of infection with cytokine administration beginning from 142-492 days post infection while the macaques were on stable optimally suppressive ART regimens. Macaques used in multiple dosing studies were rested for a minimum of 10 weeks between studies. T-cell dynamics were monitored in peripheral blood using flow cytometry up to 112 days following treatment with rsIL-7gly. No anti-IL-7 neutralizing antibodies were detected in these macaques.
In-Vivo Labeling with 5-bromo-2′-deoxyuridine (BrdU)
5-bromo-2′-deoxyuridine (BrdU) labeling was used to label cells in-vivo in macaques enrolled in the cluster dosing study as previously described (8).
Plasma Viral Load Quantification
Plasma viral load was determined using a real time RT-PCR assay essentially as described previously (63). The threshold sensitivity of the assay, as used for this study, was 30 SIVgag RNA copy eq/mL.
Flow Cytometric Analysis
Cells for flow cytometry were obtained from whole blood as previously described (64). Polychromatic flow cytometry was performed on an LSR-II (Becton Dickinson) and FlowJo (Treestar) software was used for analysis. Naïve and memory T-cell phenotypes were defined using the markers CD28, CD95, CCR5 and CCR7 after gating CD3+CD4+ or CD3+CD8+ T cells. The criteria used to identify naïve and memory T cells have been previously described (65). Briefly, naïve T cells are a uniform population expressing the following combination of markers; CD28moderate, CD95low, CCR7moderate, CCR5-, in both CD4+ and CD8+ T cells. The memory T-cell phenotype is more diverse but is generally CD95high and displays one or more non-naïve cell phenotypes, including the proteins CD28 CCR7 and CCR5. Memory T cells can be further subdivided into central memory (CM) subset (CD28high, CCR5-, CCR7+) and two effector site targeted subsets with progressive effector differentiation: transitional effector memory (TrM: CD28high, CCR5+ and/or CCR7-) and fully differentiated (EM: CD28-, CCR5dim+, CCR7-).
mAbs
The following fluorophore conjugated monoclonal antibodies were obtained from Beckton Dickinson Biosciences: SP34-2 (CD3; Alexa 700), L200 (CD4; AmCyan), SK-1 (CD8a; PeCy-7, PerCP-Cy5.5, APC-Cy7), DX2 (CD95; PE, PE-Cy7), hIL-7R-M21 (CD127; PE), 3A9 (CCR5; APC); B56 (Ki67; FITC, PE); L27 (CD20; True Red); B44 (BrDU; FITC). The clone 28.2 (CD28; PE-TexasRed) was obtained from Beckman Coulter. The purified, unconjugated antibody 15053 (CCR7) was obtained from R&D Systems and conjugated to biotin (Pierce Biotinylation kit). CCR7-biotin was detected using a pacific blue conjugated streptavidin from Invitrogen. FN-18 (CD3) was produced and purified and conjugated to Alexa 700 using an Invitrogen conjugation kit.
Results
rsIL-7gly administration in SIV-negative Rhesus and SIV+ ART treated macaques induces T-cell proliferation and increases T-cell numbers in the circulation
Previous work has demonstrated that both non-glycosylated and glycosylated recombinant macaque interleukin-7 (IL-7) exhibited induced proliferation and expansion of peripheral blood T cells (14, 52, 54, 56, 58). Here the assessment of recombinant simian IL-7 glycosylated (rsIL-7gly) was initially undertaken in a two dose regimen (30ug/kg with a 7 day interval) to assess proliferation in-vivo in uninfected macaques (grey line) and compared to results obtained in SIV+ ART treated macaques (black line) (Figure 1). Flow cytometric evaluation of the percentage of cells expressing the nuclear antigen Ki67 (a marker for cycling through S phase within the previous 4-7 days (65) revealed a consistent and robust rsIL-7gly associated increase in the percentage of Ki67+ CD4+ and CD8+ cells in the blood. This increased proliferation was observed in both the uninfected and SIV+ ART treated macaques, and within each of the T cell subsets assessed including naïve, central memory (CM), transitional memory (TrM) and effector memory (EM) (Figure 1). However, distinctions in the rsIL-7gly response were observed, as the percent of Ki67+ T cells in CM and TrM T cells increased rapidly and robustly; whereas the rsIL-7gly response in the naïve and EM cells was delayed and generally less robust (with the exception of CD8+ naïve T cells which proliferated robustly in response to rsIL-7gly (Figure 1). For the naïve, CM and TrM subsets the percentage of CD4+ and CD8+ T cells expressing Ki67 returned to near baseline levels by day 21 post-rsIL-7gly therapy, however some proliferation could be observed in the EM subset after 21 days. As EM express low levels of IL-7 receptor (CD127) on their cell surface (56, 66-76), the proliferation of this subset likely reflects subsequent differentiation of IL-7 stimulated CM and TrM T cells, rather than a direct effect on pre-exisiting EM themselves. Importantly, irrespective of the T cells subset assessed, the differences observed in Ki67 expression were not significantly different between the uninfected and SIV+ ART treated macaque (grey line compared to black line, Figure 1).
Figure 1. Comparing Ki67 induction in T-cell subsets in SIV- and SIV+ART RMs.
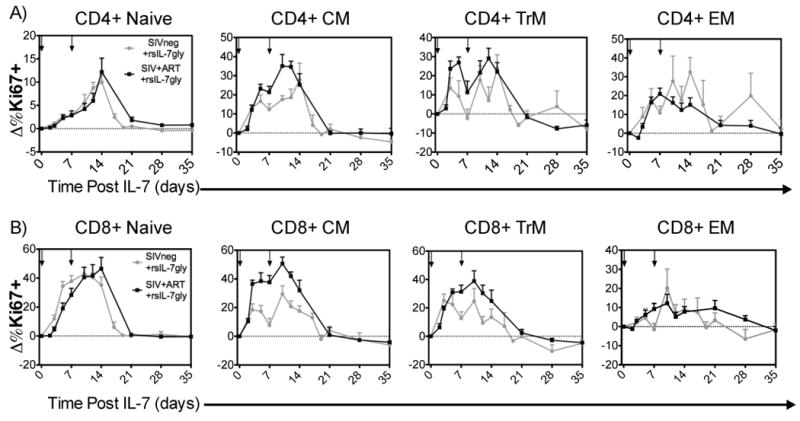
rsIL-7gly was administered where indicated [↓]. The nuclear antigen Ki67 was used to assess proliferating cells, pre-rsIL-7gly levels of proliferation were determined by averaging the percentage of Ki67+ cells in each subset over three timepoints. The difference in Ki67 expression from pre-rsIL-7gly levels was determined for each subsequent timepoint and is presented as the mean±standard error of the mean (SEM). The dashed horizontal line indicates pre-rsIL-7gly Ki67 levels. (A) Change in percent Ki67+ cells in CD4+ subsets including naïve, central memory (CM), transitional memory (TrM), and effector memory (EM); (B) change in percent Ki67+ cells in CD8+ subsets. Uninfected macaques (N=8; grey), SIV+ART treated macaques (N=6; black)
The rsIL-7gly induced proliferation of the different T cell subsets would be predicted to have an impact on the numbers of T cells in each subset. Here the changes in T cell levels are depicted as the fold change relative to the number of T cells that were present prior to the onset of rsIL-7gly therapy (therefore a 2 fold change reflects of doubling of the T cells present per microliter of blood). Comparing the uninfected (grey line) and SIV+ ART treated macaques (black line) we determined that the T cells generally respond to a greater extent in the uninfected macaques, with the exception of the CM CD4+ T cells which increased at comparable levels in both groups of macaques (Figure 2). Overall, these data indicate that even after ART treatment, the SIV+ macaques have a reduced potential to increase T cell levels in response to rsIL-7gly when compared to uninfected macaques. However, there is also indication of the potential utility of IL-7 as a therapy for HIV+ patients as a two fold or greater change was observed in the CM, TrM and EM CD4+ T cell subsets following rsIL-7gly treatment.
Figure 2. Comparing fold change in T-cell subset levels in SIV- and SIV+ART RMs.
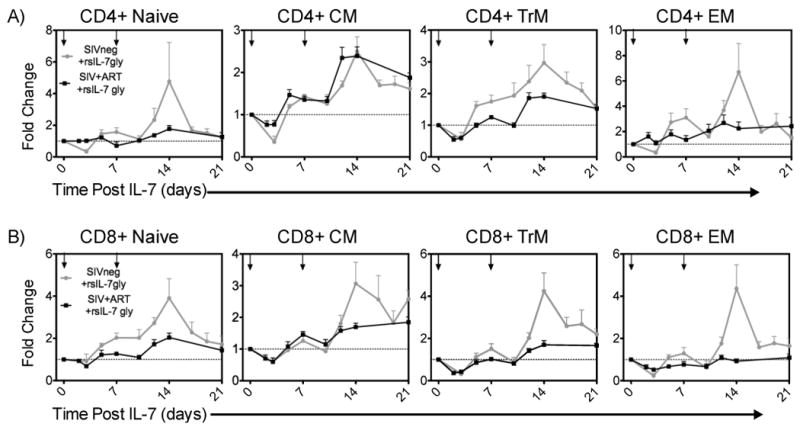
rsIL-7gly was administered where indicated [↓]. Pre-rsIL-7gly T-cell levels were determined by averaging the number cells in each subset over three timepoints. The fold change from baseline was determined for each subsequent timepoint. The dashed horizontal line indicates pre-rsIL-7gly levels. The data is presented as the mean±SEM. (A) Fold change in CD4+ subsets naïve, central memory (CM), transitional memory (TrM), and effector memory (EM); (B) Fold change in CD8+ subsets. Uninfected macaques (N=8; grey), SIV+ART treated macaques (N=6; black).
Timing of rsIL-7gly administration in ART treated SIV+ Rhesus macaques – Dosing Interval Assessment and IL-7 treatment at 6 week intervals
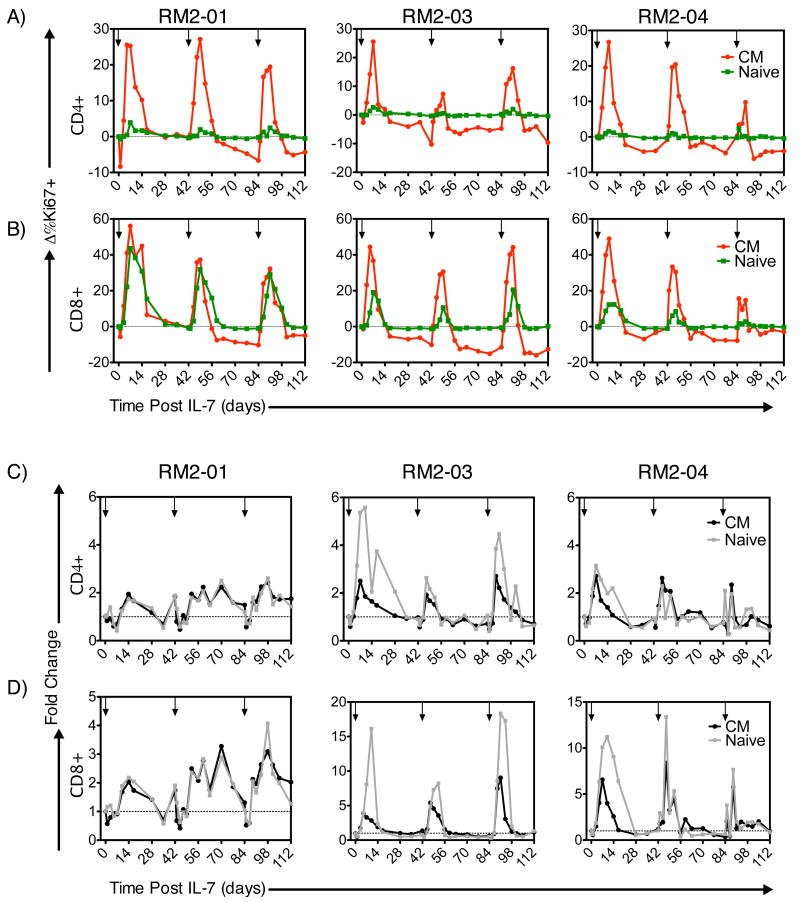
We next sought to determine the optimal spacing between sequential rounds of rsIL-7gly treatment so as to maximize the proliferative response, focusing particular attention to the CD4+ CM and naïve T cell subsets that are likely to be key for any successful CD4+ T cell recovery. Six SIV-infected macaques were treated first with two doses of rsIL-7gly seven days apart, and then the T-cell proliferative response to a third dose of IL-7 after either 2, 4 or 6 weeks was determined (Table I, rsIL-7gly 2,4,or 6 week intervals). Overall, rsIL-7gly administration in these animals was not associated with any long-term increase in plasma SIV levels, although three of the six macaques did exhibit a transient increase in plasma viral load (as high as 10 fold) at day 7 post-treatment (Table II). Administering rsIL-7gly six weeks after the previous exposure elicited robust proliferation in CD4+ and CD8+ CM subsets following each rsIL-7gly dose in RM2-05 and RM2-06 (Figure 3A; Supplemental Table 2). Indeed, following the third dose at the six week interval, macaque RM2-05 elicited the largest increase in the percent change in Ki67 levels (31.7%) when compared to the other macaques in this study. For each of the 6 week third dose interval macaque, the CD4+ CM T cell levels increased, transiently in macaque RM2-06 and in a more sustained manner for macaque RM2-05 (Figure 3D; Supplemental Table 2). Administration of the third rsIL-7gly dose at more closely spaced intervals, either 2 or 4 weeks, resulted in proliferative responses that were more variable and generally lower than those observed in the 6 week third dose macaques (Supplemental Table 2). Similar results were observed in the CD8+ CM and naïve T cells with the best third dose proliferative response being associated with the six week interval and less robust responses observed for the 2 and 4 week intervals (Fig. 3). As one might predict, the most impressive increases in levels of CD4+ CM T cells were associated with the best proliferative response following the third dose as observed in macaques from the 2 week (RM2-01), 4 week (RM2-03) and 6 week (RM2-05) dosing intervals (Fig. 3, Supplemental Table 2). These data led to the hypothesis that providing a rsIL-7gly dosing strategy that optimized proliferative responsiveness in the SIV+ ART treated macaques would provide the optimum long term CD4+ CM T cell increases. We went on to further investigate this hypothesis with two sets of experiments designed to assess a relatively long (6 week) and short (clustered 1 week) rsIL-7gly dosing approaches.
Figure 3. Ki67 response and change in T-cell levels at 2-week dosing intervals.
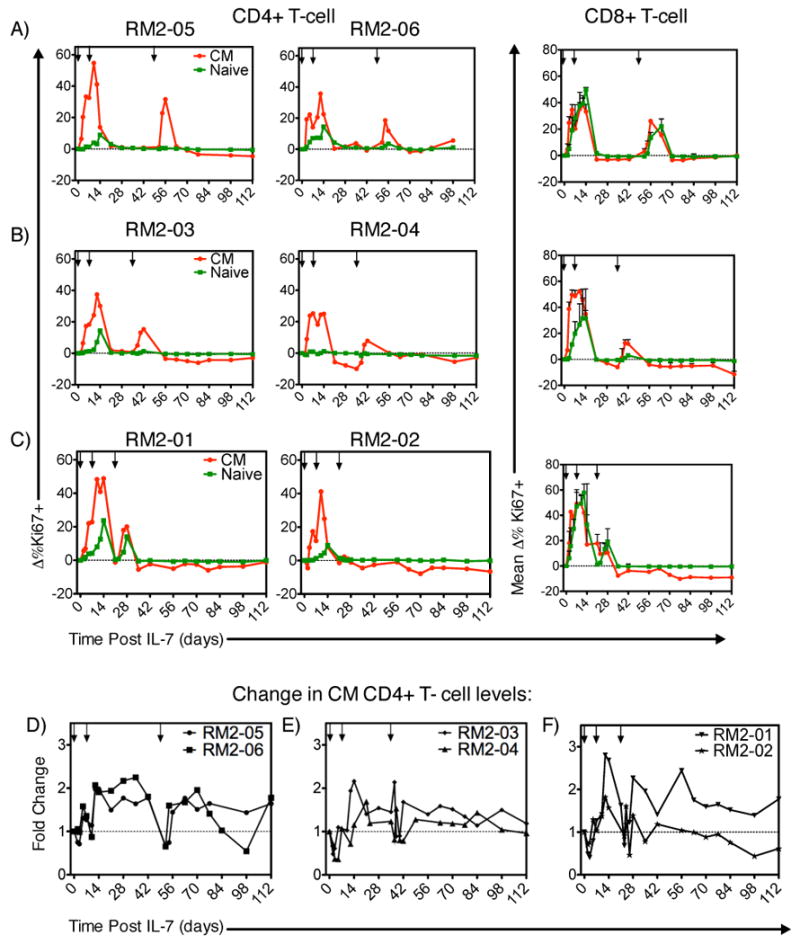
rsIL-7gly induced proliferation (Ki67+) cells was assessed by flow cytometry at 2-week intervals in groups of 2 macaques. The change from baseline was calculated for each subsequent point. rsIL-7gly administration is indicated by [↓]. Central memory (CM) (red) and naive cells (green). (A) Change in percent Ki67+ cells in RM2-05 and RM 2-06. (B) Change in percent Ki67+ cells in RM2-03 and RM 2-04 (C) Change in percent Ki67+ cells in RM2-01 and RM 2-02. The average change in Ki67 expression from both macaques is shown for CD8+ naïve and CM T cells is presented (A-C). The fold change was also determined for the absolute number of CD4+ CM T cells in each of the macaques. (D) Fold change in RM2-05 and RM2-06 (E) Fold change in RM2-03 and RM2-04 (F) Fold change in RM2-01 and RM2-02.
To test whether the 6-week interval administered to RM2-05 and RM2-06 would elicit long-term benefits after repeated exposures, we administered one dose of rsIL-7gly to three ART treated SIV+ macaques at 6 week intervals (Table I, rsIL-7gly 6-week intervals, 3 total doses). This dosing regimen was not associated with any increase in the SIV plasma viremia in these SIV+ ART treated macaques (Table II). Peak Ki67 expression occurred 5-7 days post injection and returned fully to baseline by day 14 (Figure 4). As predicted from the first dosing study (Figure 3), the second and third IL-7 administrations given at 6 week intervals resulted in increased Ki67 expression that was generally comparable to the initial IL-7 response in the CD4+ CM subset (Figure 4A; Supplemental Table 2). The absolute number of CD4+ CM T cells increased as well, averaging 2-2.5 fold increases from baseline levels, in the peripheral blood synchronous with increased Ki67 expression (Figure 4C; Supplemental Table 2). The first rsIL-7gly administration resulted in only a small increase in Ki67 expression in the CD4+ naïve cell subset (Figure 4A). The absolute number of CD4+ naive T cells did however increase in the blood following each dose (Figure 4C). In peripheral blood CD8+ T cells Ki67 expression was elevated in both naïve and CM cells at time points just following the rsIL-7gly administration (Figure 4B). In both subsets the increase in the absolute number of T cells was generally transient and returns to basal levels by 21 to 42 days post rsIL-7gly administration (Figure 4C and 4D). These data indicate that administration of rsIL-7gly in six week dosing intervals does consistently elicit robust proliferation in the CD4+ CM subset following rsIL-7gly, however a sustained increase in CD4+ T cell levels was generally not observed.
Figure 4. Ki67 response and change in T-cell levels at 6-week dosing intervals.
We assessed the change in Ki67+ T cells from pre-rsIL-7gly levels at each time point. Basal Ki67 levels were determined by averaging three rsIL-7gly time points prior to IL-7. rsIL-7gly administration is indicated by [↓]. Central memory (CM) (red) and naïve cells (green) (A) CD4+ T cells (B) CD8+ T cells. We then determined the effect of rsIL-7gly on the absolute number of T cells over time. The fold change was determined from the basal level of each T-cell subset; CM (black) and naïve cells (grey). (C) Fold change in CD4+ T cells (D) Fold change in CD8+ T cells
Assessment of rsIL-7gly utilizing a three dose cluster strategy in ART treated SIV+ macaques
An alternative hypothesis is that closely spaced, or clustered, rsIL-7gly doses as administered to RM2-01 (Figure 3C and 3F) would be able to elicit and sustain long-term increases in T cell levels when administered in multiple rounds. To evaluate the efficacy of weekly clustering rsIL-7gly administration, an experiment was designed which utilized a total of nine rsIL-7gly doses administered over 12 weeks (84 days) to two macaques stably treated with a fully suppressive ART regimen (Table I, Clustered rsIL-7gly doses). The dosing strategy utilized was to administer rsIL-7gly in 3 doses over 3 weeks in a cluster, followed by a two week wash-out period, this regimen was administered three times. As in the previous two dosing strategies, treatment with rsIL-7gly was not directly associated with any long-term increases in plasma viral levels (Table II). The initial three dose cluster of rsIL-7gly administration elicited increased Ki67 expression in CM CD4+ (Figure 5A) and CD8 + T cells (Figure 5B)(Supplemental Table 2). The increase in the percentage of Ki67+ cells resulted in a corresponding increase in the absolute number of proliferating CM cells (CD4+: Figure 5C and CD8+: Figure 5D). Peak Ki67 expression was attained approximately 7 days post rsIL-7gly administration and remained elevated until day 28 suggesting that clustered dosing elicited proliferation over a longer time than a single dose of rsIL-7gly (Figure 5A and 5B)(Supplemental Table 2). Unlike the previous study (Figure 4), rsIL-7gly induced proliferation in both CD4+ (Figure 5A) and CD8+ (Figure 5B) naive T cells, though Ki67 expression was higher in the CD8+ naive T cells. Interestingly, the rsIL-7gly induced Ki67 increase in CD4+ and CD8+ T cell subsets was attenuated in the second and third dose clusters (Figure 5A-D). This attenuated rsIL-7gly response could be observed in each of the T cell subsets with the naïve cells being most affected. The observed reduction in the Ki67 response appeared to be unrelated to CD127 (IL-7R) expression as the percentage of CD127+ cells was similar at the initiation of each cluster (data not shown). Therefore, the clustered dose strategy for administering rsIL-7gly resulted in a robust increase in the number of proliferating cells during the first three dosages that was strongly reduced during the next six rsIL-7gly administrations.
Figure 5. Ki67 response and change in Ki67+ T-cell levels with clustered dosing.
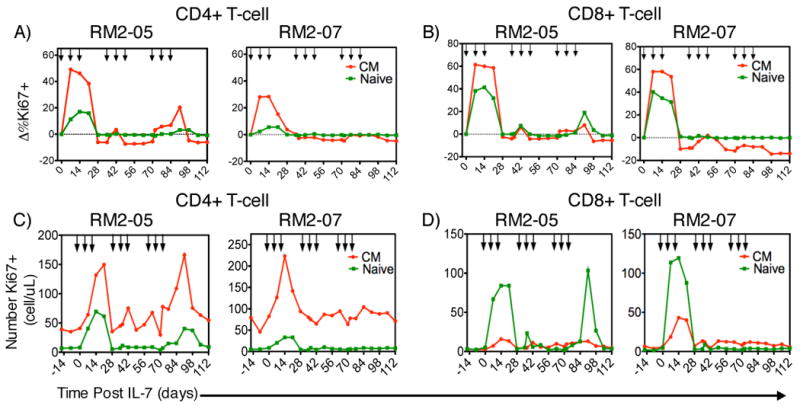
Two macaques treated with stable ART therapy were administered rsIL-7gly at weekly intervals for three weeks followed by a two-week wash out period. Proliferation in peripheral T-cell subsets was monitored by flow cytometry assessing the fraction of Ki67+ cells. Each rsIL-7gly dose is indicated by [↓]. The change in Ki67 expression from baseline levels is shown. Central memory (CM) (red); naïve cells (green) (A) CD4+ T cells (B) CD8+ T cells. We also determined the absolute number of proliferating cells in each macaque in each subset central memory (CM) (red); Naïve cells (green) (C) CD4+ T cells (D) CD8+ T cells.
The ability of rsIL-7gly administered in the clustered doses to impact peripheral blood T cell levels was assessed within the different T cell subsets. The clustered dosing regimen successfully increased peripheral blood levels of both CD4+ and CD8+ T cells within each of the virally suppressed SIV+ macaques in the naïve and CM subsets (Figure 6A and 6B; green lines). Comparing the increase in T-cell number to macaques receiving only ART demonstrates that the effect is rsIL-7gly mediated (Figure 6A and B, grey lines compared to black lines). This dosing regimen successfully increased the T-cell levels in the blood, particularly the CD4+ CM cells, for the entirety of the 112 day study (Figure 6A). In addition, despite low levels of induced Ki67 expression (Figure 5A) naïve CD4+ T cells also increased in the circulation (Figure 6A). Likewise, CD8+ CM and naïve cells showed sustained long-term increases in absolute T-cell number (Figure 6B). In summary, these data indicate that clustering doses of rsIL-7gly in SIV+ ART treated macaques induced proliferation in a broad range of peripheral T-cell subsets during and following the initial cluster of three dosings. Furthermore, the ability to induce a sustained rsIL-7gly related increase in T cell levels appears to be independent of the optimal induction of a Ki67 response in peripheral T-cell.
Figure 6. Fold change in T-cell levels following clustered dosing administration.
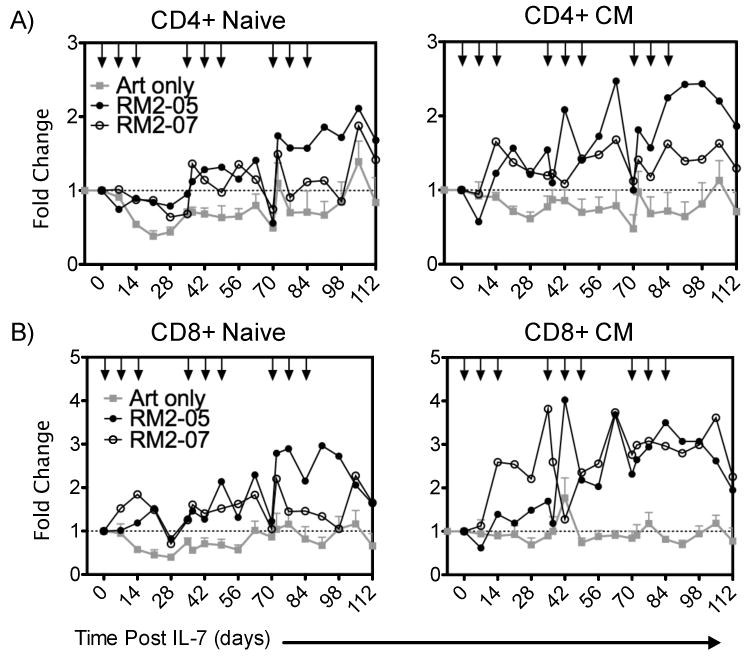
Where applicable rsIL-7gly administration is indicated [↓]. The mean absolute T-cell count of four macaques treated with ART alone (grey square), RM2-05 (closed circle) RM2-07 (open circle) is shown in each figure (mean ± SEM). (A) Fold change in CD4+ T-cell subsets naïve and central memory (CM) (B) Fold change in CD8+ subsets naïve and central memory (CM).
Assessment of BrDU retention in SIV+ ART and rsIL-7gly treated in weekly dosing clusters
To determine whether the rsIL-7gly administrations impacted the retention of proliferating cells we utilized the molecule bromo-deoxy uridine (BrDU) that is incorporated into the DNA of dividing cells and detected by flow cytometry. BrDU was administered for four days following the first of the nine rsIL-7gly doses in the clustered rsIL-7gly dosing study (Table I), as a means of labeling dividing cells. We assessed the rate at which the label was lost in memory T cells in the blood following rsIL-7gly dosing. To determine the rate of loss, the amount of BrDU detected in the T cells was normalized between macaques by setting the percentage of BrDU+ cells at one day post labeling at 100%. In the CD4+ memory cells, the BrDU label declined to 50% of the original amount by 14 to 28 days post-labeling in SIV+ macaques treated with ART only (depicted in grey) but persisted 42 to 63 days post-labeling in macaques treated with rsIL-7gly (depicted in black) (Figure 7A). rsIL-7gly administration had a greater impact on memory CD8+ T cells which retained the label past the end of the follow up period in macaques treated with rsIL-7gly (Figure 7B). Therefore, in this relatively small number of SIV+ ART treated macaques, the addition of rsIL-7gly to these macaques increased the retention of the CD4+ and CD8+ memory cells that had been labeled during the first clustered weekly dosing.
Figure 7. %BrDU Label Retained in Total Memory CD4+ T-cells in the Blood.
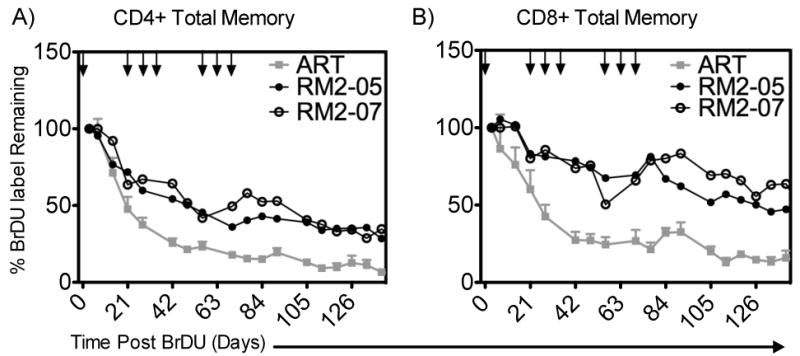
BrDU was used to label cells dividing in response to rsIL-7gly administration following the first of nine doses. The percentage of BrDU+ cell immediately post labeling was set to 100% to normalize the rate of decline in all the macaques. Where applicable rsIL-7gly administration is indicated [↓]. (A) The percentage of BrDU label retained in CD4+ memory cells in the peripheral blood (B) The percentage of BrDU label retained in CD8+ memory cells in the peripheral blood. ART controls (mean +- SEM) (grey square); RN2-05 (closed circle) and RM2-07 (open circle).
Discussion
Immunotherapeutics have the potential to compliment existing therapies that are designed to suppress HIV replication, particularly in those patients in whom CD4+ T cell reconstitution is not optimum following HAART (13-16). Cytokines involved in T cell homeostasis (e.g. IL-2, IL-7 and IL-15) have been proposed as immunotherapeutics during HIV infection (13, 15, 16, 27). The recent failure of IL-2 in clinical trials (31) highlights the challenges identifying and implementing an immune therapeutic treatment in HIV-infected patients. The strength of IL-7 for use as an HIV therapeutic lies in its ability to potentially increase the levels of two important CD4+ T cell subsets, naïve and CM (14, 22, 46-51) through both cycling (58, 77-80) and cell survival (58, 81-84). This activity has already been addressed in non human primates infected with SIV, although the IL-7 utilized in these studies was not optimum as it was not produced in mammalian cells (tended to elicit IL-7 specific neutralizing antibodies), and the dosing strategy was generally for a short duration (14, 52-57). More recently, clinical studies in humans have also been undertaken, one in cancer patients by Sportes et al (62) and two in HIV+ patients conducted by Levy et al (59) and Sereti et al (61). Both Sportes et al and Levy et al document significant expansion of CD4+ and CD8+ T cells lasting from 28 days (62) to 48 weeks (59). Notably, in agreement with the non-human primate model (14, 52-57), the human studies indicate IL-7 administration effectively expands both naïve and memory T cells (59, 62). Although both human studies reported increased cell cycling, the study by Sportes et al also indicated a role for IL-7 mediated T cell survival effects, as opposed to proliferative effects, as the principle contributor to CD4+ T cell expansion (62).
Our approach here was to use the SIV/macaque model to further elucidate the efficacy of immune therapy using rsIL-7gly treatment in a context where ongoing virally mediated killing was minimized. Previous work in the non-human primate model was conducted with 80-100ug/kg/day (14, 54, 55) or regimens of 100ug/kg/day every other day (52, 57, 72). Based on work published in 2007 by Dereuddre-Bosquet et al (57) that provided evidence the Ki67 response to IL-7 administration peaked at 7-day intervals, regardless of IL-7 administration in the interim, we chose a 7 day dosing interval as the minimum, and then further assessed the 2, 4 and 6 week dosing intervals. This study utilized a relatively low dose (30ug/kg/wk) administration of the glycosylated form of recombinant simian IL-7 as well as “wash-out” periods between doses to reduce the frequency of administration while retaining what we hypothesized would be an optimal CD4+ T cell response to rsIL-7gly. In addition, we sought to characterize further in the macaque model rsIL-7gly's impact on different T cell subsets following these different dosing intervals. The dosing interval study (Figure 3) assessed the impact of varying the third dose to a 2, 4 and 6 week spacing in SIV+ ART treated macaque that had previously been administered two weekly injections of rsIL-7gly. Overall, the third dose resulted in a muted Ki67 proliferative response, compared to the earlier doses. We observed that spacing the third dose to a six week interval resulted in best third peak Ki67 response within macaque RM2-05 and a 1.6 fold increase in CD4+ CM cells. In addition, although the 2 week interval third dose was associated with a smaller percentage of Ki67+ CM CD4+ T cells, the numbers of peripheral CM CD4+ T cells still increased to over 2 fold the pre-rsIL-7gly levels. These data enabled us to generate the hypothesis that spacing the rsIL-7gly dosages 6 weeks apart would result in robust Ki67 responses, while closely spacing of the dosages could result in superior fold increases in CM CD4+ T cells.
To more clearly assess the advantages and disadvantages of the different dosing studies we undertook two studies, the first administering rsIL-7gly every six weeks and a second administering the rsIL-7gly in clustered weekly intervals. We repeated each dosing regimen for three cycles assessing the effect of rsIL-7gly on CD4+ and CD8+ naive and CM cells after each dose. When we administered rsIL-7gly at 6-week intervals, we elicited robust proliferation, but little long-term increase in T-cell number. Whereas clustering the dosages in 1 week intervals resulted in a sustained CM CD4+ T cell increase in the two macaques studied. These observations suggest that the cumulative effect of clustering rsIL-7gly doses may be important to maintaining the self-renewing capacity of CM cells. Two further observations from these studies support the hypothesis that mechanisms other than inducing detectable increases in proliferation, for example increased cell survival in the periphery may contribute to increasing absolute cell number in macaques. First, CD4+ naïve cells increased absolute count in the periphery with little detectable change in Ki67 expression. Second, when IL-7 was administered in a three-dose cluster, despite observing a muted Ki67 response to the second and third rounds of IL-7 treatment, in the same macaques, the absolute T-cell count continued to increase with each sequential cluster of IL-7 administration (Figs. 5 and 6). These results suggest that IL-7 has therapeutic benefits contributing to expanding the peripheral T-cell pool that are not always resulting from increased proliferation but rather due to anti-apoptotic survival effects of IL-7. We conclude that the optimal dosing regimen would likely be one with closely spaced (weekly) IL-7 doses that maximizes the benefit from both proliferative and survival effects of the cytokine. Further, we would hypothesize that a cluster of IL-7 doses (three at one week intervals) could be utilized as a ‘stop-gap’ measure in HIV+ HAART treated patients that do not recover CD4+ T cell levels above a certain target, for example 500 CD4 cells/ul of blood. Frequent monitoring of these patients would be required to determine whether additional IL-7 clustered dosing were warranted and could be re-administered should the patients peripheral blood CD4 count drop below a target value at subsequent visits.
The importance of maintaining the self-renewing capacity of the CM CD4+ T cell population with regard to disease progression has been described in previous studies (24-26). These data suggest IL-7 administration can effectively regenerate the CD4+ CM subset directly countering a major effect of viral pathogenesis. Further, the results from our BrDU labeling studies indicate that the cells dividing during the first round of rsIL-7gly have a longer retention time in the peripheral blood compared to the controls. The most likely hypothesis to explain these findings is that rsIL-7gly has a direct anti-apoptotic role and is preventing apoptosis thereby prolonging the lifespan of memory CD4+ T cells. Indeed a recent study by Beq et al demonstrated that rsIL-7gly administration to macaques resulted in suppressed levels of apoptosis (measured by assessment for active form of caspases 3, 8 or 9, as well as DNA fragmentation) (58). Beq et al further demonstrated that by inducing T cell trafficking to mucosal sites, like the intestine, rsIL-7gly may actually be able to assist in repair SIV induced damage at these sites as well (58). IL-7 administration impacts naïve T-cell population dynamics in addition to memory cell populations. Increasing the absolute number of naïve T cells could potentially provide ancillary immunological benefits, such as increased memory precursor populations that may be beneficial for patients.
The studies in HIV/AIDS patients in particular those conducted by Levy et al are promising and provide strong support for further developing IL-7 as an immunotherapeutic (59, 61). The macaque models, which have thus far been highly consistent with the studies in humans, enable the undertaking of highly controlled SIV infections and more extensive sampling providing higher resolution of the events following rsIL-7gly administration and better determination of the systemic effects of rsIL-7gly administration. Of some concern from the macaque studies is the monkey-to-monkey variation that we are observing in the different rsIL-7gly treated groups, indicating that every patient might not benefit equally from the use of IL-7 as an immune therapeutic. However, it is possible that the variability could in part be due to the macaques in this study having relatively high levels of CD4+ T cells following ART treatment (ranging from 519 to 1054 CD4+ T cells/ul of blood) (Supplemental Table 1). One important positive finding in these studies is that SIV+ macaques with an ART controlled infection respond to rsIL-7gly in a manner similar to SIV-negative macaques, with proliferative bursts and a potential for a sustained increase in multiple CD4+ T cell subsets. Overall, these studies provide further evidence that IL-7 can be used effectively as an immunotherapeutic to increase the absolute number of CD4+ T cells to assist in the recovery of the immune system dysfunction induced by the SIV or HIV infections. It will be important to focus future studies on assessing the functionality of the T cells that are responding to IL-7 therapy to determine that they can perform necessary functions when exposed to antigens or pathogens.
Supplementary Material
Acknowledgments
We thank Koen Van Rompay for helpful discussions during the initiation of this project as well as Tracy Walker and Stephanie Beq for careful reading and insightful comments.
Grant support was provided by R01-AI035522 (DLS), R01-DE017541 (DLS), R37-AI054292 (LJP), P51-RR00163 (ONPRC core grant) and the Pendleton Trust (DLS).
Bibliography
- 1.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, Rupert A, Baseler M, Tagaya Y, Roby G, Rehm C, Follmann D, Lane HC. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur A, Grant RM, Means RE, McClure H, Feinberg M, Johnson RP. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J Virol. 1998;72:9597–9611. doi: 10.1128/jvi.72.12.9597-9611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 7.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 8.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 10.Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev. 2006;8:88–97. [PubMed] [Google Scholar]
- 11.Isgro A, Leti W, De Santis W, Marziali M, Esposito A, Fimiani C, Luzi G, Pinti M, Cossarizza A, Aiuti F, Mezzaroma I. Altered clonogenic capability and stromal cell function characterize bone marrow of HIV-infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin Infect Dis. 2008;46:1902–1910. doi: 10.1086/588480. [DOI] [PubMed] [Google Scholar]
- 12.Marziali M, De Santis W, Carello R, Leti W, Esposito A, Isgro A, Fimiani C, Sirianni MC, Mezzaroma I, Aiuti F. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS. 2006;20:2033–2041. doi: 10.1097/01.aids.0000247588.69438.fd. [DOI] [PubMed] [Google Scholar]
- 13.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, Hecht TT, Hill BJ, Komschlies K, Tomaszewski J, Franchini G, Mackall CL. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 15.Leone A, Picker LJ, Sodora DL. IL-2, IL-7 and IL-15 as immuno-modulators during SIV/HIV vaccination and treatment. Curr HIV Res. 2009;7:83–90. doi: 10.2174/157016209787048519. [DOI] [PubMed] [Google Scholar]
- 16.Nunnari G, Pomerantz RJ. IL-7 as a potential therapy for HIV-1-infected individuals. Expert Opin Biol Ther. 2005;5:1421–1426. doi: 10.1517/14712598.5.11.1421. [DOI] [PubMed] [Google Scholar]
- 17.Beier KC, Kallinich T, Hamelmann E. Master switches of T-cell activation and differentiation. Eur Respir J. 2007;29:804–812. doi: 10.1183/09031936.00094506. [DOI] [PubMed] [Google Scholar]
- 18.Beier KC, Kallinich T, Hamelmann E. T-cell co-stimulatory molecules: novel targets for the treatment of allergic airway disease. Eur Respir J. 2007;30:383–390. doi: 10.1183/09031936.00094406. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg AD, Evans DE, Thalhofer C, Shi T, Prell RA. The generation of T cell memory: a review describing the molecular and cellular events following OX40 (CD134) engagement. J Leukoc Biol. 2004;75:962–972. doi: 10.1189/jlb.1103586. [DOI] [PubMed] [Google Scholar]
- 20.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 21.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003;5:172–177. [PubMed] [Google Scholar]
- 22.Muthukumar A, Wozniakowski A, Gauduin MC, Paiardini M, McClure HM, Johnson RP, Silvestri G, Sodora DL. Elevated interleukin-7 levels not sufficient to maintain T-cell homeostasis during simian immunodeficiency virus-induced disease progression. Blood. 2004;103:973–979. doi: 10.1182/blood-2003-03-0874. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 24.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, Sylwester AW, Piatak M, Jr, Lifson JD, Maino VC, Sodora DL, Douek DC, Axthelm MK, Grossman Z, Picker LJ. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, Colle JH, Urrutia A, Scott-Algara D, Boufassa F, Delfraissy JF, Theze J, Venet A, Chakrabarti LA. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007;81:13904–13915. doi: 10.1128/JVI.01401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viollet L, Monceaux V, Petit F, Ho Tsong Fang R, Cumont MC, Hurtrel B, Estaquier J. Death of CD4+ T cells from lymph nodes during primary SIVmac251 infection predicts the rate of AIDS progression. J Immunol. 2006;177:6685–6694. doi: 10.4049/jimmunol.177.10.6685. [DOI] [PubMed] [Google Scholar]
- 27.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 28.Barata JT, Cardoso AA, Boussiotis VA. Interleukin-7 in T-cell acute lymphoblastic leukemia: an extrinsic factor supporting leukemogenesis? Leuk Lymphoma. 2005;46:483–495. doi: 10.1080/10428190400027852. [DOI] [PubMed] [Google Scholar]
- 29.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 31.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davey RT, Jr, Chaitt DG, Albert JM, Piscitelli SC, Kovacs JA, Walker RE, Falloon J, Polis MA, Metcalf JA, Masur H, Dewar R, Baseler M, Fyfe G, Giedlin MA, Lane HC. A randomized trial of high- versus low-dose subcutaneous interleukin-2 outpatient therapy for early human immunodeficiency virus type 1 infection. J Infect Dis. 1999;179:849–858. doi: 10.1086/314678. [DOI] [PubMed] [Google Scholar]
- 33.Davey RT, Jr, Murphy RL, Graziano FM, Boswell SL, Pavia AT, Cancio M, Nadler JP, Chaitt DG, Dewar RL, Sahner DK, Duliege AM, Capra WB, Leong WP, Giedlin MA, Lane HC, Kahn JO. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: A randomized controlled trial. JAMA. 2000;284:183–189. doi: 10.1001/jama.284.2.183. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci U S A. 1996;93:10405–10410. doi: 10.1073/pnas.93.19.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs JA, Baseler M, Dewar RJ, Vogel S, Davey RT, Jr, Falloon J, Polis MA, Walker RE, Stevens R, Salzman NP, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N Engl J Med. 1995;332:567–575. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs JA, Vogel S, Albert JM, Falloon J, Davey RT, Jr, Walker RE, Polis MA, Spooner K, Metcalf JA, Baseler M, Fyfe G, Lane HC. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 37.Lalezari JP, Beal JA, Ruane PJ, Cohen CJ, Jacobson EL, Sundin D, Leong WP, Raffanti SP, Wheeler DA, Anderson RD, Keiser P, Schrader SR, Goodgame JC, Steinhart CR, Murphy RL, Wolin MJ, Smith KA. Low-dose daily subcutaneous interleukin-2 in combination with highly active antiretroviral therapy in HIV+ patients: a randomized controlled trial. HIV Clin Trials. 2000;1:1–15. doi: 10.1310/T5FR-8JPX-0NEF-XDKD. [DOI] [PubMed] [Google Scholar]
- 38.Marchetti G, Franzetti F, Gori A. Partial immune reconstitution following highly active antiretroviral therapy: can adjuvant interleukin-2 fill the gap? J Antimicrob Chemother. 2005;55:401–409. doi: 10.1093/jac/dkh557. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuyasu R, Gelman R, Cherng DW, Landay A, Fahey J, Reichman R, Erice A, Bucy RP, Kilby JM, Lederman MM, Hamilton CD, Lertora J, White BL, Tebas P, Duliege AM, Pollard RB. The virologic, immunologic, and clinical effects of interleukin 2 with potent antiretroviral therapy in patients with moderately advanced human immunodeficiency virus infection: a randomized controlled clinical trial--AIDS Clinical Trials Group 328. Arch Intern Med. 2007;167:597–605. doi: 10.1001/archinte.167.6.597. [DOI] [PubMed] [Google Scholar]
- 40.Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, Legido A, Villinger F, Altman JD, Brown CR, Lewis MG, Katsikis PD. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008;180:350–360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, Silvera P, Villinger F, Cairns JS, Gracely EJ, Lewis MG, Katsikis PD. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J Virol. 2005;79:4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, Garcia-Hand D, Montefiori DC, Quiroz J, Rosati M, Schadeck EB, Boyer JD, Pavlakis GN, Weiner DB, Sidhu M, Eldridge JH, Israel ZR. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine. 2007;25:4967–4982. doi: 10.1016/j.vaccine.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 44.Eberly MD, Kader M, Hassan W, Rogers KA, Zhou J, Mueller YM, Mattapallil MJ, Piatak M, Jr, Lifson JD, Katsikis PD, Roederer M, Villinger F, Mattapallil JJ. Increased IL-15 production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J Immunol. 2009;182:1439–1448. doi: 10.4049/jimmunol.182.3.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenthal R, Groeper C, Bracci L, Adamina M, Feder-Mengus C, Zajac P, Iezzi G, Bolli M, Weber WP, Frey DM, von Holzen U, Oertli D, Heberer M, Spagnoli GC. Differential responsiveness to IL-2, IL-7, and IL-15 common receptor gamma chain cytokines by antigen-specific peripheral blood naive or memory cytotoxic CD8+ T cells from healthy donors and melanoma patients. J Immunother. 2009;32:252–261. doi: 10.1097/CJI.0b013e3181998e03. [DOI] [PubMed] [Google Scholar]
- 46.Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–176. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Lee SK, Surh CD. Role of interleukin-7 in bone and T-cell homeostasis. Immunol Rev. 2005;208:169–180. doi: 10.1111/j.0105-2896.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 48.Napolitano LA, Burt TD, Bacchetti P, Barron Y, French AL, Kovacs A, Anastos K, Young M, McCune JM, Greenblatt RM. Increased circulating interleukin-7 levels in HIV-1-infected women. J Acquir Immune Defic Syndr. 2005;40:581–584. doi: 10.1097/01.qai.0000187442.53708.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, Herzenberg LA, Herndier BG, Andersson J, McCune JM. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 50.Roifman CM, Zhang J, Chitayat D, Sharfe N. A partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell development and cause severe combined immunodeficiency. Blood. 2000;96:2803–2807. [PubMed] [Google Scholar]
- 51.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beq S, Nugeyre MT, Ho Tsong Fang R, Gautier D, Legrand R, Schmitt N, Estaquier J, Barre-Sinoussi F, Hurtrel B, Cheynier R, Israel N. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol. 2006;176:914–922. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- 53.Hryniewicz A, Price DA, Moniuszko M, Boasso A, Edghill-Spano Y, West SM, Venzon D, Vaccari M, Tsai WP, Tryniszewska E, Nacsa J, Villinger F, Ansari AA, Trindade CJ, Morre M, Brooks D, Arlen P, Brown HJ, Kitchen CM, Zack JA, Douek DC, Shearer GM, Lewis MG, Koup RA, Franchini G. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J Immunol. 2007;178:3492–3504. doi: 10.4049/jimmunol.178.6.3492. [DOI] [PubMed] [Google Scholar]
- 54.Nugeyre MT, Monceaux V, Beq S, Cumont MC, Ho Tsong Fang R, Chene L, Morre M, Barre-Sinoussi F, Hurtrel B, Israel N. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J Immunol. 2003;171:4447–4453. doi: 10.4049/jimmunol.171.8.4447. [DOI] [PubMed] [Google Scholar]
- 55.Lu H, Zhao Z, Kalina T, Gillespy T, 3rd, Liggitt D, Andrews RG, Maloney DG, Kiem HP, Storek J. Interleukin-7 improves reconstitution of antiviral CD4 T cells. Clin Immunol. 2005;114:30–41. doi: 10.1016/j.clim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Moniuszko M, Fry T, Tsai WP, Morre M, Assouline B, Cortez P, Lewis MG, Cairns S, Mackall C, Franchini G. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dereuddre-Bosquet N, Vaslin B, Delache B, Brochard P, Clayette P, Aubenque C, Morre M, Assouline B, Le Grand R. Rapid modifications of peripheral T-cell subsets that express CD127 in macaques treated with recombinant IL-7. J Med Primatol. 2007;36:228–237. doi: 10.1111/j.1600-0684.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 58.Beq S, Rozlan S, Gautier D, Parker R, Mersseman V, Schilte C, Assouline B, Rance I, Lavedan P, Morre M, Cheynier R. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood. 2009 doi: 10.1182/blood-2008-11-191288. [DOI] [PubMed] [Google Scholar]
- 59.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boue F, Molina JM, Rouzioux C, Avettand-Fenoel V, Croughs T, Beq S, Thiebaut R, Chene G, Morre M, Delfraissy JF. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, Asmuth DM, Tenorio AR, Altman JD, Fox L, Moir S, Malaspina A, Morre M, Buffet R, Silvestri G, Lederman MM. IL-7 administration drives T cell cycle entry and expansion in HIV-1 infection. Blood. 2009 doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 64.Walker JM, Maecker HT, Maino VC, Picker LJ. Multicolor flow cytometric analysis in SIV-infected rhesus macaque. Methods Cell Biol. 2004;75:535–557. doi: 10.1016/s0091-679x(04)75022-0. [DOI] [PubMed] [Google Scholar]
- 65.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 66.Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, Jacod S, Delfraissy JF, Theze J. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients--effects of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42:277–285. doi: 10.1097/01.qai.0000214823.11034.4e. [DOI] [PubMed] [Google Scholar]
- 67.Koesters SA, Alimonti JB, Wachihi C, Matu L, Anzala O, Kimani J, Embree JE, Plummer FA, Fowke KR. IL-7Ralpha expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur J Immunol. 2006;36:336–344. doi: 10.1002/eji.200535111. [DOI] [PubMed] [Google Scholar]
- 68.Mercier F, Boulassel MR, Yassine-Diab B, Tremblay C, Bernard NF, Sekaly RP, Routy JP. Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7Ralpha on central and effector memory CD4+ and CD8+ T cell subsets. Clin Exp Immunol. 2008;152:72–80. doi: 10.1111/j.1365-2249.2008.03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasson SC, Zaunders JJ, Zanetti G, King EM, Merlin KM, Smith DE, Stanley KK, Cooper DA, Kelleher AD. Increased plasma interleukin-7 level correlates with decreased CD127 and Increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J Infect Dis. 2006;193:505–514. doi: 10.1086/499309. [DOI] [PubMed] [Google Scholar]
- 70.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 71.Benito JM, Lopez M, Lozano S, Gonzalez-Lahoz J, Soriano V. Down-regulation of interleukin-7 receptor (CD127) in HIV infection is associated with T cell activation and is a main factor influencing restoration of CD4(+) cells after antiretroviral therapy. J Infect Dis. 2008;198:1466–1473. doi: 10.1086/592716. [DOI] [PubMed] [Google Scholar]
- 72.Moniuszko M, Edghill-Smith Y, Venzon D, Stevceva L, Nacsa J, Tryniszewska E, Tsai WP, Franchini G. Decreased number of CD4+ and CD8+ T cells that express the interleukin-7 receptor in blood and tissues of SIV-infected macaques. Virology. 2006;356:188–197. doi: 10.1016/j.virol.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 73.Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, Radziewicz H, Piedimonte G, Magnani M, Montroni M, Kaech SM, Weintrob A, Altman JD, Sodora DL, Feinberg MB, Silvestri G. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174:2900–2909. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 74.Rethi B, Fluur C, Atlas A, Krzyzowska M, Mowafi F, Grutzmeier S, De Milito A, Bellocco R, Falk KI, Rajnavolgyi E, Chiodi F. Loss of IL-7Ralpha is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 down-regulation in HIV infected patients. AIDS. 2005;19:2077–2086. doi: 10.1097/01.aids.0000189848.75699.0f. [DOI] [PubMed] [Google Scholar]
- 75.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, van Lier RA. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 76.Zhang SY, Zhang Z, Fu JL, Kang FB, Xu XS, Nie WM, Zhou CB, Zhao M, Wang FS. Progressive CD127 down-regulation correlates with increased apoptosis of CD8 T cells during chronic HIV-1 infection. Eur J Immunol. 2009;39:1425–1434. doi: 10.1002/eji.200839059. [DOI] [PubMed] [Google Scholar]
- 77.Barata JT, Cardoso AA, Nadler LM, Boussiotis VA. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1) Blood. 2001;98:1524–1531. doi: 10.1182/blood.v98.5.1524. [DOI] [PubMed] [Google Scholar]
- 78.Khaled AR, Bulavin DV, Kittipatarin C, Li WQ, Alvarez M, Kim K, Young HA, Fornace AJ, Durum SK. Cytokine-driven cell cycling is mediated through Cdc25A. J Cell Biol. 2005;169:755–763. doi: 10.1083/jcb.200409099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med. 2006;203:573–582. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109:1034–1042. doi: 10.1182/blood-2006-06-027912. [DOI] [PubMed] [Google Scholar]
- 81.Jiang Q, Li WQ, Hofmeister RR, Young HA, Hodge DR, Keller JR, Khaled AR, Durum SK. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riou C, Yassine-Diab B, Van grevenynghe J, Somogyi R, Greller LD, Gagnon D, Gimmig S, Wilkinson P, Shi Y, Cameron MJ, Campos-Gonzalez R, Balderas RS, Kelvin D, Sekaly RP, Haddad EK. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seki Y, Yang J, Okamoto M, Tanaka S, Goitsuka R, Farrar MA, Kubo M. IL-7/STAT5 cytokine signaling pathway is essential but insufficient for maintenance of naive CD4 T cell survival in peripheral lymphoid organs. J Immunol. 2007;178:262–270. doi: 10.4049/jimmunol.178.1.262. [DOI] [PubMed] [Google Scholar]
- 84.Rajnavolgyi E, Benbernou N, Rethi B, Reynolds D, Young HA, Magocsi M, Muegge K, Durum SK. IL-7 withdrawal induces a stress pathway activating p38 and Jun N-terminal kinases. Cell Signal. 2002;14:761–769. doi: 10.1016/s0898-6568(02)00026-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.