Abstract
The tumor microenvironment contains multiple cancer-supporting factors, whose joint activities promote malignancy. Here, we show that epidermal growth factor (EGF) and estrogen upregulate in an additive manner the transcription and the secretion of the angiogenic chemokine CXCL8 (interleukin 8 [IL-8]) in breast tumor cells. In view of published findings on cross-regulatory interactions between EGF receptors and estrogen receptors in breast tumor cells, we asked whether the additive effects of EGF and estrogen were due to their ability to (1) induce intracellular cross talk and amplify shared regulatory pathways or (2) act in independent mechanisms, which complement each other. We found that stimulation by EGF alone induced the release of CXCL8 through signaling pathways involving ErbB2, ErbB1, Erk, and phosphoinositide 3-kinase (PI3K). ErbB2 and Erk were also involved in estrogen activities on CXCL8 but to a lower extent than with EGF. However, in the joint stimulatory setup, the addition of estrogen to EGF has led to partial (ErbB2, ErbB1, Erk) or complete (PI3K) shutoff of the involvement of these activation pathways in CXCL8 up-regulation. Furthermore, when costimulation by EGF + estrogen was applied, the effects of estrogen were channeled to regulation of CXCL8 at the transcription level, acting through the transcription factor estrogen receptor α (ERα). In parallel, in the joint stimulation, EGF acted independently at the transcription level through AP-1, to upregulate CXCL8 expression. The independent activities of EGF and estrogen on CXCL8 transcription reinforce the need to introduce simultaneous targeting of ErbBs and ERα to achieve effective therapy in breast cancer.
Introduction
The microenvironment of breast tumors is enriched with a variety of factors, acting together to promote processes of cancer development and progression. The coordinated activities of these elements and the interactions between them may have major clinical implications. Therefore, it is important to identify cross-regulatory mechanisms that take place between different promalignancy factors in breast cancer.
In this study, we were interested in cross-regulatory interactions that may affect the release of angiogenic factors by breast tumor cells. To achieve this goal, we selected the chemokine CXCL8 as the angiogenic target and epidermal growth factor (EGF) and estrogen as stimulants.
CXCL8 was the target of choice because it is a powerful angiogenic factor that exerts a variety of additional promalignancy activities in breast cancer and is causatively involved in tumor growth and metastasis (e.g., [1–10]). In endothelial cells, the signals of CXCL8 are transmitted mainly through the G protein-coupled receptor CXCR2, leading to neovascularization in many malignant diseases, including breast cancer [1–5].
EGF and estrogen were selected as the stimulants because of increasing body of evidence, indicating that there is an intracellular cross talk between their receptors [11–16]. EGF and estrogen have multiple and well-established promalignancy roles in breast cancer, and their receptors serve as important therapeutic targets in this disease [17–25].
EGF signals are transmitted by members of the ErbB/HER family of receptor tyrosine kinases (RTKs), where ErbB2 (HER2/neu) and ErbB1 (EGFR/HER1) play important roles in EGF-induced signaling. Cell activation by EGF then leads to downstream activation of intracellular signaling molecules, primarily Erk and phosphoinositide 3-kinase (PI3K) [17–19,26–29].
The promalignancy effects of estrogen are mediated to a great extent through the intracellular receptor ERα, acting as a transcription factor that binds to estrogen response elements in target genes. However, ERα was also found to induce nongenomic signal transduction pathways, such as Erk and PI3K [11–14,30].
The cross talk between ErbBs and ERα can be mediated by genomic and nongenomic mechanisms. It is manifested by the ability of ERα to directly or to indirectly activate ErbBs—mainly ErbB2 but also ErbB1—leading to stimulation of downstream kinases. In parallel, ErbB2/ErbB1-induced activation of different intracellular kinases leads to the stimulation of ERα and of its coregulators, thus augmenting the genomic activities of ERα. Recent findings suggest that such interactions between ErbBs and ERα stand in the basis of de novo and acquired resistance to endocrine therapies in breast cancer patients [11,12,14–16].
Obviously, the cross talk between ErbBs and ERα may affect key cellular functions that promote malignancy. Therefore, in this study, we wished to identify possible interactions between EGF and estrogen in the level of regulation of angiogenic factors, specifically focusing on CXCL8. Our initial observations indicated that EGF and estrogen promoted in an additive manner the transcription and the release of CXCL8 by breast tumor cells. We therefore asked if the additive effects of EGF and estrogen on CXCL8 expression were due to (1) their ability to induce intracellular cross talk and amplify shared pathways that promote CXCL8 release or (2) their ability to act in independent pathways that complement each other, together giving rise to their additive activities on CXCL8 expression.
To answer these questions, we analyzed the involvement of signaling pathways and of transcriptional activation in the joint activities of EGF and estrogen on CXCL8 release, in comparison to the effects of EGF alone and of estrogen alone.
The findings of our study indicate that signaling events were potently involved in the ability of EGF to induce CXCL8 release by the cells, whereas estrogen less potently induced some of these activation events. When concomitant stimulation by EGF + estrogen was applied, estrogen partly or completely downregulated the ability of EGF to promote CXCL8 expression through intracellular signaling pathways. Rather, in the EGF + estrogen stimulatory setup, the effects of estrogen were channeled to transcription-related activities, mediated by ERα. Specifically, after joint stimulation by EGF + estrogen, estrogen upregulated CXCL8 by activating the transcriptional activity of ERα, whereas EGF induced the expression of CXCL8 through the activation of AP-1.
These results are novel because they indicate that when CXCL8 regulation is concerned, the stimulants EGF and estrogen probably do not act through intracellular cross talk. Rather, they act in independent transcriptional pathways that complement each other, together giving rise to additive up-regulation of CXCL8 release by breast tumor cells.
Our findings have major clinical relevance and implications. They provide insight into mechanisms that may be involved in disease course and suggest that there would be a need to introduce combination therapies directed against EGF/ErbBs and against endocrine elements together, to inhibit the promalignancy activities of the angiogenic chemokine CXCL8 in breast cancer.
Materials and Methods
Determination of CXCL8 Extracellular Expression by ELISA
Human breast carcinoma MCF-7 cells were grown overnight in growth medium [31]. Then, the cells were washed twice in phenol red- and serum-free Dulbecco modified Eagle medium and incubated overnight in phenol red-free medium containing 1% dialyzed fetal calf serum (Biological Industries, Beit Ha'emek, Israel) and estrogen (17β-estradiol, 10-8 M; Sigma, St Louis, MO). The medium was removed, and the cells were incubated in a similar medium with estrogen for additional 48 hours (estrogen was replenished every 24 hours). EGF (10 ng/ml, unless otherwise indicated; R&D Systems, Minneapolis, MN) was added during the last 24 hours of incubation with estrogen. In parallel, the treatments included stimulation by estrogen alone or EGF alone. Control cells were grown under similar conditions, with ethanol (the solubilizer of estrogen) used as a control for estrogen. No EGF and/or estrogen were added to control cells.
This procedure of tumor cell stimulation was selected based on preliminary kinetics studies (data not shown), performed under the following conditions:
Stimulation by EGF—In preliminary studies, we found that stimulation of the cells by EGF for 24 or 48 hours induced up-regulation of CXCL8 release. Thereafter, throughout the study, the actual stimulations were performed for 24 hours only to reduce the EGF-induced proliferative effects. The stimulation length was not reduced to less than 24 hours because sufficient time was required to enable chemokine accumulation in the cell supernatants.
Stimulation by estrogen—Because the activation of ERα takes time to evolve, we first tested the effects of estrogen for 48 and 72 hours of stimulation. Although both conditions have induced CXCL8 up-regulation, we performed all experiments at 72 hours of stimulation because we found that 72 hours was required for induction of the additive effects of EGF + estrogen on CXCL8 up-regulation.
Joint stimulation by EGF and estrogen—The time points were based on the same guidelines indicated above: 24 hours for EGF and 72 hours for estrogen.
In specific parts of the study, pharmacological inhibitors were used in conventional concentrations: AG825 (10 εM; A. G. Scientific, San Diego, CA), AG1478 (0.5 εM; A. G. Scientific), PD98059 (50 εM; Cayman Chemical Company, Ann Arbor, MI), LY294002 (20 εM; Alexis, San Diego, CA), ICI-182,780 (1 εM; Sigma), and pyrrolidinedithio-carbamate ammonium (PDTC, 60 εM; Sigma). The procedures of incubation with the drugs are given in detail in the legends to figures. Control cells were incubated with dimethyl sulfoxide (DMSO; Sigma), the solubilizer of the drugs (except for PDTC that was diluted in water). The cells were exposed to the inhibitors for time points that would allow maximal exposure to the inhibitors and, in parallel, would not damage the proliferation or the morphology of the cells (based on kinetics analyses). Of note, in all assays, the findings obtained by the use of inhibitors were confirmed by experiments showing the involvement of the relevant proteins in CXCL8 up-regulation by the different stimulants in ELISA assays.
CXCL8 expression in the supernatants of the cells was determined by ELISA, using standard curves with recombinant human CXCL8 (Peprotech, Rocky Hill, NJ), at the linear range of absorbance. The following antibodies were used: coating antibodies—mouse monoclonal antibodies against human CXCL8 (508402; BioLegend, San Diego, CA); and detecting antibodies—biotinylated goat anti-human CXCL8 antibodies (BAF208; R&D Systems). After the addition of streptavidin-horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA), the substrate TMB/E solution (Chemicon, Temecula, CA) was added. The reaction was stopped by the addition of 0.18 M H2SO4 and was measured at 450 nm. P values were calculated by Student's t test.
Determination of CXCL8 Transcription by Real-time Polymerase Chain Reaction
MCF-7 cells were stimulated by EGF (6 hours), estrogen (48 hours), or EGF + estrogen (estrogen 48 hours and EGF during the last 6 hours). Essentially similar results were obtained when EGF stimulation was performed for 3 hours. These stimulatory conditions were based on kinetics analyses performed as follows:
Stimulation by EGF—EGF signals are induced by RTK; therefore, it is expected that its effects on the messenger RNA (mRNA) level would be relatively rapid. Thus, exposure to EGF was performed for 3 or 6 hours, with essentially similar results.
Stimulation by estrogen—The conditions were selected to allow the transcriptional activities of ERα to come into effect. Because 72 hours of stimulation by estrogen was required to induce the additive effects of EGF + estrogen in ELISA, 48 hours of stimulation was used in the real-time analysis to guarantee effects at the mRNA levels.
Joint stimulation by EGF and estrogen—The time points were based on the same guidelines indicated above: 3 or 6 hours for EGF and 72 hours for estrogen.
Total RNA was isolated from the cells using the EZ-RNA kit (Biological Industries). RNA samples were used for generation of first-strand complementary DNA synthesis using the M-MLV reverse transcriptase (Ambion, Austin, TX). Quantification of complementary DNA targets by real-time polymerase chain reaction (PCR) was performed on Rotor Gene 6000 (Corbett Life Science, Concorde, NSW, Australia), using Rotor Gene 6000 series software. Transcripts were detected using SYBR Green I (Thermo Fisher Scientific, Abgene, England) according to the manufacturer's instructions. In each reaction, two pairs of specific primers were used, designed for different axons. The sequences of the primers for CXCL8 (accession no. NM_000584) were as follows: forward 5′-TTCTGCAGCTCTGTGTGAAG-3′, reverse 5′-CAGTGTGGTCCACTCTCAAT-3′. The sequences of the primers for the normalizing gene rS9 (accession no. NM_001013) were as follows: forward 5′-TTACATCCTGGGCCTGAAGAT-3′ and reverse 5′-GGGATGTTCACCACCTGCTT-3′. PCR amplification was performed over 40 cycles (95°C for 15 seconds, 59°C for 20 seconds, 72°C for 15 seconds). Dissociation curves for each primer set indicated a single product, and no-template controls were negative after 40 cycles.
Western Blot Analysis
MCF-7 cells were stimulated by EGF and/or estrogen (see “Comment” below for stimulation conditions), and were then lysed in RIPA lysis buffer. Lysis was followed by conventional Western blot procedures. The following antibodies were used: phosphorylated AKT (AF887; R&D Systems), AKT (9272; Cell Signaling Technology, Danvers, MA), phosphorylated Erk (F1018; R&D Systems), Erk (sc-154; Santa Cruz Biotechnology, Santa Cruz), phosphorylated ERα (sc-12915; Santa Cruz Biotechnology), ERα (sc-543, Santa Cruz Biotechnology), IκBα (4814; Cell Signaling Technology), p38 (M0800; Sigma), GAPDH (MAB374; Chemicon), phosphorylated c-Jun (sc-822; Santa Cruz Biotechnology), and c-Jun (610326; BD Transduction Laboratories, San Jose, CA). ErbB2 was immunoprecipitated by mouse antibodies (OP15L; Calbiochem, Gibbstown, NJ), followed by detection of phosphorylated ErbB2 by antibodies to phospho-tyrosine (SC-7020, Santa Cruz Biotechnology). Protein loading was determined by antibodies to ErbB2 (SC-284, Santa Cruz Biotechnology).
After washings, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, as appropriate: sheep anti-mouse-HRP (NA931; Amersham Pharmacia Biotech, Buckinghamshire, UK), goat anti-rabbit-HRP (111-035-003; Jackson ImmunoResearch Laboratories), and donkey anti-goat-HRP (705-035-003; Jackson ImmunoResearch Laboratories). The membranes were subjected to enhanced chemiluminescence (Amersham Pharmacia Biotech), and bands on immunoblots were quantitated by densitometry.
Comment. All experiments were preceded by preliminary kinetics analyses, which included time points expected to allow optimal response, based on published literature and, on our experience, in the specific cell systems. The time points in the preliminary studies included short exposure times for phosphorylation events (minutes) and were extended to the hour time range for transcription factors as follows (data not shown): ErbB2, 1.5 and 10 minutes; Erk and AKT, different time points in the range of 1 to 40 minutes; ERα, 10, 20, and 30 minutes; IκBα, 3 to 6 hours for EGF and 3 to 54 hours for estrogen; c-Jun, 1.5, 5, and 10 minutes. On the basis of these preliminary analyses, selected time points were used in the actual sets of experiments presented in the figures.
Dual-Luciferase Assays
The assays were performed with the following constructs: 1) Constructs coding for firefly luciferase under the control of CXCL8 promoter, including the 5′-flanking region from -558 to +98 bp. We have used the CXCL8 promoter expressing either WTAP-1 binding site (5′-AAG TGT GAT GAC TCA GGT TTG CCC TGA-3′) or AP-1-mutated binding site (5′-AAG TGT GATATC TCAGGT TTG CCC TGA-3′). Both constructs were kindly provided by Dr Muhl (University Hospital Goethe-University Frankfurt), and these are described in detail in Hellmuth et al. [32]. 2) A construct coding for renilla luciferase was used for normalization of the results according to transfection yields (kindly provided by Dr Zor, Tel Aviv University).
The different constructs were transiently transfected to MCF-7 cells by ICA Fectin (In Cell Art, Nantes, France). Forty-eight hours after transfection, the cells were stimulated by EGF for 4 or 8 hours (with essentially similar results; on the basis of preliminary studies and because of technical reasons related to the length of estrogen stimulation, these experiments were not performed with estrogen alone or with estrogen + EGF) and processed with the reagents provided in Dual-Luciferase Assay System Kit (Promega, Madison, WI). Luciferase activity was determined using the same kit according to the manufacturer's instructions.
Results
EGF and Estrogen Additively Promote CXCL8 Release and Transcription in Breast Tumor Cells
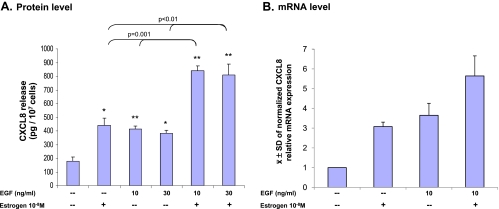
Human breast carcinoma MCF-7 cells are the classic model system used in studies of possible interactions between EGF and estrogen in breast cancer. On the basis of kinetics analyses (see Materials and Methods), the cells were stimulated by EGF (24 hours), estrogen (72 hours), or EGF + estrogen (estrogen 72 hours; EGF during last 24 hours). The results in Figure 1A show that EGF and estrogen, each alone, has induced a significant up-regulation in CXCL8 release by these tumor cells. Moreover, additive effects of the two stimulants were observed when the cells were costimulated by EGF and estrogen concomitantly. The extent of additivity ranged in different experiments and reached up to two-folds, compared with stimulation by estrogen alone or by EGF alone (e.g., Figure 1A).
Figure 1.
Stimulation by EGF + estrogen additively upregulates the release and transcription of CXCL8 in MCF-7 breast carcinoma cells. (A) CXCL8 release by the tumor cells. MCF-7 cells were grown in the presence of estrogen (72 hours), EGF (last 24 hours), EGF + estrogen (estrogen 72 hours; EGF last 24 hours), or ethanol as control (ethanol did not affect CXCL8 release compared with untreated cells) (time points were selected based on kinetics analyses, as described in Materials and Methods). CXCL8 extracellular expression was determined in the supernatants of the cells by ELISA and was analyzed in the linear range of absorbance. A representative experiment of n > 3 is presented. *P < .05, **P < .01 for the difference between treated and untreated cells. (B) CXCL8 transcription. MCF-7 cells were grown in the presence of estrogen (48 hours), EGF (last 6 hours), EGF + estrogen (estrogen: 48 hours; EGF: last 6 hours), or ethanol as control (time points were selected based on kinetics analyses, as described in Materials and Methods). The CXCL8 transcript level was determined by quantitative real-time PCR analysis. The results were standardized by rS9 levels. The figure presents the mean () ± SD of normalized values of mRNA levels in three independent experiments, all showing similar results.
After determination of the effects of EGF, estrogen, and EGF + estrogen on CXCL8 release by the cells, we have determined their effects on CXCL8 mRNA levels, in conditions selected after kinetics analyses (see Materials and Methods). This analysis has shown that the pattern of CXCL8 transcription was similar to that of CXCL8 protein release (Figure 1, B and A, respectively). Each of the two factors, EGF and estrogen, induced CXCL8 transcription, and additive effects of EGF and estrogen were observed at this level. These results indicate that the basis for the additive increase in CXCL8 release by the tumor cells was increased transcription of the gene, induced additively by EGF and estrogen.
Additive Effects of EGF + Estrogen on CXCL8 Release: Involvement of Signaling Pathways
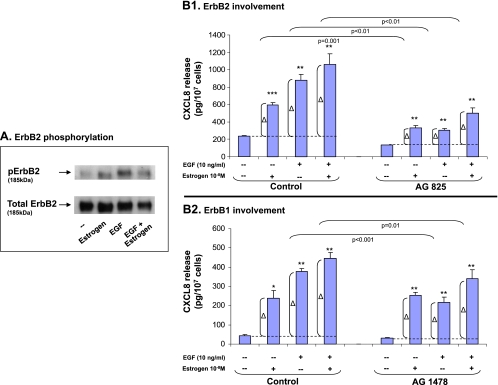
Increased transcription of CXCL8 by EGF and estrogen may reflect the ability of the two stimulants to induce signaling events that eventually lead to transcriptional activation. To determine the roles of stimulatory signaling processes in CXCL8 regulation by EGF and/or estrogen, we first analyzed the activation of membrane-proximal events. Specifically, we focused on ErbB2 because of its major contribution to signal amplification in response to EGF [22,28,29].
As expected from the potent activities of EGF on the tumor cells, ErbB2 was activated in response to EGF stimulation (Figure 2A). The activation of ErbB2 by EGF was higher than after stimulation by estrogen (Figure 2A). We then asked whether ErbB2 was involved in the ability of the different stimulants to elevate the release of CXCL8 by the tumor cells. To address this question, we determined the effects of the specific ErbB2 inhibitor AG825 (used in conventional concentrations) on the net increase in CXCL8 amounts in the cell supernatants after stimulation by estrogen, EGF, or both. The net increase is indicated as Δ in the graph (Figure 2B1).
Figure 2.
The additive up-regulation of CXCL8 induced by EGF + estrogen: Involvement of ErbB2 and ErbB1. (A) ErbB2 activation. MCF-7 cells were stimulated by EGF (10 ng/ml), estrogen (10-8 M), or EGF + estrogen (EGF: 10 ng/ml; estrogen: 10-8 M) or ethanol as control for 10 minutes. Time points were selected based on kinetics analyses as described in Materials and Methods. ErbB2 phosphorylation was determined by Western blot analysis. A representative experiment of n > 3 (with estrogen used for 1.5 or 10 minutes) is presented. (B) The involvement of ErbB2 (B1) and ErbB1 (B2) in EGF-, estrogen-, and EGF + estrogen-induced up-regulation of CXCL8 expression. MCF-7 cells were grown as detailed in Figure 1A. Two hours before addition of estrogen, the cells were pretreated with conventional concentrations of the ErbB2 inhibitor AG825 (10 εM; B1), the ErbB1 inhibitor AG1478 (0.5 εM; B2), or DMSO (control = the drug's solubilizer). Then, the growth of the cells was continued in absence or presence of the drug (or DMSO as control) as required. CXCL8 extracellular expression was determined in the supernatants of the cells by ELISA and was analyzed in the linear range of absorbance. Δ, The net amount of CXCL8 added to cell supernatant on stimulation. These values were used for statistical evaluations of differences between the control group and the inhibitor-treated group. A representative experiment of n > 3 is presented. *P < .05, **P < .01, ***P < .001 for the difference between cells stimulated by EGF/estrogen/EGF + estrogen and untreated cells.
Figure 2B1 shows that ErbB2 played an important role in EGFinduced CXCL8 release by the cells. In parallel, estrogen activities on CXCL8 were partly mediated through ErbB2 activation, being in line with cross talk studies showing that estrogen-induced signaling can lead to ErbB2 activation [11–16]. However, in the joint stimulation by EGF + estrogen, the roles of ErbB2 in CXCL8 up-regulation were lower compared with its roles on stimulation by EGF alone. Therefore, the addition of estrogen to EGF has led to a partial reduction of the involvement of this signaling pathway in CXCL8 regulation.
In parallel, we have analyzed the involvement of ErbB1 in the activities of EGF, estrogen, and EGF + estrogen, leading to CXCL8 release by the tumor cells. Because ErbB1 levels in MCF-7 cells are relatively difficult to detect technically, we focused on the inhibition of its activities by AG1478, the specific ErbB1 inhibitor (used in conventional concentrations). We found that although EGF activities were partly mediated through ErbB1, those of estrogen were not (Figure 2B2). Furthermore, when costimulation by EGF + estrogen was applied, ErbB1 has shown reduced involvement in CXCL8 up-regulation compared with its roles in EGF-induced effects.
Together, the above results indicate that EGF has induced the upregulation of CXCL8 expression through activation of ErbB2 and ErbB1. However, the addition of estrogen to EGF has led to partial down-regulation of the roles played by these stimulatory signaling processes in CXCL8 promotion.
Next, we addressed the roles of downstream signaling events in EGF-, estrogen-, and EGF + estrogen-induced CXCL8 up-regulation. Here, we have analyzed several mediators that are known to be involved in EGF- and/or estrogen-induced pathways. Preliminary analyses of such mediators—including Src [12,19], cAMP [33], and GPR30 [34, 35]—did not provide substantial basis to pursue their roles in the joint effects of EGF + estrogen on CXCL8 release by the tumor cells (data not shown).
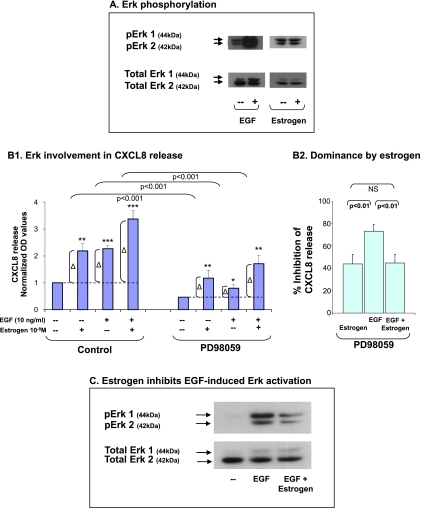
In addition, we have determined the roles of Erk and PI3K in the abilities of EGF, estrogen, and EGF + estrogen to upregulate CXCL8 release by the tumor cells. To this end, we analyzed the ability of each stimulant to induce Erk or AKT (PI3K substrate) phosphorylation; in parallel, we determined the effects of most conventional and specific Erk and PI3K inhibitors (PD98059 and LY294002, respectively, used at standard concentrations) on CXCL8 release after exposure to each of the stimulants.
Erk phosphorylation assays indicated that EGF potently activated Erk (Figure 3A), and its ability to upregulate CXCL8 release was largely dependent on Erk (Figure 3B1). In contrast, when the stimulation was performed by estrogen (following preliminary kinetics analyses, see Materials and Methods), estrogen induced only a minor activation of Erk (in the range of x1.24-x1.6 in four experiments; Figure 3A). Although minimal and relatively difficult to detect, this estrogen-induced Erk activation level probably enabled a partial participation of Erk in estrogen-induced CXCL8 up-regulation, as determined by the use of PD98059 (Figure 3B1). Of interest, and along the lines that were already found before, the addition of estrogen to EGF has led to reduced involvement of Erk in CXCL8 up-regulation (Figure 3B1) compared with the effects of EGF alone. Figure 3B2 illustrates the relative roles of Erk in each of the stimulatory setups, indicated by percent inhibition of CXCL8 release obtained by the Erk inhibitor PD98059 (average and SD of three experiments). This figure clearly shows that estrogen dictated the magnitude of Erk-mediated responses when joint stimulation by EGF + estrogen was introduced. Whereas, in EGF-stimulated cells Erk was involved by 73.6% in CXCL8 induction, its involvement was downregulated to 44.8% when estrogen was added to EGF. This “dominance” of estrogen over EGF-induced signaling was due to the ability of the hormone to reduce EGF-induced activation of Erk, indicated by substantial inhibition of EGF-induced Erk phosphorylation (Figure 3C).
Figure 3.
The additive up-regulation of CXCL8 induced by EGF + estrogen: Involvement of Erk. (A) Erk phosphorylation by EGF and estrogen. MCF-7 cells were stimulated by EGF (10 ng/ml, 4–7 minutes) or estrogen (10-8 M; the results are of 20–35 minutes of stimulation; no substantial phosphorylation was obtained at shorter or longer exposure times; time points were selected based on kinetics analyses, as described in Materials and Methods). Erk phosphorylation was determined by Western blot analysis. A representative experiment of n = 4 is presented. (B) The involvement of Erk in EGF-, estrogen- and EGF + estrogen-induced up-regulation of CXCL8 expression. MCF-7 cells were grown as detailed in Figure 1A. Two hours before addition of EGF, the cells were pretreated with conventional concentrations of the Erk inhibitor PD98059 (50 εM) or DMSO (control = the drug's solubilizer). Then, the growth of the cells was continued in the absence or presence of the drugs (or DMSO as control). (B1) CXCL8 extracellular expression was determined by ELISA and was analyzed in the linear range of absorbance. Δ, The net amount of CXCL8 added to cell supernatant on stimulation. These values were used for statistical evaluations of differences between the control group and the inhibitor-treated group. A representative experiment of n = 3 is presented. *P < .05, **P < .01, and ***P < .001 for the difference between cells stimulated by EGF/estrogen/EGF + estrogen and untreated cells. P values were calculated based on original values before normalization. (B2) Dominance of estrogen over EGF in combined stimulation. The figure demonstrates a summary of the results of the experiments presented in B1 using PD98059. An average inhibition value (% ± SD) of n = 3 is presented. (C) Estrogen downregulates EGF-induced Erk activation. MCF-7 cells were stimulated by EGF (10 ng/ml), EGF + estrogen activation (estrogen 10-8 M + EGF 10 ng/ml), or ethanol as control for 5 to 12 minutes. Time points were selected based on kinetics analyses, as described in Materials and Methods. Erk phosphorylation levels were determined by Western blot analysis. A representative experiment of n = 3 is presented.
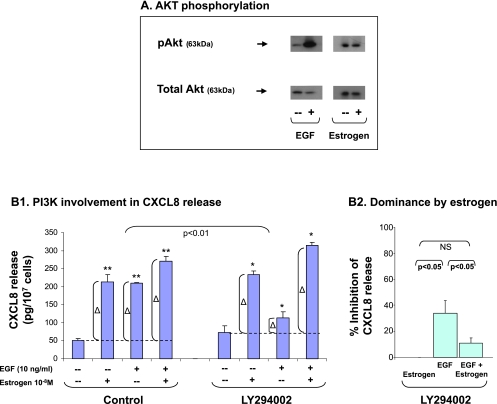
We then analyzed PI3K and found that EGF induced potent activation of this enzyme, as indicated by AKT phosphorylation (Figure 4A). Accordingly, PI3Kwas largely involved in EGF-induced CXCL8 release (Figure 4B1). In contrast, estrogen did not induce reproducible PI3K activation (Figure 4A; estrogen was tested in a large variety of conditions), and PI3K did not take part in estrogen-induced release of CXCL8 (Figure 4B1). When estrogen was added to the EGF stimulation, the involvement of PI3K was diminished and actually mirrored the lack of PI3K involvement in estrogen activities on CXCL8 (Figure 4B1).
Figure 4.
The additive up-regulation of CXCL8 induced by EGF + estrogen: Involvement of PI3K. (A) AKT phosphorylation by EGF and estrogen. MCF-7 cells were stimulated by EGF (10 ng/ml, 4–7 minutes) or estrogen (10-8 M; the results are of 20 to 35 minutes of stimulation; no substantial phosphorylation was obtained at shorter or longer exposure times; time points were selected based on kinetics analyses, as described in Materials and Methods). AKT phosphorylation was determined by Western blot analysis. A representative experiment of n > 3 is presented. (B) The involvement of PI3K in EGF-, estrogen-, and EGF + estrogen-induced up-regulation of CXCL8 expression. MCF-7 cells were grown as detailed in Figure 1A. Two hours before addition of EGF, the cells were pretreated with conventional concentrations of the specific PI3K inhibitor LY294002 (20 εM) or DMSO (control = the drug's solubilizer). Then, the growth of the cells was continued in the absence or presence of the drugs (or DMSO as control). (B1) CXCL8 extracellular expression was determined in the supernatants of the cells by ELISA, and was analyzed in the linear range of absorbance. Δ, The net amount of CXCL8 added to cell supernatant on stimulation. These values were used for statistical evaluations of differences between the control group and the inhibitor-treated group. A representative experiment of n > 3 is presented. *P < .05, **P < .01 for the difference between cells stimulated by EGF/estrogen/EGF + estrogen and untreated cells. (B2) Dominance of estrogen over EGF in combined stimulation. The figure demonstrates a summary of the results of the experiments presented in B1, using LY294002. An average inhibition value (% ± SD) of n > 3 is presented.
The involvement of PI3K in CXCL8 regulation is illustrated also in Figure 4B2, showing that, in the presence of estrogen, the ability of EGF to induce CXCL8 up-regulation through PI3K activation was significantly shutoff; therefore, estrogen dictated the response and “dominated” over the activities of EGF. It is possible that this downregulation of PI3K activities was due to the ability of estrogen to partly reduce AKT phosphorylation because we have obtained in some of the experiments an inhibitory effect of estrogen on EGF-induced AKT activation (data not shown).
To conclude this part of the study, we found that EGF activities on CXCL8 were mediated by ErbB2 and ErbB1 and thereafter by the downstream mediators Erk and PI3K. Whereas estrogen could partly induce CXCL8 through some of these signaling pathways, its addition to EGF has led to partial or complete shutoff of the involvement of these activation events in CXCL8 regulation.
Additive Effects of EGF + Estrogen on CXCL8 Release: Involvement of Transcription Factors
The above results indicate that signaling pathways were not the key mediators of CXCL8 up-regulation after stimulation by estrogen, either alone or together with EGF. Because the prime activities of estrogen are mediated by the transcriptional activation of its receptor ERα [11–14,30], the thus-far obtained findings suggested that the activities of estrogen on CXCL8 may have been channeled to the level of transcription.
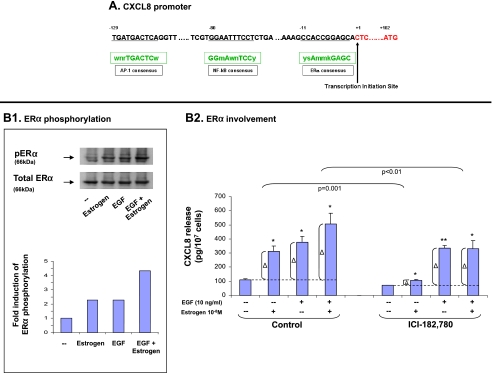
This possibility was supported by the fact that the promoter of the CXCL8 gene contains a binding site for ERα (Figure 5A). Because the transcriptional activation of ERα depends to a large extent on its phosphorylation at serine 118 [11–14,30,36,37], we asked whether estrogen induced the phosphorylation of ERα at this site in our experimental system. Our analyses indicated that indeed, estrogen induced the phosphorylation of ERα at serine 118 (Figure 5B1). Furthermore, by the use of the antagonist ICI-182,780 (fulvestrant) that induces ERα degradation [38,39], we could show that ERα had a very important role in estrogen-induced CXCL8 up-regulation (Figure 5B2). Taken together with the low competence of estrogen in inducing CXCL8 release through signaling pathways, the results obtained herein—on the importance of ERα in estrogen-induced CXCL8 up-regulation and on the phosphorylation of ERα on serine 118—indicate that estrogen activities through ERα were indeed channeled to the control of CXCL8 at the transcription level.
Figure 5.
The additive up-regulation of CXCL8 induced by EGF + estrogen: Involvement of ERα. (A) A region of the CXCL8 promoter. The consensus sequences of ERα, NF-κB, and AP-1 are indicated in boxes, and their corresponding sequences in the CXCL8 promoter are underlined. Small letters: n = A/T/C/G, w = A/T, r = A/G, m = A/C, y = T/C, s = G/C, k = G/T. (B) The activation of ERα and its roles in CXCL8 regulation. (B1) Phosphorylation of ERα on serine 118. MCF-7 cells were stimulated by estrogen (10-8 M), EGF (10 ng/ml), EGF + estrogen (estrogen 10-8M + EGF 10 ng/ml), or ethanol as control for 10 minutes. Time points were selected based on kinetics analyses, as described in Materials and Methods. ERα phosphorylation was determined by Western blot analysis. A representative experiment of n = 3 is presented. (B2) ERα partly mediates the additive effects of EGF + estrogen on CXCL8 expression. MCF-7 cells were grown as detailed in Figure 1A. Two hours before addition of estrogen, the cells were pretreated with conventional concentrations of ICI-182,780 (1 εM) or DMSO (control = the drug's solubilizer). Then, the growth of the cells was continued in the absence or presence of the drug (or DMSO as control). CXCL8 extracellular expression was determined in the supernatants of the cells by ELISA and was analyzed in the linear range of absorbance. Δ, The net amount of CXCL8 added to cell supernatant on stimulation. These values were used for statistical evaluations of differences between the control group and the inhibitor-treated group. A representative experiment of n > 3 is presented. *P < .05, **P < .01 for the difference between cells stimulated by EGF/estrogen/EGF + estrogen and untreated cells.
Published findings indicate that the cross talk between EGF stimulation and ERα activation is manifested by the ability of EGF to induce the phosphorylation of ERα on serine 118 [36,37,40–42]. Accordingly, we observed that EGF induced the activation of ERα indicated by ERα phosphorylation on this serine residue (Figure 5B1). However, this EGF-induced activation of ERα was not “productive” in terms of CXCL8 up-regulation because the ability of EGF to promote CXCL8 release was not affected by ICI-182,780 (Figure 5B2). The “incompetence” of EGF to act through ERα to promote CXCL8 release was also demonstrated in EGF + estrogen costimulation: Under the costimulatory conditions, ERα was only partly involved in CXCL8 up-regulation. Because ERα was not at all involved in the sole activities of EGF (Figure 5B2), we concluded that, in the concomitant stimulation by EGF + estrogen, only estrogen has led to “productive” ERα activation as a transcription factor, through which it promoted CXCL8 release. In addition, one cannot exclude the possibility that the activated ERα could induce low and indirect activation of the signaling pathways that were found to be involved in CXCL8 up-regulation, specifically of Erk (Figure 3).
Because of these results, showing that EGF did not upregulate CXCL8 expression by activating ERα, we determined the involvement of other transcription factors in EGF activity on CXCL8. Here, we focused on NF-κB and AP-1 because they are largely involved in EGF activities, and they are the most substantial factors regulating the transcription of inflammatory chemokines, including of CXCL8 in breast cancer [43–46]. Accordingly, the CXCL8 promoter expresses the corresponding binding sites for NF-κB and AP-1 (Figure 5A).
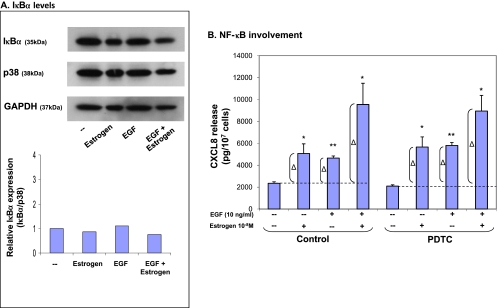
The translocation of NF-κB to the nucleus is enabled by the degradation of its inhibitor, IκBα [47]. Therefore, the level of intracellular expression of IκBα is used as a conventional estimate of NF-κB activation, and similarly, we have used this approach in a kinetics-based analysis (see Materials and Methods). The results in Figure 6A show that IκBα levels were not at all affected by exposure to EGF, and that they were not changed by estrogen or EGF + estrogen stimulations, suggesting that NF-κB is not involved in CXCL8 up-regulation by these stimulants. Supporting these results were experiments in which the activities of NF-κB were inhibited by the specific inhibitor PDTC (Figure 6B). The inhibitor was used at conventional concentrations for MCF-7 cells (60 εM; and was tested preliminary in higher concentration with similar results). The results in Figure 6B show that PDTC did not affect the release of CXCL8 in response to EGF, estrogen, or EGF + estrogen. Here, we wish to note that we have ensured that the inhibitor was active by using it in a parallel set of experiments of an unrelated study, where the inhibitor was indeed functional (data not shown). On the basis of these results, suggesting that NF-κB is not involved in EGF- and/or estrogen-induced CXCL8 up-regulation, we did not follow this direction any further.
Figure 6.
The additive up-regulation of CXCL8 induced by EGF + estrogen: Involvement of NF-κB. (A) Activation of NF-κB determined by expression levels of IκBα. MCF-7 cells were grown in the presence of estrogen (10-8 M, 54 hours), EGF (10 ng/ml, last 6 hours), EGF + estrogen (estrogen: 10-8 M, 54 hours; EGF: 10 ng/ml, last 6 hours), or ethanol as control. The expression levels of IκBα were determined by Western blot analysis. The experiment represents similar stimulations, preformed under varying exposure times of EGF, estrogen and both together (see Materials and Methods). (B) Inhibition of NF-κB activities by PDTC. MCF-7 cells were grown as detailed in Figure 1A. Two hours before addition of EGF, the cells were pretreated with conventional concentrations of the specific NF-κB inhibitor PDTC (60 εM; diluted in water; the drug was active in other experimental designs—data not shown). Then, the growth of the cells was continued in the absence or presence of the drug. CXCL8 extracellular expression was determined in the supernatants of the cells by ELISA and was analyzed in the linear range of absorbance. Δ, The net amount of CXCL8 added to cell supernatant on stimulation. These values were used for statistical evaluations of differences between the control group and the inhibitor-treated group. A representative experiment of n = 3 is presented. *P < .05, **P < .01 for the difference between cells stimulated by EGF/estrogen/EGF + estrogen and untreated cells.
As indicated above, the promoter of CXCL8 expresses a binding site for the transcription factor AP-1, composed of dimers of c-Jun with other Jun proteins/AP-1 members [47,48]. Joined by the fact that AP-1 is a potent inducer of CXCL8 transcription in conditions that are related to immune activities, we raised the possibility that AP-1 is involved in CXCL8 up-regulation in response to EGF, estrogen, or EGF + estrogen stimulation in the tumor cells. This possibility was supported by chromatin immunoprecipitation studies that we have performed with antibodies to c-Jun compared with isotype control antibodies. These preliminary studies have provided initial solid evidence to specific binding of c-Jun to the endogenous CXCL8 promoter in MCF-7 cells and also in human breast cancer MDA-MB-231 cells (data not shown; these studies are intended to serve as basis for future analyses of regulation of CXCL8 transcription, which are beyond the scope of this study).
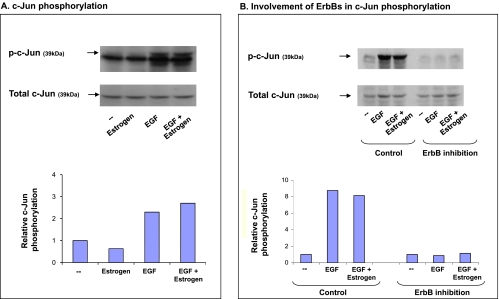
On the basis of the above, we asked whether the different stimulatory setups—EGF, estrogen, or EGF + estrogen—activate c-Jun. The results in Figure 7A indicate that EGF induced potent activation of c-Jun; however, estrogen did not. The phosphorylation of c-Jun in response to costimulation by EGF + estrogen was similar to that with EGF alone (Figure 7A), indicating that, in the joint stimulatory setup, it was actually EGF that induced AP-1 activation.
Figure 7.
The additive up-regulation of CXCL8 induced by EGF: Involvement of AP-1. (A) Activation of AP-1. MCF-7 cells were stimulated by estrogen (10-8 M), EGF (10 ng/ml), EGF + estrogen (estrogen 10-8 M + EGF 10 ng/ml), or ethanol as control for 10 minutes. Time points were selected based on kinetics analyses, as described in Materials and Methods. c-Jun phosphorylation was determined by Western blot analysis. A representative experiment of n = 4 is presented. (B) Inhibition of AP-1 activation by ErbB1 + ErbB2 inhibitors. MCF-7 cells were pretreated with AG825 (10 εM) + AG1478 (0.5 εM) or DMSO (control = the drug's solubilizer) for 2 hours. Then, the cells were incubated for additional 10 minutes in the presence of EGF (10 ng/ml), estrogen + EGF (estrogen 10-8 M; EGF 10 ng/ml), or ethanol as control. c-Jun phosphorylation was determined by Western blot analysis. A representative experiment of n = 2 is presented.
Additional experiments have shown that the activation of c-Jun was completely shutoff by inhibition of ErbBs when the cells were stimulated by EGF alone (Figure 7B). This finding is in line with our previous findings, showing that ErbBs—and mainly ErbB2—are required for EGF-induced CXCL8 expression, suggesting that the events flow from EGF stimulation, through ErbB activation to c-Jun activation, and then to CXCL8 up-regulation.
In parallel, additional results in Figure 7B indicate that, also in the joint stimulatory setup, EGF was active through ErbB stimulation that leads to c-Jun activation. These results suggest that, in the joint stimulation, EGF acts through ErbBs and than c-Jun, to upregulate CXCL8 expression, whereas estrogen acts through ERα (as indicated by Figure 5). Taken together, these findings explain why ErbB2 was only partly involved in EGF + estrogen-induced CXCL8 upregulation (as shown in Figure 2), and support the involvement of c-Jun in the ability of EGF to induce CXCL8 up-regulation.
The above results indicated that it was actually EGF that has induced c-Jun activation in the tumor cells. On the basis of these results, we wished to determine the possibility that EGF promoted through AP-1 the transcription of CXCL8, leading to increased synthesis of the chemokine and to its elevated release by the tumor cells. To see if this was indeed the case, we looked for appropriate manners to determine the roles of AP-1 in regulating EGF-induced CXCL8 expression. In the absence of a specific inhibitor of c-Jun, we took the small hairpin RNA approach and also used the dominant negative variant of c-Jun, TAM67. In agreement with the fact that c-Jun is essential for cell proliferation [48,49], we could not obtain transient or stable transfectants in which the expression/activation of c-Jun was downregulated.
Therefore, we took an alternate approach and used constructs expressing the luciferase reporter, under the regulation of CXCL8 promoter, where the promoter expressed the AP-1 binding site in a WT or a mutated form. The use of the luciferase reporter enabled us to determine to which extent the activation of AP-1 was required for basal and EGF-induced CXCL8 transcription in our system of MCF-7 cells.
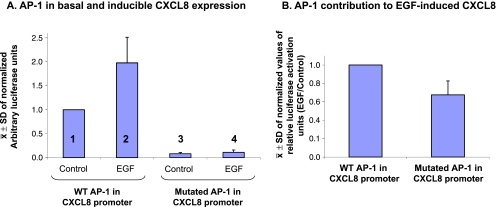
When the constructs of CXCL8 promoters were expressed in the tumor cells, the basal transcription of the CXCL8 gene was extremely reduced in the context of mutated AP-1 binding site (Figure 8A, lane 1 vs lane 3). These results indicate that the basal transcription of CXCL8 is highly dependent on AP-1. Furthermore, in the context of the AP-1-mutated CXCL8 promoter, EGF had considerably lower ability to promote transcription (Figure 8A, lane 2 vs lane 4; and Figure 8B; Conditions selected based on kinetics analyses, as indicated in Materials and Methods). These results indicate that, in breast tumor cells, the ability of EGF to induce the transcription of CXCL8 is highly dependent on AP-1 activation (similar analyses were not performed for estrogen-induced activation of AP-1, because the findings of Figure 7A indicated that the hormone did not activate the AP-1 component c-Jun).
Figure 8.
CXCL8 transcription is regulated by the AP-1 binding site. MCF-7 cells were transfected with 1) constructs of firefly luciferase under the control of promoters expressing WT or mutated AP-1 binding sites and 2) construct of renilla luciferase. The cells were either not stimulated (= Control) or stimulated for 4 hours with EGF (10 ng/ml). Time points were selected based on kinetics analyses, as described in Materials and Methods. (A) The figure shows the ± SD of normalized results obtained in four independent experiments, all having similar results. In each of the experiments, the expression of luciferase firefly was normalized relatively to renilla firefly. (B) The results of part A are presented in each of the AP-1 promoters, namely WT and mutated, as “Fold induction of luciferase units (EGF/control).” The figure presents the ± SD of normalized values of luciferase activation levels in four independent experiments, all showing similar results.
Together, the results of this part of the study indicate that in the combined stimulation of breast tumor cells by EGF + estrogen, EGF promoted the transcription of CXCL8 by activating AP-1, whereas estrogen acted through ERα. Therefore, the two stimulatory factors act in independent pathways to upregulate the transcription of CXCL8, together giving rise to the additive production and secretion of CXCL8 by the tumor cells.
Discussion
In this study, we have provided novel insights to the molecular mechanisms involved in interactions between promalignancy factors in breast cancer. Our findings indicate that EGF and estrogen can each upregulate the release of the angiogenic chemokine CXCL8 by breast tumor cells, and furthermore, that they can act in an additive manner to promote CXCL8 transcription and expression.
Our findings strengthen published studies suggesting the existence of interactions between CXCL8 and EGF, as well as between CXCL8 and estrogen. Previous studies with neutralizing antibodies have shown that EGF and CXCL8 act in a cooperative manner to promote the metastatic potential of breast tumor cells [4]. Along these lines, it was found that the overexpression of ErbB2 in breast tumor cells has led to an increase in CXCL8 release by the cells and that CXCL8 was expressed to a higher degree in ErbB2-positive than in ErbB2-negative patients [8,50].
In parallel, the study of Bendrik and Dabrosin [51] has shown a significantly positive correlation between the plasma levels of CXCL8 and of estrogen in normal breast tissues, and that there was an increased secretion of CXCL8 by normal human breast tissue biopsies and breast tumor cells on exposure to estrogen.
Other studies have addressed the expression and roles of CXCL8 in association with the expression of ERα. Here, it is important to note that the roles of estrogen and of its receptor in breast cancer are complex, and whereas estrogen is a growth factor to breast tumor cells, the lack of ERα is used in the clinic as a marker of poor prognosis [52–54]. Accordingly, ERα-negative breast tumor cells are known to have an aggressive phenotype, and recent findings indicate that they express higher CXCL8 levels than ERα-positive cells [3,8,55,56]. The invasion potential of ERα-negative cells was regulated by CXCL8 in vitro, and CXCL8 promoted the growth and metastatic potential of ERα-negative breast tumor cells in vivo [5,6,55].
Overall, these studies indicate that CXCL8 stands in the focal point of EGF and estrogen activities. Our findings indicate that there is triple-factor net between these elements because EGF can act in additivity together with estrogen to upregulate the release of CXCL8 by breast tumor cells.
Our study has taken this issue further and has performed a detailed analysis of the molecular mechanisms involved in CXCL8 regulation by the sole or joint activities of EGF and estrogen. Our investigation has provided novel insights to the complexity of EGF and estrogen activities and of CXCL8 regulation. When EGF acted alone, it upregulated CXCL8 by activating signaling pathways (Erk and, to some extent, also PI3K) and the transcription of the CXCL8 gene through AP-1. In contrast, when estrogen was the sole stimulant, its ability to promote CXCL8 release was only partly mediated by the Erk pathway, and its activities were channeled to the activation of ERα, acting in this context as a transcription factor on the CXCL8 gene.
Of interest were the events taking place in joint stimulation of the cells by EGF + estrogen. Under these conditions, the relatively low potential of estrogen to control CXCL8 expression by the signaling pathways dominated over the more potent effects of EGF on Erk and PI3K activation. Specifically, the addition of estrogen to EGF has led to partial (Erk) or complete (PI3K) reduction in the involvement of these pathways in CXCL8 regulation. Nevertheless, it is possible that the remaining activities of Erk in the joint stimulation reflect both direct activation by EGF, as well as indirect stimulation due to estrogeninduced ERα activation, that could lead through intracellular cross talk to the stimulation of this enzyme.
These observations indicate that EGF and estrogen can act independently to promote CXCL8 release by breast tumor cells and that their activities are additive. Therefore, in conditions where both ErbBs and ERα are active, there would be a need to target both pathways to achieve an effective inhibition of CXCL8 release by the tumor cells. On the basis of the literature, such an approach would be important in two clinical conditions:
Breast cancer patients expressing both ErbBs and ERα: The expression patterns of ERα and ErbBs differ considerably in breast cancer patients. Whereas the expression of ERα prevails in 70% to 80% of the patients, amplification and overexpression of ErbB2 are denoted in 20% to 25% of the patients. On the basis of immunohistochemistry analysis, it was found that approximately half of the ErbB2-positive patients are ERα-positive, that is, approximately 10% of the patients overall [57]. In this specific subpopulation of patients, EGF and estrogen could each act independently through ErbBs and ERα, respectively, to promote the release of CXCL8 by the tumor cells.
-
Patients manifesting interactions between ErbBs and ERα: The regulation of estrogen, its receptors, and their interactions with RTKs—such as ErbBs—are complex and much is still to be learnt. However, recent studies suggest that each of the two pathways can regulate the other at the level of receptor expression/activation. Between others, it was found that anti-RTK therapies can activate the estrogen-related pathway and vice versa. To give only few examples, the study by Xia et al. has shown that long-term treatment of breast cancer patients with lapatinib, the dual inhibitor of ErbB1/ErbB2, was associated with increased signaling by estrogen receptors. Also, lapatinib induced ER signaling in tumor biopsies from patients with ErbB2-overexpressing breast cancers [20,58]. In parallel, endocrine treatment of estrogen receptor-positive breast cancer has led to endocrine resistance, associated with increased expression and signaling by ErbB1/ErbB2 [20,59].
The above findings suggest that, in patients treated with inhibitors to only one of the two pathways, the other pathway may be amplified. Therefore, in patients treated with anti-RTK drugs, the endocrine pathway may be activated and vice versa. Under these conditions, the tumor cells may express elevated levels of both ErbBs and ERα, enabling them to respond to signals provided by EGF and estrogen. On the basis of our results, this may lead to an additive increase in the release of CXCL8 by the tumor cells.
Our results reinforce the recently considered therapeutic approach, suggesting treating specific groups of breast cancer patients with combined therapies directed against ErbBs and ERα together [11,16,20,30]. When CXCL8 regulation is concerned, patients belonging to the above-mentioned two clinical subgroups would benefit from such simultaneous treatment, as it would inhibit each of the two arms responsible for elevated release of CXCL8 at the tumor site: the RTK pathway, which is mediated by ErbB1/ErbB2, and the endocrine pathway of ERα.
Acknowledgments
The authors thank H. Muhl, University Hospital Goethe-University Frankfurt, for kindly providing the firefly luciferase constructs with the WT and mutated AP-1 binding site in the CXCL8 promoter [32]. The authors thank E. Bacharach, A. Katz, C. Gllit-Santar (Tel Aviv University), and F. Lantner (Weizmann Institute) for their assistance in setting up the chromatin immunoprecipitation analyses. The authors also thank T. Zor and D. Avni from Tel Aviv University for their assistance in the luciferase assays and cAMP analyses.
Abbreviations
- ERα
estrogen receptor α
- PDTC
pyrrolidinedithio-carbamate ammonium
- PI3K
phosphoinositide 3-kinase
- RTK
receptor tyrosine kinase
Footnotes
This research was supported (in part) by grant no. 300000-4877 from the Weinkselbaum Family Medical Research Fund (through the Chief Scientist Office of the Ministry of Health Israel), and by Federico Foundation.
References
- 1.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 3.Chen JQ, Russo J. ERα-negative and triple negative breast cancer: molecular features and potential therapeutic approaches. Biochim Biophys Acta. 2009;1796:162–175. doi: 10.1016/j.bbcan.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salcedo R, Martins-Green M, Gertz B, Oppenheim JJ, Murphy WJ. Combined administration of antibodies to human interleukin 8 and epidermal growth factor receptor results in increased antimetastatic effects on human breast carcinoma xenografts. Clin Cancer Res. 2002;8:2655–2665. [PubMed] [Google Scholar]
- 5.Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121:1949–1957. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- 6.Yao C, Lin Y, Ye CS, Bi J, Zhu YF, Wang SM. Role of interleukin-8 in the progression of estrogen receptor-negative breast cancer. ChinMed J (Engl) 2007;120:1766–1772. [PubMed] [Google Scholar]
- 7.Snoussi K, Mahfoudh W, Bouaouina N, Fekih M, Khairi H, Helal AN, Chouchane L. Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer. 2010;10:283. doi: 10.1186/1471-2407-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Baruch A. Pro-malignancy and Putative Anti-malignancy Chemokines in the Regulation of Breast Cancer Progression. New York, NY: Nova Science Publishers; 2006. [Google Scholar]
- 10.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox EM, Andrade J, Shupnik MA. Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids. 2009;74:622–627. doi: 10.1016/j.steroids.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietras RJ. Interactions between estrogen and growth factor receptors in human breast cancers and the tumor-associated vasculature. Breast J. 2003;9:361–373. doi: 10.1046/j.1524-4741.2003.09510.x. [DOI] [PubMed] [Google Scholar]
- 14.Osborne CK, Schiff R. Growth factor receptor cross-talk with estrogen receptor as a mechanism for tamoxifen resistance in breast cancer. Breast. 2003;12:362–367. doi: 10.1016/s0960-9776(03)00137-1. [DOI] [PubMed] [Google Scholar]
- 15.Santen RJ, Fan P, Zhang Z, Bao Y, Song RX, Yue W. Estrogen signals via an extra-nuclear pathway involving IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer cells. Steroids. 2009;74:586–594. doi: 10.1016/j.steroids.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Lichtner RB. Estrogen/EGF receptor interactions in breast cancer: rationale for new therapeutic combination strategies. Biomed Pharmacother. 2003;57:447–451. doi: 10.1016/j.biopha.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Amit I, Wides R, Yarden Y. Evolvable signaling networks of receptor tyrosine kinases: relevance of robustness to malignancy and to cancer therapy. Mol Syst Biol. 2007;3:151. doi: 10.1038/msb4100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Phy siol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 19.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 20.Johnston SR. Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer. 2009;9(suppl 1):S28–S36. doi: 10.3816/CBC.2009.s.003. [DOI] [PubMed] [Google Scholar]
- 21.Fischer OM, Streit S, Hart S, Ullrich A. Beyond Herceptin and Gleevec. Curr Opin Chem Biol. 2003;7:490–495. doi: 10.1016/s1367-5931(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Brdlik C, Jin P, Shepard HM. A pan-HER approach for cancer therapy: background, current status and future development. Expert Opin Biol Ther. 2009;9:97–110. doi: 10.1517/14712590802630427. [DOI] [PubMed] [Google Scholar]
- 23.Janni W, Hepp P. Adjuvant aromatase inhibitor therapy: outcomes and safety. Cancer Treat Rev. 2010;36:249–261. doi: 10.1016/j.ctrv.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Cleator SJ, Ahamed E, Coombes RC, Palmieri C. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9(suppl 1):S6–S17. doi: 10.3816/CBC.2009.s.001. [DOI] [PubMed] [Google Scholar]
- 25.Howell A. The endocrine prevention of breast cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:615–623. doi: 10.1016/j.beem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Prenzel N, Zwick E, Leserer M, Ullrich A. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2000;2:184–190. doi: 10.1186/bcr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudelist G, Singer CF, Manavi M, Pischinger K, Kubista E, Czerwenka K. Co-expression of ErbB-family members in human breast cancer: Her-2/neu is the preferred dimerization candidate in nodal-positive tumors. Breast Cancer Res Treat. 2003;80:353–361. doi: 10.1023/A:1024929522376. [DOI] [PubMed] [Google Scholar]
- 28.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 29.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 30.Johnston SR, Martin LA, Leary A, Head J, Dowsett M. Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J Steroid Biochem Mol Biol. 2007;106:180–186. doi: 10.1016/j.jsbmb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093–1102. [PubMed] [Google Scholar]
- 32.Hellmuth M, Wetzler C, Nold M, Chang JH, Frank S, Pfeilschifter J, Muhl H. Expression of interleukin-8, heme oxygenase-1 and vascular endothelial growth factor in DLD-1 colon carcinoma cells exposed to pyrrolidine dithiocarbamate. Carcinogenesis. 2002;23:1273–1279. doi: 10.1093/carcin/23.8.1273. [DOI] [PubMed] [Google Scholar]
- 33.Levin ER. Membrane oestrogen receptor α signalling to cell functions. J Physiol. 2009;587:5019–5023. doi: 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 35.Mizukami Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J. 2010;57:101–107. doi: 10.1507/endocrj.k09e-332. [DOI] [PubMed] [Google Scholar]
- 36.Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, Nicholson RI. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12(suppl 1):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 37.Murphy LC, Weitsman GE, Skliris GP, Teh EM, Li L, Peng B, Davie JR, Ung K, Niu Y-L, Troup S, et al. Potential role of estrogen receptorα (ERα) phosphorylated at serine118 in human breast cancer in vivo. J Steroid Biochem Mol Biol. 2006;102:139–146. doi: 10.1016/j.jsbmb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Kabos P, Borges VF. Fulvestrant: a unique antiendocrine agent for estrogen-sensitive breast cancer. Expert Opin Pharmacother. 2010;11:807–816. doi: 10.1517/14656561003641982. [DOI] [PubMed] [Google Scholar]
- 39.Johnston SJ, Cheung KL. Fulvestrant—a novel endocrine therapy for breast cancer. Curr Med Chem. 2010;17:902–914. doi: 10.2174/092986710790820633. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly JM, et al. Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–4931. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- 41.Joel PB, Traish AM, Lannigan DA. Estradiol-induced phosphorylation of serine 118 in the estrogen receptor is independent of p42/p44 mitogen-activated protein kinase. J Biol Chem. 1998;273:13317–13323. doi: 10.1074/jbc.273.21.13317. [DOI] [PubMed] [Google Scholar]
- 42.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. Embo J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 43.Bièche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, Guinebretière JM, Burlinchon S, Lidereau R, Lazennec G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer. 2007;14:1039–1052. doi: 10.1677/erc.1.01301. [DOI] [PubMed] [Google Scholar]
- 44.Fong YC, Maa MC, Tsai FJ, Chen WC, Lin JG, Jeng LB, Yang RS, Fu WM, Tang CH. Osteoblast-derived TGF-β1 stimulates IL-8 release through AP-1 and NF-κB in human cancer cells. J Bone Miner Res. 2008;23:961–970. doi: 10.1359/jbmr.080206. [DOI] [PubMed] [Google Scholar]
- 45.Freund A, Jolivel V, Durand S, Kersual N, Chalbos D, Chavey C, Vignon F, Lazennec G. Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene. 2004;23:6105–6114. doi: 10.1038/sj.onc.1207815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chavey C, Muhlbauer M, Bossard C, Freund A, Durand S, Jorgensen C, Jobin C, Lazennec G. Interleukin-8 expression is regulated by histone deacetylases through the nuclear factor-κB pathway in breast cancer. Mol Pharmacol. 2008;74:1359–1366. doi: 10.1124/mol.108.047332. [DOI] [PubMed] [Google Scholar]
- 47.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent IκBα phosphorylation marks the NF-κB inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartl M, Bader AG, Bister K. Molecular targets of the oncogenic transcription factor jun. Curr Cancer Drug Targets. 2003;3:41–55. doi: 10.2174/1568009033333781. [DOI] [PubMed] [Google Scholar]
- 49.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Martin A, Colomer R, Menendez JA. Her-2/neu-induced “cytokine signature” in breast cancer. Adv Exp Med Biol. 2008;617:311–319. doi: 10.1007/978-0-387-69080-3_29. [DOI] [PubMed] [Google Scholar]
- 51.Bendrik C, Dabrosin C. Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. J Immunol. 2009;182:371–378. doi: 10.4049/jimmunol.182.1.371. [DOI] [PubMed] [Google Scholar]
- 52.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Keen JC, Davidson NE. The biology of breast carcinoma. Cancer. 2003;97:825–833. doi: 10.1002/cncr.11126. [DOI] [PubMed] [Google Scholar]
- 54.Fuqua SA. The role of estrogen receptors in breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2001;6:407–417. doi: 10.1023/a:1014782813943. [DOI] [PubMed] [Google Scholar]
- 55.Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, Vignon F, Lazennec G. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22:256–265. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, Huang RP. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109:507–515. doi: 10.1002/ijc.11724. [DOI] [PubMed] [Google Scholar]
- 57.Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr Relat Cancer. 2001;8:191–195. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- 58.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulazzo G, Lyass L, Trusk P, Hill J, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M. Enhanced estrogen receptor (ER) α, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long-term estrogen deprivation. J Biol Chem. 2003;278:30458–30468. doi: 10.1074/jbc.M305226200. [DOI] [PubMed] [Google Scholar]