Abstract
Background and Aims
The association between hygiene and prevalence of autoimmune disease has been attributed in part to enteric helminth infection. A pilot study of experimental infection with the hookworm Necator americanus was undertaken among a group of otherwise healthy people with celiac disease to test the potential of the helminth to suppress the immunopathology induced by gluten.
Methods
In a 21-week, double-blinded, placebo-controlled study, we explored the effects of N. americanus infection in 20 healthy, helminth-naïve adults with celiac disease well controlled by diet. Staged cutaneous inoculations with 10 and 5 infective 3rd stage hookworm larvae or placebo were performed at week-0 and -12 respectively. At week-20, a five day oral wheat challenge equivalent to 16 grams of gluten per day was undertaken. Primary outcomes included duodenal Marsh score and quantification of the immunodominant α-gliadin peptide (QE65)-specific systemic interferon-γ-producing cells by ELISpot pre- and post-wheat challenge.
Results
Enteric colonisation with hookworm established in all 10 cases, resulting in transiently painful enteritis in 5. Chronic infection was asymptomatic, with no effect on hemoglobin levels. Although some duodenal eosinophilia was apparent, hookworm-infected mucosa retained a healthy appearance. In both groups, wheat challenge caused deterioration in both primary and several secondary outcomes.
Conclusions
Experimental N. americanus infection proved to be safe and enabled testing its effect on a range of measures of the human autoimmune response. Infection imposed no obvious benefit on pathology.
Trial Registration
ClinicalTrials.gov NCT00671138
Introduction
The “hygiene hypothesis” proposes that the increasing prevalence of allergic and autoimmune diseases in developed countries may be due to the reduction in the incidence of infectious diseases [1]; epidemiologic and genetic studies suggest that the decline in the prevalence of helminth infections is a major factor [2]–[5]. Observational studies supporting this hypothesis include a report that infection with the nematode Strongyloides stercoralis is associated with protection against autoimmune liver disease [6], and another showing that unspecified helminth infections with peripheral eosinophilia are associated with reduced autoantigen-specific responses and disease progression in multiple sclerosis [7]. Co-infection with helminths is also known to attenuate murine models of autoimmunity and inflammatory bowel disease [8], [9].
Despite being classed as pathogens [10], helminth parasites are being proposed as treatments for allergic and autoimmune diseases [11], [12]. Preliminary observations suggest that intentional infection with the zoophilic Trichuris suis is safe [13], and reduces the activity of inflammatory bowel disease [13], [14]. However, dosing with T. suis every three weeks is required to maintain ongoing infection because the human host does not sustain development of T. suis to maturity [14].
Necator americanus (NA) is a long-lived hematophagous, human-specific (anthropophilic) gastrointestinal nematode that infects over 500 million people in developing countries where heavy infection causes iron deficiency anemia, and is associated with reduced physical and intellectual development [15]. Experimental infection of healthy volunteers with NA infective third-stage larvae (L3) may cause an acute, painful enteropathy [16]–[18]. Recent published data indicate that low-dose inocula of NA are better tolerated [19], and because they do not proliferate in humans, a defined dose can be administered and later fully eliminated with anthelmintic therapy [19]. A further advantage is infected individuals pose no risk to others because hookworms are soil-transmitted (geohelminths) and cannot be propagated in modern sanitary environments.
We chose NA and celiac disease (CD) to explore the relationship between helminth infection and intestinal inflammation due to a well characterised dietary antigen, gluten. Our previous studies with this hookworm have provided us with a pure source of infective larvae [18], [20]. Individuals carrying chronic infection with hookworms in endemic settings demonstrate parasite-specific TH2 responses, but TH1 and TH2 immune responses to other antigens are diminished [21].
Celiac disease is uniquely suited to explore the effects of helminth infection; it is common [22] and remission is achieved with elimination of dietary gluten allowing host-parasite interaction to be studied free of potential artefacts caused by medications. Clinical studies in volunteers with CD also have the advantage that the effects of deliberate gluten exposure can be measured by symptom response, in blood and intestinal tissue [23]–[26]. Over 90% of people with CD possess genes encoding the major histocompatability (MHC) Class II molecule, HLA-DQ2, and HLA DQ2-restricted CD4+ T cells specific for deamidated gluten peptides can be isolated from intestinal tissue [26]. HLA DQ2-restricted CD4+ TH1 cells specific for deamidated gluten including the immunodominant α-gliadin 17-mer p57-73 (QE65) peptide are also present in blood after oral wheat challenge [27].
In this study, we undertook a clinical trial to test whether NA infection reduces the immunotoxic effects of gluten in CD.
Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1.
Ethics Statement
The Princess Alexandra Hospital, Queensland Institute of Medical Research and Townsville Hospital Human Research Ethics Committees approved the study. Written informed consent was obtained from all subjects.
Patients
Healthy people with CD aged 18 years or older were invited to participate through the Queensland Coeliac Society. Inclusion criteria included: 1) pre-treatment histological diagnosis of Marsh grade 3 celiac disease [25], 2) positive immunoglobulin (Ig) A anti-tissue transglutaminase (tTG) or anti-endomysial antibody, 3) HLA-DQ2 phenotype, and 4) adherence to a gluten-free diet (GFD) for at least 6 months pre-enrolment [28]. Subjects were excluded if they had 1) insulin dependent diabetes mellitus or Addison's disease; 2) life threatening allergy; 3) treatment with immunomodulatory therapies within the six months prior to enrolment (including aspirin, non-steroidal anti-inflammatory drugs, celocoxib inhibitors, statins or intramuscular and intravenous steroids); 4) an unmanaged risk of pregnancy; 5) historical, fecal or serological evidence of a prior helminth infection; 6) iron deficiency anemia, 7) any vaccination within the 30-day period prior to study commencement, or 8) an elevated level of tTG IgA.
Study Design
This was a prospective, randomized, double-blinded, placebo-controlled trial evaluating the safety, tolerability and immunological effects of NA infection in subjects with CD in remission on gluten free diet and during wheat challenge. Participants were matched by age and gender (Table 1). Using a random number generation sequence, the researcher preparing inocula independently of the clinical support, assigned participants to the “hookworm” and “control” groups. Primary end-points included duodenal histology Marsh scores and systemic interferon-γ measured by QE65-ELISpot pre- and post-wheat challenge. Secondary end-points included a) the clinical response to inoculation with hookworm, and b) subsequent response to the in vivo wheat challenge, as measured by symptom and laboratory indices, intraepithelial duodenal CD4+, CD8+ and CD3+ lymphocyte counts (IEL), and duodenal villus height/crypt depth (Vh/Cd) ratios. Because this was a Phase 1b/2a clinical trial assessing safety, the hookworm and control sample sizes were deliberately limited to ten volunteers each. Subjects and investigators remained blinded throughout the trial.
Table 1. Participant demographics.
| Control Subjects (n = 10) | Hookworm Subjects (n = 10) | |
| Age (years)1 , 2 | 44 (25–58) | 47 (25–62) |
| Gender (M/F) | 2/8 | 2/8 |
| BMI (kg/m2)3 , 2 | 27 (22–32) | 26 (18–31) |
| IgA tTG3 (U/ml)4 , 2 | 9 (5–15) | 8 (5–19) |
| Duration (months)5 , 2 | 70 (10–325) | 77 (15–143 |
Age at enrolment,
mean value (range),
Body Mass Index,
Pre-enrolment IgA anti-tissue transglutaminase (normal range below 20 U/ml),
duration of gluten free diet.
Inocula and inoculation
Inoculations were performed at wk 0 (either 10 L3 or placebo) and wk 12 (either 5 L3 or placebo). Inocula of five or ten NA L3 were prepared as previously described [18]. Placebo consisted of an identically presented inoculum containing 0.2 ml McIlhenny Co Tabasco Pepper Sauce® to mimic the pruritus that accompanies skin penetration by L3. A control sample of L3 was submitted with every batch of inoculum including placebo, and was inspected microscopically for larval motility to verify viability. All vials and pipettes were examined following inoculation to ensure that there were no residual hookworm larvae. Evidence of hookworm infection was provided by peripheral blood eosinophil count (×109/L), and documenting hookworm eggs in feces by microscopy and/or by sighting a hookworm at endoscopy.
A strict GFD was maintained from weeks 0 to 20. Endoscopy was performed at week 20 using an Olympus high resolution digital endoscope; five single-bite biopsies were collected from the third part of the duodenum (D3), and seven from the second part (D2). Volunteers consumed two 50 g slices of white bread twice a day (equivalent to 16 g of gluten daily) for five days. On the sixth day, blood was collected and a second endoscopy was performed (referred to as the week 21 timepoint).
Monitoring, assessments and wheat challenge
Clinical reviews occurred at weeks 0, 4, 12, 20 and 21, and symptom diaries were recorded weekly. From weeks 0–20 and daily during the wheat challenge, participants were asked to complete a symptom score rating from 0 (none) to 3 (severe) bloating, constipation, flatulence, headaches, lethargy, mouth ulcers, nausea, skin rash, skin itch, and vomiting, and to record their number of bowel motions, loose bowel motions and urgent bowel motions, and the number of episodes of pain per day. Also recorded were pain intensity and general wellbeing on a visual analogue scale from “no pain at all” to “very severe” and “very well” to “severely ill” respectively.
Histopathological Analysis
Biopsies were fixed in neutral buffered formalin, processed and carefully orientated and embedded in paraffin wax. Sections (3 µm) were stained with hematoxylin and eosin (H&E) and immunostained with anti-CD8, anti-CD4 and anti-CD3 antibodies (all from Novocastra Laboratories Ltd). The Marsh scores were graded by two independent researchers [25]. The IELs per 100 nucleated enterocytes (100NE) were counted at 24 randomly selected sites between the villus tip to the base of the crypt in each biopsy. The Vh/Cd ratios were measured independently of the Marsh grading [24] and were performed on 10 randomly selected well-orientated sites.
Peripheral blood interferon-γ ELISpot responses to the immunodominant α-gliadin peptide p57–73 QE65
Peripheral blood mononuclear cells (PBMCs) were incubated with p57–73 QE65 (50 µg/ml) in overnight IFN-γ ELISpot assays, as previously described [27]. Briefly, ELISpot plates were coated with anti-IFN-γ (clone 1-D1K, Mabtech) and then blocked with 5% fetal calf serum (FCS). Freshly isolated PBMCs were added to wells containing medium alone (RPMI 1640, Invitrogen, 10% fetal bovine serum, 100 U/µl penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine) or p57–73 QE65 in medium, and cultured for 24 h at 37°C in 5% CO2. Cells were removed and the plates were incubated with anti-IFN-γ-biotin (clone 7-B6-1, Mabtech), then streptavidin-alkaline phosphatase, and developed with BCIP/NBT substrate. Spots were counted on an iSpot ELISpot reader (Autoimmun Diagnostika GmbH).
Statistical Analyses
Statistical analyses were performed using Prism5 (GraphPad). When comparing non-continuous variables (eg. symptom scores), area under the curve analysis from week 1 to week 20 followed by Mann-Whitney U test was used to compare between groups. For continuous variables (eg. duodenal IEL counts), two-way ANOVA was used to analyse between time-points and groups. In the figures, * = p<0.05, ** = p<0.01, *** = p<0.001.
Results
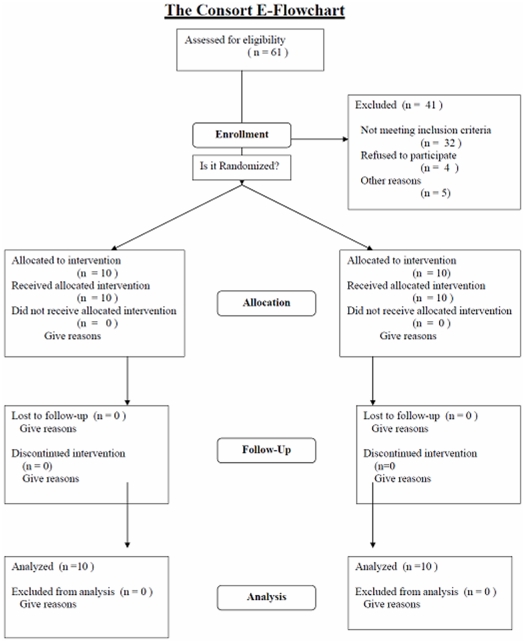
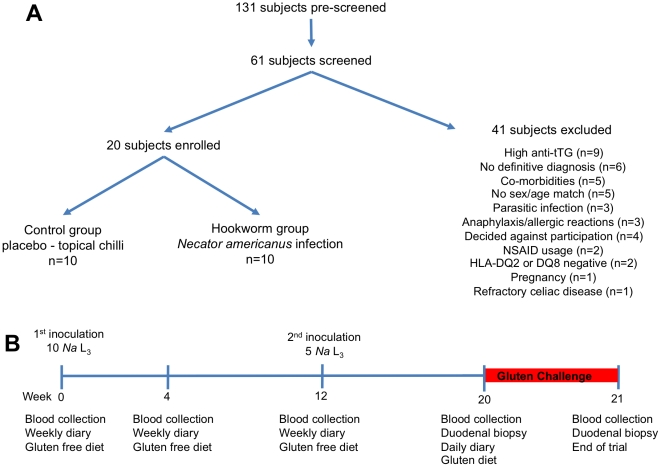
Between October 2007 and March 2008, 131 potential subjects indicated interest, 61 were screened and twenty were enrolled and completed the study without protocol violations (Figure 1). Reasons for exclusion of subjects are summarised in Figure 2.
Figure 1. CONSORT flowchart.
Figure 2. Recruitment and Protocol.
Recruitment and summation of those excluded post screening (A), and trial outline (B).
Hookworm Tolerance and Safety
Inoculation efficacy
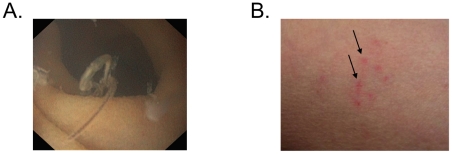
Mature hookworm infection was confirmed in each of the hookworm group by the identification of eggs in feces (n = 5), or by endoscopic visualisation of at least one adult worm (n = 8; Figure 3A), or by both (n = 3). In five subjects with proven infection, patency could not be confirmed by fecal microscopy despite the testing of 17 of 18 scheduled collections from wk 12–21. No control subject had evidence of hookworm infection and no subject had evidence of any other helminth infection.
Figure 3. Representative photographs of parasitic infection (A) Adult hookworm (B) Inoculation site.
Clinical response to N. americanus
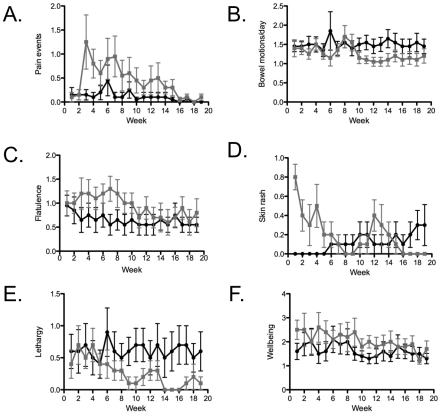
Symptom scores: No serious adverse event relating to hookworm infection occurred. All subjects in the hookworm group developed multiple tiny papules shortly after the application of L3 (Figure 3B). Symptoms were scored by questionnaire over the 20 weeks of hookworm infection (Figure 4A–F). The hookworm group experienced pain during the initial colonisation of the intestine, but this resolved completely by week 16 (Figure 4A). There were trends towards the hookworm group having less frequent bowel actions after colonisation was established (Figure 4B), and more flatulence (4C), nausea and bloating (data not shown) at earlier timepoints. The inoculation site remained itchy until week 4 (Figure 4D). Unexpectedly, the hookworm group experienced less lethargy than the controls (Figure 4E), and wellbeing was unaffected (Figure 4F). At week 21, 5 of 10 subjects in the control group incorrectly guessed that they were in the active hookworm-infected group, while 8 of the 10 in the hookworm group confidently and correctly identified his or her infection status. At the end of the study all infected subjects were offered anthelminthic treatment but declined.
Figure 4. Symptom responses to hookworm infection.
Control group is indicated by black circles, hookworm group by grey squares, values are mean +/− SEM. (A) pain events, (B) bowel motions/day, (C) flatulence, (D) skin rash, (E) lethargy, (F) wellbeing. Area under the curve analysis followed by Mann-Whitney U test to compare between groups showed that pain events (A) was significantly different between the groups.
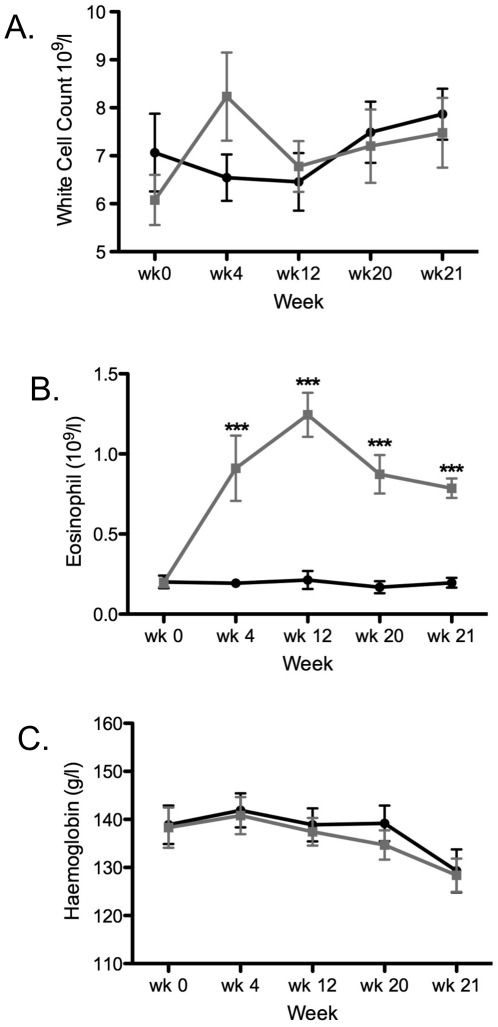
Laboratory Indices: A modest leukocytosis with an eosinophilia was noticeable from week 4 after hookworm inoculation (Figure 5A–B). Hemoglobin levels were stable from week 0 to 20, but dropped at week 21 in both groups after wheat challenge (Figure 5C).
Figure 5. Blood cell and marker levels.
(A) white cell, (B) eosinophil (C) hemoglobin. Control group is indicated by black circles, hookworm group by grey squares, values are mean +/− SEM. Data was analyzed by two-way ANOVA: significant effects of time and interaction was shown in (B), and post-hoc one-way ANOVA on each group showed the differences between timepoints as indicated.
Oral wheat challenge
Primary outcomes
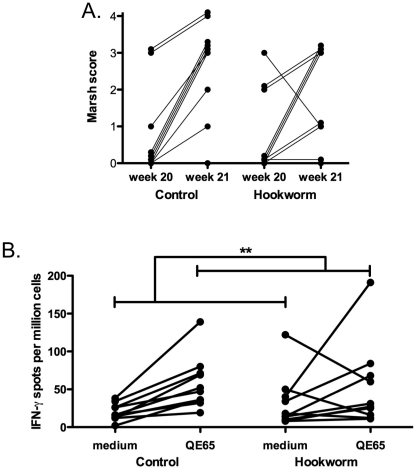
Mucosal damage measured by Marsh score: In both the hookworm and control groups, mucosal damage deteriorated after the 5-day wheat challenge (Figure 6A). Although this deterioration was pronounced in the control group (p = 0.02), there was no statistical difference in Marsh score between hookworm and control groups.
Figure 6. Histological and immunological results.
(A) Marsh score and (B) IFN-γ ELISpot. Data was analyzed by two-way ANOVA: significant effects of time only were shown as indicated, no significant effect of group or interaction was found in (A) or (B).
Systemic inflammatory immune responses: Gluten-specific IFN-γ-producing PBMCs were measured by ELISpot before and after wheat challenge (Figure 6B). As expected, T cells secreting IFN-γ in response to p57–73 QE65 were absent prior to wheat challenge (data not shown), but were mobilised into blood following the challenge. The frequency of IFN-γ secreting T cells specific for p57–73 QE65 increased significantly only in the control group (p = 0.01), but the difference between the groups was not significant.
Secondary Outcomes
Clinical response to wheat challenge — Symptom scores: Nine subjects (4 with hookworm and 5 controls) developed abdominal discomfort, bloating and vomiting 1–2 hours after the oral wheat challenge. These symptoms resolved within 2–4 hours (data not shown). These symptoms did not recur despite continuation and completion of the five day wheat challenge. There was no significant difference between the groups during week 20 in level of pain intensity or episodes of pain, number or urgency of bowel motions, vomiting, nausea bloating, headaches, lethargy or well-being.
Mucosal inflammatory response — Duodenal histological measurements: After the 5-day in vivo wheat challenge, the total, CD3+ and CD8+ duodenal IEL counts increased in both groups (Table 2). The CD4+ IEL counts were not significantly affected by either in vivo wheat challenge or hookworm infection. A robust but almost identical decrease in the Vh∶Cd ratio after gluten was measured in the hookworm and control groups (Table 2).
Table 2. Duodenal cellular responses following in vivo gluten challenge.
| Control | Hookworm | p values | |||||
| Week 20 | Week 21 | Week 20 | Week 21 | Time | Group | Interaction | |
| Total IEL | 30.5(14.8–46.2) | 52.55(32.5–72.6) | 26.4(19.4–33.4) | 43.5(32.7–54.3) | 0.0037 | 0.30 | 0.70 |
| CD8+ IEL | 32.95(19.5–46.4) | 45.75(33.6–57.9) | 38.55(31.8–45.3) | 54.05(36.6–71.5) | 0.019 | 0.24 | 0.82 |
| CD4+ IEL | 0.85(−0.043–1.74) | 2.75(−0.43–5.93) | 0.55(0.090–1.01) | 0.7(0.087–1.31) | 0.18 | 0.13 | 0.25 |
| CD3+ IEL | 37.65(23.4–51.9) | 58.9(44.4–73.4) | 42.45(34.0–50.9) | 60(42–78) | 0.0039 | 0.64 | 0.77 |
| Villus Height | 40.44(32.9–48.0) | 28.23(21.8–34.6) | 38.27(34.6–41.9) | 28.43(19.6–37.3) | 0.0009 | 0.75 | 0.70 |
| Crypt Depth | 15.9(14.1–17.8) | 18.08(15.8–20.3) | 15.38(13.5–17.3) | 18.54(15.5–21.6) | 0.014 | 0.98 | 0.64 |
| Vh/Cd ratio | 2.669(2.0–3.3) | 1.644(1.2–2.1) | 2.575(2.1–3.0) | 1.683(1.0–2.3) | 0.0006 | 0.91 | 0.79 |
Levels were analyzed by two-way ANOVA. p values considered significant are indicated in bold.
Discussion
The “hygiene hypothesis” is a plausible explanation for the increasing incidence of autoimmune and allergic diseases in affluent societies, but it has been difficult to directly elucidate the mechanism by which infectious agents such as nematodes alter disease-causing immune responses in humans. In common with many other immune diseases, the prevalence of CD has risen dramatically. We propose that chronic helminthiasis, such as hookworm infection, may be immunomodulatory and alter pathogenic immune responses in vivo.
In this Phase 1b/2a trial, we have established an experimental model allowing us to explore how hookworm infection alters the effects of gluten in CD. Chronic hookworm infection can be reliably and safely established in subjects with well controlled disease. Lacking precedent, the size of this demanding study was small. We observed at best weak trends towards reduced numbers of gluten peptide-specific T cells in blood and histological damage following wheat challenge in CD.
As has been reported in another trial, standard fecal microscopy is relatively insensitive in light infection; a negative test does not exclude colonization [29]. All patients inoculated acquired adult hookworms in the intestine. The pathognomic findings of a transient papular rash at the site of inoculation, together with the common development of mild abdominal pain in the period soon after the initial inoculation hampered attempts to blind the hookworm-infected participants and investigators. In each control participant however, genuine confusion as to status was usual. It is inherently difficult to mask the hallmark features of hookworm inoculation, just as it is to mimic them. Rather than inoculating every participant and subsequently treating the controls with an anthelminthic, we felt obliged to accept this compromise. The occurrence of abdominal pain has implications when evaluating hookworms as therapy in clinical trials, especially during the establishment phase where symptom scores to measure outcomes may be confounded [20]. Because infection with NA typically persists for years [30], [31], the morbidity occurring during early infection should be accounted for by undertaking studies after chronic infection is established.
The refusal of all participants in the active arm to take anthelminthic therapy after completion of the trial was not unique to this study [20], [32] and supports the contention that chronic light hookworm infection does not compromise wellbeing, an argument congruous with the trend in the improved lethargy score. Although it is well recognized that heavy hookworm infection causes clinically significant blood loss [15], the legitimate concern that experimental infection would cause anemia in patients already predisposed with CD did not eventuate. As anticipated from a previous experimental inoculation study utilising capsule endoscopy [18], the hookworm group acquired peripheral and mucosal eosinophilia but the mucosa at week 20 was not obviously damaged.
Following the epidemiological evidence of a causal association between the disappearance of helminths from societies with advanced sanitary infrastructure and the apparent rise in incidence of autoimmune and allergic diseases [3], a number of interventional clinical trials have been undertaken [12], [20], [32], [33], with inconsistent results. The porcine whipworm, T. suis, has been reported as beneficial in Crohn's disease and ulcerative colitis [33], both conditions which share genetic traits with CD [34]. However, a recently reported controlled trial using this helminth in patients with allergic rhinitis demonstrated that while an immunological response to whipworm was elicited, no therapeutic benefit was apparent [12]. Similarly, in a trial where NA infection was tested for an effect among patients with asthma, no significant benefit from helminth infection was reported [32]. While our experience from a proof of concept study where patients with active Crohn's disease were infected with hookworm suggested an early benefit, their wellbeing was reliant on continuation of immunosuppressive therapies [20].
Our study establishes that hookworm infection on its own will not obviate the necessity for a restricted diet in CD. However, this experimental human challenge system appears to be a safe way of investigating the effect helminth parasites might impact on immune pathology. The advantages are that it directly addresses the human response, the disease process is not affected by the clinical imperative to use immune modulating therapy, intestinal tissue as well as blood is available for analyses and antigen stimulation testing can be effected both in vivo and in vitro.
Supporting Information
CONSORT Checklist.
(PDF)
Study Protocol.
(PDF)
Acknowledgments
The authors wish to gratefully acknowledge the valued contribution made by Dr Owen Harris who agreed to be the clinical monitor for this trial. They also wish to acknowledge the contribution made by Dr Linda Fletcher for assistance with specimen handling.
Footnotes
Competing Interests: The authors have declared that no competing interests exist. Envoi Specialist Pathologists, Brisbane, QLD, Australia contributed to the processing and the interpretation (Prof. Clouston) of the histological material, but neither the organisation nor any employee has any commercial interest beyond this support nor a competing interest. Envoi's involvement does not affect or alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The study was funded by the Broad Medical Research Program of The Broad Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Prof. Andrew Clouston is a principal of and clinical pathologist at Envoi Specialist Pathologists, Brisbane, QLD, Australia, which processed tissue sections for histological grading. Prof. Clouston contributed to the interpretation and grading of the histology, and the preparation of the manuscript. Neither Prof. Clouston nor Envoi Specialist Pathologists has any patent or financial interest in the product of this clinical trial.
References
- 1.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. The N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- 3.Altmann DM. Review series on helminths, immune modulation and the hygiene hypothesis: nematode coevolution with adaptive immunity, regulatory networks and the growth of inflammatory diseases. Immunology. 2009;126:1–2. doi: 10.1111/j.1365-2567.2008.03006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206(6):1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyama H, Hirata T, Sakugawa H, Watanabe T, Miyagi S, et al. An inverse relationship between autoimmune liver diseases and Strongyloides stercoralis infection. Am J Trop Med Hyg. 2007;76:972–976. [PubMed] [Google Scholar]
- 7.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 8.Elliott DE, Weinstock JV. Helminthic therapy: using worms to treat immune-mediated disease. Adv Exp Med Biol. 2009;666:157–166. doi: 10.1007/978-1-4419-1601-3_12. [DOI] [PubMed] [Google Scholar]
- 9.Ruyssers NE, De Winter BY, De Man JG, Ruyssers ND, Van Gils AJ, et al. Schistosoma mansoni proteins attenuate gastrointestinal motility disturbances during experimental colitis in mice. World J Gastroenterol. 2010;16:703–712. doi: 10.3748/wjg.v16.i6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musgrove P, Hotez PJ. Turning neglected tropical diseases into forgotten maladies. Health Aff (Millwood) 2009;28:1691–1706. doi: 10.1377/hlthaff.28.6.1691. [DOI] [PubMed] [Google Scholar]
- 11.Feary J, Venn A, Brown A, Hooi D, Falcone FH, et al. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study. Clin Exp Allergy. 2009;39:1060–1068. doi: 10.1111/j.1365-2222.2009.03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bager P, Arnved J, Ronborg S, Wohlfahrt J, Poulsen LK, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125:123–130. doi: 10.1016/j.jaci.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Summers RW, Elliott DE, Qadir K, Urban JF, Jr, Thompson R, et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 14.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, et al. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell C, Hussain R, Nutman TB, Poindexter RW, Little MD, et al. The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am J Trop Med Hyg. 1987;37:126–134. doi: 10.4269/ajtmh.1987.37.126. [DOI] [PubMed] [Google Scholar]
- 17.Wright V, Bickle Q. Immune responses following experimental human hookworm infection. Clin Exp Immunol. 2005;142:398–403. doi: 10.1111/j.1365-2249.2005.02945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croese J, Wood MJ, Melrose W, Speare R. Allergy controls the population density of Necator americanus in the small intestine. Gastroenterology. 2006;131:402–409. doi: 10.1053/j.gastro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Mortimer K, Brown A, Feary J, Jagger C, Lewis S, et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am J Trop Med Hyg. 2006;75:914–920. [PubMed] [Google Scholar]
- 20.Croese J, O'Neil J, Masson J, Cooke S, Melrose W, et al. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut. 2006;55:136–137. doi: 10.1136/gut.2005.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinnell RJ, Bethony J, Pritchard DI. The immunoepidemiology of human hookworm infection. Parasite Immunol. 2004;26:443–454. doi: 10.1111/j.0141-9838.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 22.Leffler DA, Dennis M, Edwards George J, Jamma S, Cook EF, et al. A validated disease-specific symptom index for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7:1328–1334. doi: 10.1016/j.cgh.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 24.Catassi C, Fabiani E, Iacono G, D'Agate C, Francavilla R, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 2007;85:160–166. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 25.Marsh MN, Crowe PT. Morphology of the mucosal lesion in gluten sensitivity. Baillieres Clin Gastroenterol. 1995;9:273–293. doi: 10.1016/0950-3528(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 26.Tye-Din J, Anderson R. Immunopathogenesis of celiac disease. Curr Gastroenterol Rep. 2008;10:458–465. doi: 10.1007/s11894-008-0085-9. [DOI] [PubMed] [Google Scholar]
- 27.Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AV. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–342. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 28.Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41–49. doi: 10.1172/JCI30253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blount D, Hooi D, Feary J, Venn A, Telford G, et al. Immunologic profiles of persons recruited for a randomized, placebo-controlled clinical trial of hookworm infection. Am J Trop Med Hyg. 2009;81:911–916. doi: 10.4269/ajtmh.2009.09-0237. [DOI] [PubMed] [Google Scholar]
- 30.Palmer ED. Course of egg output over a 15 year period in a case of experimentally induced necatoriasis americanus, in the absence of hyperinfection. Am J Trop Med Hyg. 1955;4:756–757. doi: 10.4269/ajtmh.1955.4.756. [DOI] [PubMed] [Google Scholar]
- 31.Beaver PC. Light, long-lasting Necator infection in a volunteer. Am J Trop Med Hyg. 1988;39:369–372. doi: 10.4269/ajtmh.1988.39.369. [DOI] [PubMed] [Google Scholar]
- 32.Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy. 2010;40:299–306. doi: 10.1111/j.1365-2222.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glas J, Stallhofer J, Ripke S, Wetzke M, Pfennig S, et al. Novel genetic risk markers for ulcerative colitis in the IL2/IL21 region are in epistasis with IL23R and suggest a common genetic background for ulcerative colitis and celiac disease. Am J Gastroenterol. 2009;104:1737–1744. doi: 10.1038/ajg.2009.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Checklist.
(PDF)
Study Protocol.
(PDF)