Abstract
Previous systematic arrangement on the ciliate order Urostylida was mainly based on morphological data and only about 20% taxa were analyzed using molecular phylogenetic analyses. In the present investigation, 22 newly sequenced species for which alpha-tubulin, SSU rRNA genes or ITS1-5.8S-ITS2 region were sampled, refer to all families within the order. Following conclusions could be drawn: (1) the order Urostylida is not monophyletic, but a core group is always present; (2) among the family Urostylidae, six of 10 sequenced genera are rejected belonging to this family; (3) the genus Epiclintes is confirmed belonging to its own taxon; (4) the family Pseudokeronopsidae undoubtedly belongs to the core portion of urostylids; however, some or most of its members should be transferred to the family Urostylidae; (5) Bergeriellidae is confirmed to be a valid family; (6) the distinction of the taxon Acaudalia is not supported; (7) the morphology-based genus Anteholosticha is extremely polyphyletic; (8) ITS2 secondary structures of Pseudoamphisiella and Psammomitra are rather different from other urostylids; (9) partition addition bootstrap alteration (PABA) result shows that bootstrap values usually tend to increase as more gene partitions are included.
Introduction
Ciliates, eurychoric unicellular eukaryotes, are characterized by complexes of cilia and a nuclear dimorphism [1]. In last 25 years, molecular phylogenetic analyses, especially based on small subunit rRNA (SSU rRNA) gene sequences, provided resolution of a number of important questions on the phylogenetic relationships within this group (for example, [2], [3]–[7]). However, many questions remain open, mostly related to a number of spirotrichean lineages, either on the assignment of certain species to one or another group or, more importantly, on the phylogenetic relationships within certain orders/families that contain a large number of taxa.
Among these, the order Urostylida is one of the most confused and diverse and is increasingly attractive for the researchers working on morphogenetic, taxonomic and molecular fields (for example, [4], [8], [9]–[13]). The most important apomorphy for urostylids is a zig-zagging ventral cirral pattern originating from more than six anlagen evolved possibly convergently for several times (for example, [ 1,9]). There are more than ten studies that include details of interrelationships within this order (for example, [ 1], [9], [14]–[21]), which are mainly based on morphological/morphogenetic data, but none reaches the same conclusions as another.
In his monograph of the Urostyloidea, Berger [9] recognised 154 valid species, and assigned most of them to four families (Holostichidae, Bakuellidae, Urostylidae and Epiclintidae) using the frontal ciliature and the midventral complex as the main features. Recently, another systematic classification was proposed by Lynn [1], which also divided the order Urostylida into four families (Epiclintidae, Pseudokeronopsidae, Pseudourostylidae, Urostylidae). Between these two systems, there is only agreement over the classification of Epiclintidae. In order to investigate further the evolutionary relationships among the urostylids, molecular phylogenetic analyses based on SSU rRNA gene sequences have been increasingly used in recent few years [4], [12], [22]–[27]. Although these investigations undoubtedly show that Urostylida is a large group within the Hypotricha, the monophyly of this order is not yet certain, and relationships within it are still confused. Furthermore, molecular phylogenies based on other gene markers, albeit with sparse taxon sampling, have produced rather different results compared to SSU rRNA phylogenies [22], [28], [29].
Comparison between different molecular trees is an essential step to reveal the evolution within investigated groups, even when independent datasets yield congruent results. The combined phylogenetic analyses of multiple genes have become popular due to the poor resolution of phylogenies based on single loci [30], and have successfully inferred better-resolved phylogenies within the major taxonomic groups, including animals [31], plants [32], fungi [33] and bacteria [34]. However, there are few ciliate phylogenies based on combined gene partitions [6]. With the advent of multi-gene phylogenies, particular emphasis has been placed on congruence or combinability of independent and possibly heterogeneous datasets [35]–[37]. To date, the only molecular urostylid phylogeny based on combined genes is that of Hewitt et al. [26] who used SSU-5.8S-LSU rRNA. There are only three congruent phylogenies, based on different genes that include few urostylid taxa [38]–[40].
The present study was initiated to improve our understanding of evolutionary relationships within the order Urostylida by extending the SSU rRNA gene, ITS1-5.8S-ITS2 region, and alpha-tubulin gene database. Moreover, molecular phylogenies are discussed with critical consideration of the taxonomic literature. In addition, statistical tests, i.e. incongruence length difference (ILD) test, Shimodaira-Hasegawa (S-H test) and partition addition bootstrap alteration (PABA) approach, are performed to detect incongruence among these three gene partitions.
Results
Analyses of Sequences and Secondary Structures
A total of one SSU rRNA gene, eight ITS1-5.8S-ITS2 regions, and 13 alpha-tubulin genes were sequenced in our analyses (Table 1).
Table 1. Urostylid Species for Which SSU rRNA Gene, ITS1-5.8S-ITS2 Regions and Alpha-Tubulin Gene Were Newly Sequenced in the Present Work.
| Species | SSU rRNA gene | ITS1-5.8S-ITS2 | Alpha-tubulin gene | |||
| Accession No. | Length in bp | Accession No. | Length in bp | Accession No. | Length in bp | |
| Anteholosticha gracilis | - | - | - | - | GQ258104 | 1074 |
| Anteholosticha manca | - | - | - | - | GQ258111 | 1074 |
| Anteholosticha parawarreni | FJ870074 | 1784 | - | - | - | - |
| Anteholosticha eigneri | - | - | - | - | GQ258105 | 1074 |
| Apokeronopsis bergeri | - | - | - | - | GQ258112 | 1074 |
| Bergeriella ovata | - | - | GQ246479 | 552 | GQ258113 | 1074 |
| Epiclintes auricularis auricularis | - | - | - | - | GQ262001 | 1074 |
| Epiclintes auricularis rarisetus | - | - | GQ246480 | 483 | - | - |
| Holosticha diademata | - | - | - | - | GQ258106 | 1074 |
| Metaurostylopsis cheni | - | - | GQ246481 | 537 | GQ258114 | 1074 |
| Nothoholosticha fasciola | - | - | - | - | GQ258107 | 1074 |
| Parabirojimia multinucleata | - | - | GQ246483 | 517 | GQ258108 | 1074 |
| Psammomitra retractilis | - | - | GQ246483 | 478 | - | - |
| Pseudoamphisiella quadrinucleata | - | - | GQ246484 | 483 | GQ258109 | 1074 |
| Pseudokeronopsis carnea | - | - | - | - | GQ258110 | 1074 |
| Pseudourostyla cristata | - | - | GQ246486 | 504 | GQ258115 | 1074 |
| Thigmokeronopsis stoecki | - | - | GQ246485 | 480 | - | - |
The SSU rRNA gene had the most characters (1,635 bp unambiguously aligned), followed by alpha-tubulin (1,071 bp), then ITS1-5.8S-ITS2 (427 bp) for the 14-taxon datasets. The nucleotide sequences of all three genes among 14 urostylids share similarities of 90.59–99.26%, 52.77–94.03%, and 77.40–91.96%, respectively (Tables S1, S2). It is noteworthy that alpha-tubulin amino acid sequences share similarities of 97.13–100.00% (Table S2), so phylogenetic trees were constructed using alpha-tubulin nucleotide sequences instead of amino acid sequences in our analyses.
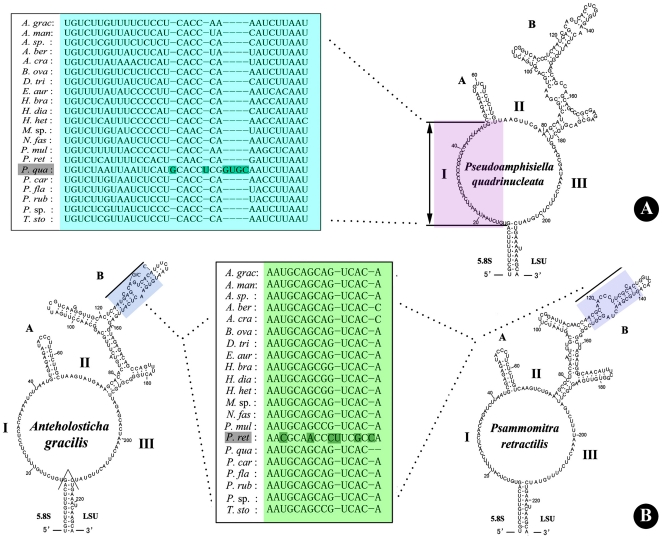
Comparisons of the ITS2 region sequences as well as secondary structures (Figure S1) show that there are two unique regions for Pseudoamphisiella quadrinucleata, and one for Psammomitra retractilis. As shown in Fig. 1, the main loop is divided into three parts (viz. I, II, and III) by Helix A and B, and there are 37 nucleotides in part I of Pseudoamphisiella, whereas there are only 31 ones in other species (Fig. 1A). Helix A in Pseudoamphisiella contains 19 nucleotides, whereas that of other species is constantly composed of 20 nucleotides. This is caused by one nucleotide deletion in the terminal loop of Helix A for Pseudoamphisiella (data not shown). Previous investigations [41]–[43] showed that for spirotricheans, 11 out of 12 paired nucleotides were identical in the labeled 15 nucleotides stretch of Helix A. However, our current analysis (Fig. 1B) indicates that Psammomitra has rather different sequences and secondary structure in this region.
Figure 1. Secondary structures of the internal transcribed spacer 2 (ITS2) RNA transcript of three representative urostylid species (Viz. Anteholosticha gracilis, Psammomitra retractilis, Pseudoamphisiella quadrinucleata), and sequence alignments of two unique regions.
The diagrams illustrate the two helices, labeled A and B, present in the class Spirotrichea [41]. Three parts of the biggest loop are labeled I, II and III, respectively. Lines beside A. gracilis and Psammomitra retractilis denote the region of greatest primary sequence conservation for class Spirotrichea [41]. Unique nucleotide sites are highlighted.
ILD tests for all combined datasets (viz. Datasets 4, 5, 9–11) show that most of the partitioned datasets contain conflicting signal (P = 0.001), with only Dataset 9 being congruent (P = 0.256). In an attempt to further clarify the incongruence, each taxon was deleted in turn to determine if one or a few taxa were particularly problematic. However, in no dataset did this approach indicate that conflict is potentially caused by a specific taxon (Table 2).
Table 2. Results of the ILD Test of Congruence of Datasets.
| Taxa | Dataset 5 | Dataset 9 | Dataset 10 | Dataset 11 |
| All taxa | 0.001 | 0.256 | 0.001 | 0.001 |
| Excluded: | ||||
| Anteholosticha eigneri | 0.001 | 0.969 | 0.001 | 0.001 |
| Anteholosticha gracilis | 0.001 | 0.178 | 0.001 | 0.001 |
| Anteholosticha manca | 0.001 | 0.151 | 0.001 | 0.001 |
| Apokeronopsis bergeri | 0.001 | 0.171 | 0.001 | 0.001 |
| Bergeriella ovata | 0.001 | 0.404 | 0.001 | 0.001 |
| Holosticha diademata | 0.001 | 0.601 | 0.001 | 0.001 |
| Metaurostylopsis cheni | 0.001 | 0.147 | 0.001 | 0.001 |
| Nothoholosticha fasciola | 0.001 | 0.109 | 0.001 | 0.001 |
| Parabirojimia multinucleata | 0.001 | 0.998 | 0.001 | 0.001 |
| Psammomitra retractilis | 0.001 | 0.312 | 0.001 | 0.001 |
| Pseudoamphisiella quadrinucleata | 0.001 | 0.122 | 0.001 | 0.001 |
| Pseudokeronopsis carnea | 0.001 | 0.231 | 0.001 | 0.001 |
| Pseudourostyla cristata | 0.001 | 0.108 | 0.001 | 0.001 |
| Thigmokeronopsis stoecki | 0.001 | 0.211 | 0.001 | 0.001 |
NOTE.-Significant P values≥0.05 in bold.
Phylogenetic Analyses Inferred from Dataset 1 (SSU rRNA, 89 Taxa)
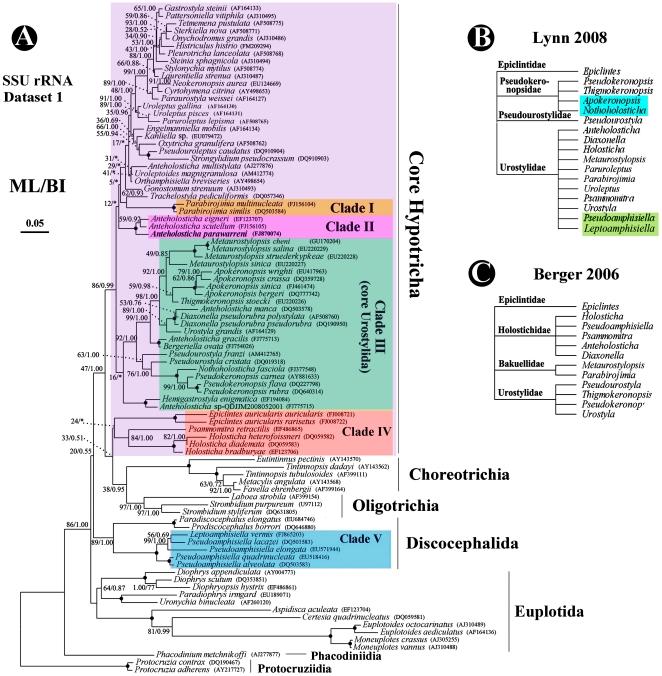
In our analyses (Fig. 2A), the outgroup Protocruziidia is followed by Phacodiniidia and Euplotida, then the sister clade forming by Oligotrichia and Choreotrichia. Hypotricha seems to be paraphyletic: most species group together, and others cluster with Oligotrichia, Choreotrichia, and the core discocephalids, respectively.
Figure 2. Phylogeny of the class Spirotrichea inferred by ML of SSU rRNA gene sequences (A), and two systems (B, C).
A. Urostylids are labeled in colours. Species newly sequenced in the present study is shown in bold type. BP for ML tree and PP for BI tree are given near nodes, respectively. Asterisks show different node topologies between BI and ML trees. Fully supported (100%/1.00) branches are marked with solid circles. The scale bar corresponds to 5 substitutions per 100 nucleotide positions; black dot marks the genus Hemigastrostyla which is a non-urostylid. B. System of Lynn [1] containing only sequenced urostylid genera, with several highlighted genera not included by Lynn [1]. C. System of Berger [9] containing only sequenced urostylid genera.
Though Uroleptus and Paruroleptus are assigned into the family Urostylidae according to Lynn [1], they are undoubtly classified out of the order Urostylida in our SSU rRNA gene tees (Fig. 2), which is congruent with previous investigations [9], [12], [23], [24], [44]. Considering exclusion of these two genera from the order Urostylida, all available SSU rRNA gene sequences of urostylids were included in our phylogenetic analyses, and they refer to 15 genera representing all four urostylid families (sensu Lynn [1]) and four unclassified genera (Fig. 2). In both analyses, the order appears to be always paraphyletic, and species fall into six clades, except for Anteholosticha multistilata, the position of which is unresolved. Clade I consists of two Parabirojimia species (family Urostylidae), which group with Trachelostyla, a non-urostylid genus. Clade II consists of three Anteholosticha species. Clade III is the “core” urostylid clade, and it is composed of seven genera which belong to the family Urostylidae (viz. Metaurostylopsis, Urostyla, Diaxonella and Anteholosticha), two genera of the family Pseudokeronopsidae (Pseudokeronopsis, Thigmokeronopsis), one genus of the family Pseudourostylidae (Pseudourostyla), three unclassified urostylid genera (Apokeronopsis, Bergeriella and Nothoholosticha), and the non-urostylid genus Hemigastrostyla [1], [45]. Clade IV has a closer relationship with Oligotrichia and Choreotrichia than with other urostylids, and consists of two genera of the family Holostichidae (viz. Holosticha and Psammomitra), and the type genus of the family Epiclintidae, Epiclintes. Clade V falls into the order Discocephalida, and consists of Pseudoamphisiella (family Holostichidae) and the unclassified genus Leptoamphisiella (see Discussion). Among the four urostylid families, the Epiclintidae is monotypic whereas the other three are multi-generic and paraphyletic. All species of Pseukeronopsidae and Pseudourostylidae fall into Clade III, and urostylid species appear in all six clades. Among 15 sequenced urostylid genera, species of Anteholosticha are the most diverse and representatives could be found in both Clades II and V. Of the other 14 genera, none have representatives in more than one clade.
Phylogenetic Analyses Inferred from Dataset 2 (ITS1-5.8S-ITS2, 31 Taxa), Dataset 3 (Alpha-Tubulin, 26 Taxa) and Dataset 4 (Three-Gene Combined, 25 Taxa)
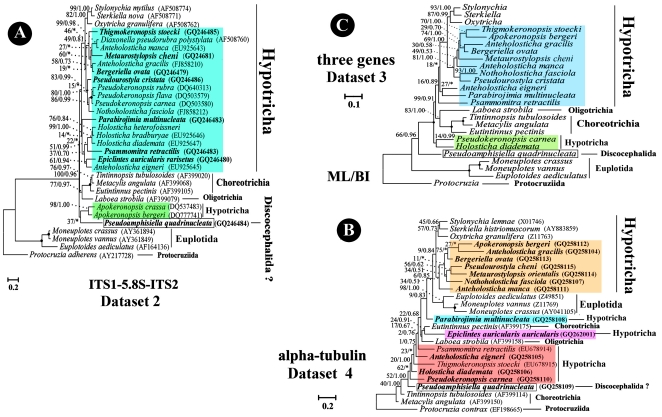
As revealed in trees based on Dataset 1 (Fig. 2A), analyses inferred from Datasets 2 and 4 (Fig. 3A and 3C) also indicate that: (1) the outgroup Protocruziidia is followed by Euplotida, Oligotrichia, Choreotrichia; (2) Hypotricha is separated into several clades; (3) the core urostylid group contains only genera/species of Clade IV derived from Dataset 1 (Fig. 2A), namely Anteholosticha gracilis, A. manca, Bergeriella, Diaxonella (absent from Dataset 4), Metaurostylopsis, Thigmokeronopsis, Apokeronopsis (which does not cluster with this group in trees based on Dataset 2), Pseudokeronopsis, Pseudourostyla, and Nothoholosticha; (4) Pseudoamphisiella is rather distant from other urostylids in Datasets 2, 4. However, the cluster pattern of species outside the core urostylid group is rather different among trees based on Datasets 1, 2, and 4.
Figure 3. Phylogeny of the class Spirotrichea inferred by ML of Datasets 2–4 (A–C).
Urostylids are labeled in colours. Species newly sequenced in the present study are shown in bold type. BP for ML tree and PP for BI tree are given near nodes, respectively. Asterisks show different node topologies between BI and ML trees. Fully supported (100%/1.00) branches are marked with solid circles. The scale bar corresponds to 10/20 substitutions per 100 nucleotide positions.
In analyses inferred from Dataset 3, the subclass Protocruziidia branches at the deepest level, however, compared to trees based on Datasets 1, 2, and 4, the clade comprising the euplotids is more closely related to the “core” Hypotricha. The monophyly of Choreotrichia is rejected. In addition, Thigmokeronopsis and Pseudokeronopsis, which belong to the core urostylids in analyses based on Datasets 1, 2, and 4, fall outside the core Urostylida.
Comparison of Phylogenetic Analyses Inferred from 14-Taxa Datasets
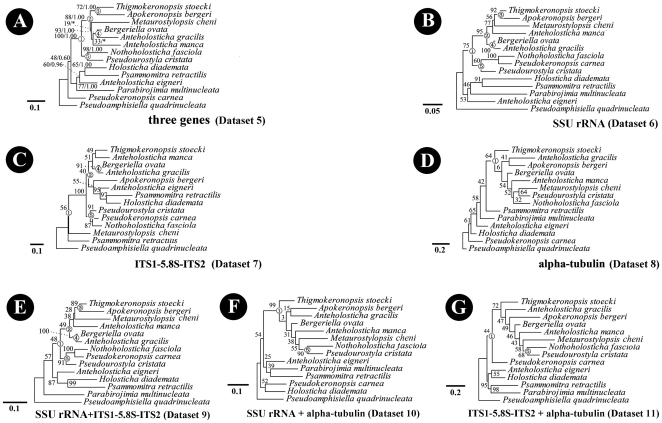
ML tree topologies inferred from seven 14-taxa datasets (Datasets 5–11) (Fig. 4) were not identical to each other. However, as revealed by trees based on Datasets 1–4, these analyses also strongly indicate that: (1) Pseudoamphisiella should be excluded from urostylids, and; (2) the core urostylid group contains Anteholosticha manca, A. gracilis, Bergeriella, Metaurostylopsis, Thigmokeronopsis, Apokeronopsis, Pseudokeronopsis, Pseudourostyla and Nothoholosticha.
Figure 4. Phylogeny of the class Spirotrichea inferred by ML of 14-taxa Datasets 5–11 (A–G).
The scale bar corresponds to 5/10 substitutions per 100 nucleotide positions. Circled numbers refer to node numbers in PABA approach.
Using the S-H approach, out of 42 possible comparisons, 15 ones result in a P value above 0.05, signaling that congruence is not rejected, whereas 27 comparisons reject congruence (P<0.05) (Table 3). Dataset 5 rejects all topologies inferred from other 14-taxa datasets, however, two topologies among them are not totally rejected. Conversely, topology based on Dataset 5 is only rejected by Dataset 7. Interestingly, all topologies obtained by datasets including alpha-tubulin are accepted by other datasets also including alpha-tubulin (Fig. 4A, D, F, G), but are rejected by all other datasets (Fig. 4B, C, E).
Table 3. Results of the SH Test of Congruence of Datasets.
| Datasets | Topology (ML) | ||||||
| Dataset 5 | Dataset 6 | Dataset 7 | Dataset 8 | Dataset 9 | Dataset 10 | Dataset 11 | |
| Dataset 5 | – | 0.140 | <0.001 | 0.036 | 0.212 | 0.150 | 0.275 |
| Dataset 6 | 0.002 | – | <0.001 | <0.001 | 0.669 | <0.001 | <0.001 |
| Dataset 7 | <0.001 | 0.001 | – | <0.001 | 0.053 | <0.001 | <0.001 |
| Dataset 8 | <0.001 | <0.001 | <0.001 | – | <0.001 | 0.415 | 0.355 |
| Dataset 9 | <0.001 | 0.442 | 0.233 | <0.001 | – | <0.001 | <0.001 |
| Dataset 10 | 0.038 | 0.005 | <0.001 | 0.285 | 0.003 | – | 0.702 |
| Dataset 11 | 0.049 | <0.001 | <0.001 | 0.603 | 0.004 | 0.508 | – |
NOTE.-Significant P values≥0.05 in bold.
Five, three, one, five, two and two of five nodes selected based on 14-taxa three-gene combined datasets could be found in trees inferred from Datasets 6–11, respectively (Fig. 4B–G). For Node 1, the addition of ITS1-5.8S-ITS2 region data causes the bootstrap values to decrease (Table S3). For Nodes 2–4, the addition of alpha-tubulin gene data does the same thing (Tables S4, S5, S6). By contrast, the addition of SSU rRNA gene data always increases the support values (Tables S3, S4, S5, S6, S7). Considering all five nodes, the PABA approach also shows that bootstrap values tend to increase as more data or data partitions are added except when alpha-tubulin gene data is added as the second partition (Table 4).
Table 4. Alteration of Bootstrap Support δ to Nodes in Fig. 4 as Gene Partitions Are Added.
| <?tvs=-2.5pt?>Nodes | BP value of Dataset 5 | Gene partitions | |||||
| Alpha-tubulin | ITS1-5.8S-ITS2 | SSU rRNA | |||||
| 2nd | 3rd | 2nd | 3rd | 2nd | 3rd | ||
| 1 | 100 | 6 | 52 | −24 | 0 | 14 | 56 |
| 2 | 88 | −58 | 39 | −13 | 88 | 5 | 88 |
| 3 | 72 | −46 | −17 | −2 | 72 | 45 | 72 |
| 4 | 93 | −96 | −7 | 0 | 93 | 5 | 93 |
| 5 | 98 | 4 | 7 | 50 | 8 | 45 | 30 |
| Average over all nodes | −38 | 15 | 2 | 52 | 23 | 68 | |
Discussion
This study represents one of the few attempts to reconstruct generic-level relationships within Urostylida with molecular characters from multiple genes, and the only phylogenetic analysis that includes all four urostylid families. Though the phylogenetic results based on different datasets are mixed, and support values for some nodes are not high (Fig. 2– 4), some conclusions could be drawn following by comparison between our phylogenetic trees and system of Lynn [1].
The Current Status of the Phylogenetic Relationships within the Order Urostylida
Recent molecular phylogenetic investigations (for example, [ 4], [12], [22]–[27]), as well as the current work based on both single gene (Datasets 1–3, Fig. 2A, 3A, B) and multiple genes (Dataset 4, Fig. 3C) shows that the urostylid assemblage is not monophyletic and thus raises serious challenges to the classification of the order Urostylida [1], [8], [9], [14]–[16], [18], [21]. This is consistent with the conclusion that there is a considerable amount of convergence in urostylid morphology [9] which brings into question current classification scheme [1]. In the present work, several datasets, with SSU rRNA, alpha-tubulin and ITS1-5.8S-ITS2 gene/region sequences for all known urostylid genera, were used in order to re-evaluate phylogenetic relationships within this assemblage and to make a comparison between molecular phylogeny and the system of Lynn [1] which is mainly based on morphological/morphogenetic data.
Classification of Four Unclassified Genera
The systematic positions of four recently reported genera, namely Bergeriella, Leptoamphisiella, Apokeronopsis and Nothoholosticha, were not included in any of updated systems although they were putatively assigned to the order Urostylida based on either morphological/morphogenetic or molecular information in the original descriptions [10], [11], [46], [47]. Among them, a new family, Bergeriellidae, was erected for the type genus Bergeriella [11]. In the present investigation, Bergeriella always falls into core urostylid group in all the trees, and is not closely related to any of the four urostylid families [1] (Fig. 2– 4). Thus, according to both molecular and morphological/morphogenetic data, all the evidence supports the conclusion that Bergeriella should represent a distinct family within the order Urostylida [11].
The results presented here show that the genus Leptoamphisiella is most related to Pseudoamphisiella, the type genus of the family Pseudoamphisiellidae, which is, however, assigned to the family Urostylidae in Lynn's system [1]. Our analyses firmly support the conclusion that this family should be excluded from the order Urostylida, but rather belongs to a group of its own which clusters to the well-known discocephalids [23], [48].
Both Apokeronopsis and Nothoholosticha are confirmed as true urostylids belonging to the family Pseudokeronopsidae [10], [47].
Classification of the Family Urostylidae
Nine genera included in our analyses (viz. Anteholosticha, Diaxonella, Holosticha, Metaurostylopsis, Parabirojimia, Psammomitra, Urostyla, Pseudoamphisiella and Leptoamphisiella), all of which are assigned to the family Urostylidae in Lynn's [1] system, are distributed among Clades I–VI in the present analysis (Fig. 2A).
As revealed in previous molecular and morphological investigations [1], [4], [9], [12], [24] and in our SSU rRNA gene trees (Fig. 2A), Uroleptus and Paruroleptus should be removed from the urostylid family Urostylidae [for details see discussion in 24] to the non-urostylid family Uroleptidae [44]. Similarly, Pseudoamphisiella and Leptoamphisiella, two urostylid genera according to Lynn [1], should be placed in the suborder Discocephalina since they consistently cluster with Discocephalina (Fig. 2A). This is consistent with the results of previous studies based on molecular data [6], [23], [48], and supports the findings that some morphological/morphogenetic features of these genera, e.g. the cirri of the midventral complex are not arranged in the zig-zag pattern, and the general developmental process of the ciliary structure, are more similar to those of discocephalines than urostylids [49].
The phylogenetic position of Parabirojimia is slightly variable according to different datasets, however, it always falls outside of the “core” urostylid group and does not have a robust relationship with any other typical urostylids (Fig. 2– 4). Considering the extremely unusual mode of development of the cortical structure during morphogenesis, especially the formation of the somatic ciliature, e.g. the transverse cirri, the right marginal rows, etc. [50], it is reasonable to assign this genus/family to its own group, that is the suborder Parabirojimina, as suggested by Yi et al. [23].
The genus Metaurostylopsis is only included in three systems [1], [9], [51]. Among those genera included in the present investigation, Shi et al. [51] considered that Metaurostylopsis has a close relationship with Urostyla and Pseudourostyla, Berger [9] placed it together with Parabirojimia in family Bakuellidae, and five other (non-sequenced) genera, whereas Lynn [1] suggested that Metaurostylopsis could be related to Anteholosticha, Holosticha, Diaxonella, Parabirojimia, Psammomitra, Pseudoamphisiella, and Uroleptus. However, among these hypotheses, only the sister relationship between Metaurostylopsis and Pseudourostyla is hinted by Dataset 3 (Fig. 3B), indicating that none of the assignments of Metaurostylopsis in these three systems are reasonable.
As noted by Berger [9], the systematic position of Diaxonella is complicated since the type species, D. pseudorubra, has been repeatedly reported under different generic and specific names (for example, [ 9], [18], [52]–[56]). This genus has only been included in two systems [1], [9], since it was established by Jankowski [20]. Based on the redescription of D. pseudorubra (as D. trimarginata by Shao et al. [55]), it was assigned to the family Pseudourostylidae, thus as an urostylid species. This report also included a description of morphogenesis and the unusual mode of formation of left marginal rows, which has been reported in only another hypotrich genus, that is, Pseudourostyla. However, the present and previous molecular investigations [4], [12], [22], [23], [27], [40], [57], [58] did not recover a close relationship between Diaxonella and Pseudourostyla, thus supporting Berger's [9] hypothesis that this unusual morphogenetic process is very likely a result of convergent evolution and should not be regarded as a family level character as suggested by Eigner and Foissner [8]. In addition, the placement of Diaxonella in family Holostichidae (sensu Berger [9]) is also clearly rejected by the molecular data in both the present and previous investigations [4], [12], [22], [23], [27], [40]. This is consistent with the morphological finding that Diaxonella has more than two marginal rows, and is hence rather different from other holostichid genera (sensu Berger [9]). According to Lynn [1], Diaxonella should be assigned into the family Urostylidae. However, only the connection between this genus and Urostyla, and Anteholosticha manca is accepted in the present work (Fig. 2A, 3A) and previous investigations [4], [12], [22], [23], [27], [40], [57], [58]. All this evidence indicates that Diaxonella is undoubtedly an urostylid, however its family-level assignment in both Berger's [9] and Lynn's [1] systems is highly questionable and needs to be re-evaluated.
Of the final four genera, viz. Holosticha, Psammomitra, Urostyla and Anteholosticha which are also assigned to the family Urostylidae by Lynn [1], the first three are located in two separate clades in our trees (Fig. 2– 4). The relationship between Holosticha and Psammomitra hypothesized by Lynn [1] and Berger [9] was confirmed by both previous [22] and present analyses except in trees based on single-gene datasets and in those based on datasets containing alpha-tubulin information with two genes combined (Fig. 2– 4). By contrast, the genus Anteholosticha appears to be heterogeneous and highly divergent, with species falling into different clades in all our trees (Fig. 2– 4). In addition, distinct from other genera, seven Anteholosticha species share no unique nucleotides at semi-conserved, parsimony-information sites in the alignment of SSU rRNA gene sequences. These findings indicate that Anteholosticha is probably a convergent assemblage of species as predicted also by Berger [9], [59] and a revision of this genus is urgently needed.
In summary, the family Urostylidae (sensu Lynn [1]) seems to be a huge “melting pot” containing over 24 nominal genera, the monophyly of which is strongly rejected by the present analyses (Fig. 2). Currently, a complete re-arrangement for its classification remains impossible partly because molecular information is lacking for too many taxa. Nevertheless, the following conclusions can be drawn based on our analyses: 1) as revealed in previous investigations [9], [12], [22]–[24], [44], Parabirojimia, Psammomitra, Pseudoamphisiella, Leptoamphisiella, Uroleptus and Paruroleptus should be removed from this family and the last four genera are not even members of the order Urostylida. 2) Holosticha should also be excluded from this family; 3) Diaxonella and Urostyla/Parabirojimia respectively might represent two isolated families; 4) the genus Anteholosticha is extremely diverse, polyphyletic and should be revised when more information becomes available.
Classification of the Family Pseudokeronopsidae
Two of the six genera in the family Pseudokeronopsidae (sensu Lynn [1]), viz. Pseudokeronopsis and Thigmokeronopsis, and another two genera which should be included in this family, viz. Apokeronopsis and Nothoholosticha, group consistently into two clades in SSU rRNA trees (Fig. 2A, 4B), and into more then two clades in other trees (Fig. 3, 4A, C–G). Thus, all of these analyses reject the monophyly of this family.
Berger [9] synonymised Apokeronopsis begeri as Thigmokeronopsis crassa, due to the genus Apokeronopsis was not erected then. However, phylogenetic trees based on Datasets 2, 3, 7, 8, 10, and 11, none of which contain SSU rRNA gene sequences except Dataset 10, failed to recover a close relationship between these two genera (Fig. 3A, B, 4C, D, F, G ), although they did group together in other trees including SSU rRNA gene (Fig. 2A, 3C, 4A, B, E), which indicates that the connecting of Apokeronopsis with Thigmokeronopsis is probably due to inclusion of SSU rRNA. Considering the separation of these two genera is supported by some morphological/morphogenetic data, for example, presence or absence of thigmotactic cirri and the fusion pattern of macronuclear segments prior to division [47], [60], the distinction of both genera is reliable but their systematic positions remain unresolved.
Although Thigmokeronopsis and Pseudokeronopsis are placed into the family Pseudokeronopsidae by most investigators e.g., [ 1], [8], [9,14], a sister relationship between these two genera is not revealed in any of our trees (Fig. 2– 4), nor in previous molecular phylogenetic analyses [11], [12], [22], [40]. The relationship between Pseudokeronopsis and Nothoholosticha is clearly supported both by morphological (viz. midventral pairs arranged in a zig-zag pattern, distinctly fewer transverse cirri than midventral cirral pairs, and one marginal row on each side of the body) and phylogenetic trees based on SSU rRNA gene and ITS-5.8S-ITS2 region sequences [Figs 2A, 3A, 4B, C, E in present investigation,] [40]. However, no close relationship is recovered in trees containing alpha-tubulin gene sequences (Fig. 3B, C, 4A, D, F, G).
As a primary conclusion, it appears that the family Pseudokeronopsidae is not monophyletic although most of its members almost certainly belong to the core portion of urostylids. Very likely, some or most pseudokeronopsids should be transferred to the family Urostylidae, although a taxonomic revision of this group must await further data.
Classification of Acaudalia and the Family Pseudourostylidae
The family Pseudourostylidae comprises three genera, Hemicycliostyla, Trichotaxis and Pseudourostyla (sensu Lynn [1]). SSU rRNA gene sequence data is available for only two pseudourostylids, viz. Pseudourostyla franzi and P. cristata. This classification is consistent with that of Berger [9]. In our SSU rRNA gene trees, two Pseudourostyla species group with the Pseudokeronopsis-Nothoholosticha cluster, which is a sister group to other typical urostylids, e.g. Anteholosticha, Metaurostylopsis, Apokeronopsis etc. (Fig. 2A). And Chen et al. [61] observed that, Pseudourostyla is morphologically similar to Urostyla and Metaurostylopsis, albeit with some minor morphological and morphogenetic differences. The latter two, however, were assigned to the family Urostylidae by Lynn [1]. According to Berger [9], Pseudourostyla, Thigmokeronopsis, Apokeronopsis (syn. Thigmokeronopsis), and Pseudokeronopsis are included in the unranked higher taxon Acaudalia Berger, 2006. The monophyly of Acaudalia, however, is not recovered in any of our trees (Fig. 2– 4), and is rejected by AU tests (P<0.05), which is consistent with several previous reports [12], [22], [27], [40], [58], although close relationships between Thigmokeronopsis and Apokeronopsis, and between Pseudourostyla and Pseudokeronopsis, were recovered in some trees (Fig. 2– 4).
Classification of the Family Epiclintidae
The family Epiclintidae (Wicklow & Borrow 1990) contains two genera, viz. Epiclintes and Eschaneustyla [1], [9]. Due to the absence of gene sequences for Eschaneustyla, however, the evolutionary relationships of these genera cannot be evaluated using molecular data.
The phylogenetic position of Epiclintes is subject to a long and ongoing dispute due to its unusual cirral pattern. As referred in Berger [9], it has been historically assigned to the families Oxytrichidae [20], [62]–[64], Urostylidae [64]–[67], Amphisiellidae [68], Keronidae [15], [69]–[73], Spirofilidae [74], [75], or as incertae sedis within the order Stichotrichida [76]. Based on morphological and ultrastructural specializations, Wicklow and Borror [77] established the family Epiclintidae for this genus, and supposed that Epiclintes is a specialized descendent from Kahliella-like stichotrichines. In a recent study, Hu et al. [57] rejected the placement of Epiclintes in the families Oxytrichidae, Amphisiellidae, and Spirofilidae, or in the order Stichotrichida. Furthermore, several morphological and morphogenetic features of Epiclintes were found to be inconsistent with those of urostylids, including: (1) many oblique ventral rows originating from cirral anlagen but no zigzagic pattern formed, (2) a short row of frontal cirri deriving from UM-anlage, (3) partial replacement of the old adoral zone, (4) de novo formation of the oral primordium, the anlagen for marginal rows and dorsal kineties [57]. The results of the present study are consistent with these findings and also reject a close relationship between Epiclintes and Kahliella (Fig. 2A). As a basal clade, it branches deeply from the assemblage of three Holosticha and one Psammomitra species. Thus, all the available evidence supports the separation of the Epiclintidae at family/suborder level as suggested previously [57], [77], [78].
Congruence/Incongruence among Different 14-Taxa Datasets
Seven phylogenies based on seven different datasets (Datasets 5-11) with same taxa were topologically incongruent, however, a “core” urostylid group is revealed in each tree (Fig. 4). Anteholosticha manca, A. gracilis, Bergeriella, Metaurostylopsis, Thigmokeronopsis, Nothoholosticha and Pseudourostyla, always fall into this core group, whereas Pseudokeronopsis, Holosticha and Psammomitra only cluster within this group in some Datasets (Fig. 4). Among the core group, five nodes are chosen to test congruence among partitions (Fig. 4).
In these seven 14-taxa analyses, ILD, S-H and PABA tests were used to detect congruence/incongruence among different partitions (Tables 2– 4, Tables S3, S4, S5, S6, S7). The ILD test fails to show congruence among most datasets, and only Dataset 9 is suggested to be combined (Table 2). By contrast, the S-H test shows that none of the tree topologies based on combined datasets (Datasets 5, 9, 10, 11) are totally rejected by all other datasets (Table 3). Furthermore, the PABA approach revealed that, apart from the addition of alpha-tubulin gene as the second partition, all additions of partitions increase average BP over all five selected nodes (Table 4). This is consistent with previous investigations [37], [79], [80], the ILD test appears to be too conservative, and should only used as a measure of heterogeneity between gene partitions rather than a measure for a combinability test. The ILD test indicates that SSU rRNA and ITS1-5.8S-ITS2 are congruent, and that alpha-tubulin is incongruent with them, whereas the S-H tests fail to pinpoint the cause of conflict.
For the PABA approach, the mean bootstrap alteration values in Table 4 suggest that in general the SSU rRNA gene contributed the most signal, followed by ITS1-5.8S-ITS2, and then alpha-tubulin. This is consistent with results of all five separated nodes, which shows that all partitions increase BP for Node 5 (Table S7), whereas ITS1-5.8S-ITS2 decrease BP for Node 1 (Table S3), and alpha-tubulin decrease BP for the other three nodes (Tables S4, S5, S6). This is reasonable, considering that the SSU rRNA and ITS1-5.8S-ITS2 genes locate near each other, and SSU rRNA possesses most characters in our analyses.
Materials and Methods
Selection and Identification of Ciliates
The taxa in this study were selected to represent the morphological and morphogenetic diversity of Urostylida. Although the current taxon sampling does not cover all genera in Urostylida, representative taxa for each family were included.
Bergeriella ovata (Liu et al. 2010), Parabirojimia multinucleate (Chen et al. 2010), and Pseudoamphisiella quadrinucleata (Shen et al. 2008) were collected from the coast near Guangzhou, southern China (22°42′N; 114°32′E). Other species and strains were collected from the coast near Qingdao, northern China (36°08′N; 120°43′E). All isolates were identified by the methods of Shao et al. [81] and Li et al. [10]. Terminology and systematic classification used in the current paper follow Berger [82] and Lynn [1], respectively.
Extraction and Sequencing of DNA
Genomic DNA was extracted according to methods described in Yi et al. [22]. Eukaryotic universal A (5′-AACCTGGTTGATCCTGCC AGT-3′) or 82F (5′-GAAACTGCGAATGGCTC-3′) and Eukaryotic universal B (5′-TGATCCTTCTGCAGGTTCACCTAC-3′) primers were used for amplification of the SSU rRNA gene [83] by polymerase chain reaction (PCR). Cycling parameters for the SSU rRNA gene were as follows: 5 min initial denaturation (94°C), followed by 35 cycles of 1 min at 95°C, 1 min 30 s at 56°C, and 2 min at 72°C, with a final extension of 15 min at 72°C. A fragment of approximately 500 bp containing the ITS1, 5.8S ribosomal gene, and ITS2 was amplified using primers ITS-F (5′-GTAGGTGAACCTGCGGAAGGATCATTA-3′) and ITS-R (5′-TACTGATATGCTTAAGTTCAGCGG-3′) [84], with the following cycling parameters: 5 min initial denaturation (94°C), followed by 35 cycles of 30 s at 95°C, 1 min at 56°C, and 1 min at 72°C, with a final extension of 15 min at 72°C. A fragment of approximately 1,000 bp comprising part of the alpha-tubulin gene was amplified using ciliate-specific primers Tub-1 (5′-AAGGCTCTCTTGGCGTACAT-3′) and Tub-2 (5′-TGATGCCTTCAACACCTTCTT-3′) [22]. Cycling parameters were as follows: 5 min initial denaturation (94°C), 35 cycles of 30 s at 94°C, 1 min at 56°C, and 1.5 min at 72°C, with a final extension of 15 min at 72°C. Purified PCR product of appropriate size was inserted into the pUCm-T vector (Shanghai Sangon Biological Engineering & Technical Service Company, China) and sequenced at the Invitrogen sequencing facility in Shanghai, China.
Databases Selection
Eight datasets were evaluated in our analyses: (1) SSU rRNA gene sequences including all available urostylid sequences plus some other spirotricheans (89 sequences in total); (2) ITS1-5.8S-ITS2 region sequences including all available urostylid sequences plus some other spirotricheans (31 sequences in total); (3) alpha-tubulin gene sequences including all available urostylid sequences plus some other spirotricheans (26 sequences in total); (4) three-gene combined dataset including all spirotrichean species available, and Protocruzia adherens, Stylonychia mytilus and Sterkiella nova for SSU rRNA and ITS1-5.8S-ITS2, and Protocruzia contrax, Stylonychia lemnae and Sterkiella cavicola for alpha-tubulin (25 sequences in total); (5) three-gene combined dataset including all available urostylid species (14 sequences in total); (6) SSU rRNA gene sequences including all taxa in Dataset 5; (7) ITS1-5.8S-ITS2 region sequences including all taxa in Dataset 5; (8) alpha-tubulin gene sequences including all taxa in Dataset 5; (9) two-gene combined dataset composed of Datasets 6 and 7; (10) two-gene combined dataset composed of Datasets 6 and 8; (11) two-gene combined dataset composed of Datasets 7 and 8.
Secondary Structure Prediction and ITS2 Sequence Alignment
The default settings of the mfold website (http://frontend.bioinfo.rpi.edu/applications/mfold) [85] were used to produce the secondary structure and sequence in dot-bracket structural format of ITS2 RNA transcripts. The structures were edited for aesthetic purposes with RnaViz 2.0 [86].
The ITS2 sequences with the secondary structure format were aligned using the MARNA web server (http://biwww2.informatik.uni-freiburg.de/Software/MARNA/index.html) [87], based on both the primary and secondary structures.
Phylogenetic Analyses
Sequences (except for ITS2 sequences) were aligned using the ClustalW implemented in Bioedit 7.0.0 [88] and further modified manually using Bioedit.
The final alignment of Dataset 1 included 1,607 positions, and the alignment is available from the authors upon request. A Bayesian inference (BI) analysis was performed with MrBayes 3.1.2 [89] using the GTR+I+G model selected by MrModeltest 2 [90] under the AIC criterion. Markov chain Monte Carlo (MCMC) simulations were run with two sets of four chains using the default settings: chain length 2,000,000 generations, with trees sampled every 100 generations. The first 5,000 trees were discarded as burn-in. The remaining trees were used to generate a consensus tree and to calculate the posterior probabilities (PP) of all branches using a majority-rule consensus approach. A Maximum Likelihood (ML) analysis was performed with PhyML V2.4.4 [91] using the GTR+G+I model selected under the AIC criterion by Modeltest v.3.7 [92]. The reliability of internal branches was assessed using a non-parametric bootstrap method with 1,000 replicates.
The following evolutionary models were selected by MrModeltest 2 for single datasets: GTR+I model for Datasets 2 and 7; GTR+I+G for Datasets 3, 6, and 8. Using these selected models, Bayesian trees for Datasets 2, 3, 4 were built as above. For Dataset 4, individual coding regions were treated as ‘unlinked’, so that separate parameter estimates as specified above were obtained for each gene partition for all runs.
The following evolutionary models were selected by Modeltest v.3.7 for different datasets: GTR+I model for Datasets 2 and 7; GTR+I+G for Datasets 3– 6, 8–11. Using these selected models, ML trees for Datasets 2–4 were constructed as above.
Phylogenetic trees were visualized with TreeView v1.6.6 [93] and MEGA 4 [94].
Identifying of Congruence or Incongruence
Congruence of different data partitions (in this case genes) was tested with both the incongruence length difference (ILD) test [95] and Shimodaira-Hasegawa (S-H) test [96] as implemented in PAUP*4.0b. We excluded taxa with missing data in some gene partitions, and performed the ILD tests with Dataset 4 and Dataset 5, respectively. Six gene-by-gene comparisons were conducted based on 1,000 ILD replicates. In interpreting the results of ILD tests, recent studies have shown that the utility of the ILD test is limited as a measure of the incongruence among data partitions [79], [80]. Therefore, we used the ILD tests as a measure of heterogeneity between gene partitions and the results of ILD tests were not interpreted as a measure for a combinability test [79]. In the case of S-H tests, variance estimations of the difference in the likelihood values of given topologies to the best topology were used to test whether the topology produced by a given partition was accepted or rejected by different data partitions [80], [97]. Therefore, the major-rule consensus topologies obtained by the 7 different 14-taxon datasets were compared to each other based on each of these datasets using the S-H test. RELL approximations with 1,000 replicates and ML methods described above were conducted.
Because neither of these two approaches sufficiently described the source of possible incongruence and its influence in the dataset, the partition addition bootstrap alteration (PABA) approach [80] was used to evaluate the influence of combining genes on nodal support of “core Urostylida”, five nodes with high supports in three gene combined tree (Fig. 4A) were selected.
Supporting Information
ITS2 region secondary structures of 21 sequenced urostylid species.
(TIF)
Evolutionary Similarities among Dataset 6 and 7, and Expressed as Percentages.
(DOC)
Evolutionary Similarities among Dataset 9, and Expressed as Percentages.
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 1 (See Figure 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 2 (See Fig. 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 3 (See Fig. 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 4 (See Fig. 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 5 (See Fig. 4 ).
(DOC)
Acknowledgments
Our deepest gratitude goes to Dr. Alan Warren, Natural History Museum, UK, for his help in improving written English. Many thanks are due to Ms. Feng Gao and Jie Huang, Laboratory of Protozoology, OUC for gene sequencing.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Natural Science Foundation of China (Project No. 31030059, 41006098). Its website is http://www.nsfc.gov.cn/Portal0/default124.htm. It was also supported by Foundation for Distinguished Yong Talents in Higher Education of Guangdong, China (Project No. LYM10060). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lynn DH. The Ciliated Protozoa. New York: Pergamon Press; 2008. [Google Scholar]
- 2.Stoeck T, Foissner W, Lynn DH. Small-subunit rRNA phylogenies suggest that Epalxella antiquorum (Penard, 1922) Corliss, 1960 (Ciliophora, Odontostomatida) is a member of the Plagyopylea. J Eukaryot Microbiol. 2007;54:436–42. doi: 10.1111/j.1550-7408.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 3.Elwood HJ, Olsen GJ, Sogin ML. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol Biol Evol. 1985;2:399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt SL, Bernhard D, Schlegel M, Foissner W. Phylogeny of the Stichotrichia (Ciliophora; Spirotrichea) reconstructed with nuclear small subunit rRNA gene sequences: discrepancies and accordances with morphological data. J Eukaryot Microbiol. 2007;54:201–9. doi: 10.1111/j.1550-7408.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 5.Strüder-Kypke MC, Wright AD, Foissner W, Chatzinotas A, Lynn DH. Molecular phylogeny of litostome ciliates (Ciliophora, Litostomatea) with emphasis on free-living haptorian genera. Protist. 2006;157:261–78. doi: 10.1016/j.protis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Yi Z, Song W, Clamp J, Chen Z, Gao S, et al. Reconsideration of systematic relationships within the order Euplotida (Protista, Ciliophora) using new sequences of the gene coding for small-subunit rRNA and testing the use of combined data sets to construct phylogenies of the Diophrys-complex. Mol Phylogenet Evol. 2009;50:599–607. doi: 10.1016/j.ympev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Cameron S, Adlard R, O'Donoghue PJ. Evidence for an independent radiation of endosymbiotic litostome ciliates within Australian Marsupial Herbivores. Mol Phylogenet Evol. 2001;20:302–310. doi: 10.1006/mpev.2001.0986. [DOI] [PubMed] [Google Scholar]
- 8.Eigner P, Foissner W. Divisional morphogenesis in Bakuella pampinaria nov. spec. and reevaluation of the classification of the urostylids (Ciliophora, Hypotrichida). Eur J Protistol. 1992;28:460–470. doi: 10.1016/S0932-4739(11)80011-8. [DOI] [PubMed] [Google Scholar]
- 9.Berger H. Monograph of the Urostyloidea (Ciliophora, Hypotricha). Monographiae Biologicae. 2006;85:1–1304. [Google Scholar]
- 10.Li L, Zhang Q, Hu X, Warren A, Al-Rasheid K, et al. A redescription of the marine hypotrichous ciliate, Nothoholosticha fasciola (Kahl, 1932) nov. gen., nov. comb. (Ciliophora: Urostylida) with brief notes on its cellular reorganization and SS rRNA gene sequence. Eur J Protistol. 2009;45:237–248. doi: 10.1016/j.ejop.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Shao C, Gong J, Li J, Lin X, et al. Morphology, morphogenesis and molecular phylogeny of a new marine urostylid ciliate (Ciliophora, Stichotrichia) from the South China Sea, and a brief overview of the convergent evolution of the midventral pattern within the Spirotrichea. Zool J Linn Soc. 2009;158:697–710. [Google Scholar]
- 12.Yi Z, Song W, Shao C, Warren A, Al-Rasheid K, et al. Phylogeny of some systematically uncertain urostyloids – Apokeronopsis, Metaurostylopsis, Thigmokeronopsis (Ciliophora, Stichotrichia) estimated with small subunit rRNA gene sequence information: Discrepancies and agreements with morphological data. Eur J Protistol. 2008;44:254–262. doi: 10.1016/j.ejop.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Chen XR, Gao S, Song W, Al-Rasheid KAS, Warren A, et al. Parabirojimia multinucleata spec. nov. and Anteholosticha scutellum (Cohn, 1866) Berger, 2003, two marine ciliates (Ciliophora, Hypotrichida) from tropical waters in southern China, with note on their SSU rRNA gene sequences. Int J Syst Evol Microbiol. 2010;60:234–243. doi: 10.1099/ijs.0.008037-0. [DOI] [PubMed] [Google Scholar]
- 14.Lynn DH, Small EB. Phylum Ciliophora Doflein, 1901. In: Lee JJ, Leedale GF, Bradbury P, editors. (2002) An illustrated guide to the protozoa I: Allen Press Inc; 2002. [Google Scholar]
- 15.Corliss JO. The ciliated protozoa: characterization, classification and guide to the literature. Oxford: Pergamon Press; 1979. [Google Scholar]
- 16.Tuffrau M, Fleury A. Classe des Hypotrichea Stein, 1859. Traite de Zoologie. 1994;2:83–151. [Google Scholar]
- 17.Borror AC. Redefinition of the Urostylidae (Ciliophora, Hypotrichida) on the basis of morphogenetic characters. J Protozool. 1979;26:544–550. [Google Scholar]
- 18.Borror AC, Wicklow BJ. The suborder Urostylina Jankowski, (Ciliophora, Hypotrichida): morphology, systematics and identification of species. Acta Protozool. 1983;22:97–126. [Google Scholar]
- 19.de Puytorac P. Phylum Ciliophora Doflein, 1901. In: de Puytorac P, editor. (1994) Traité de Zoologie,Tome II, Infusoires Ciliés, Fasc. 2, Systématique Paris: Masson; 1994. pp. 1–15. [Google Scholar]
- 20.Jankowski AW. Revision of the order Hypotrichida Stein, 1859. Generic catalogue, phylogeny, taxonomy. Proc Acad Sci USSR. 1979;86:48–85. [Google Scholar]
- 21.Shi X. Systematic revision of the order Hypotrichida II. Urostylina (Ciliophora). Acta Zootaxon Sin. 1999;24:361–371. [Google Scholar]
- 22.Yi Z, Song W, Stoeck T, Al-Rasheid K, Al-Khedhairy A, et al. Phylogenetic analyses suggest that Psammomitra (Ciliophora, Urostylida) should represent an urostylid family, based on SSrRNA and alpha-tubulin gene sequence information. Zool J Linn Soc. 2009;157:227–236. [Google Scholar]
- 23.Yi Z, Song W, Warren A, Roberts DM, Al-Rasheid KA, et al. A molecular phylogenetic investigation of Pseudoamphisiella and Parabirojimia (Protozoa, Ciliophora, Spirotrichea), two genera with ambiguous systematic positions. Eur J Protistol. 2008;44:45–53. doi: 10.1016/j.ejop.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Foissner W, Moon-van der Staay SY, van der Staay GWM, Hackstein JHP, Krautgartner W-D, et al. Reconciling classical and molecular phylogenies in the stichotrichines (Ciliophora, Spirotrichea), including new sequences from some rare species. Eur J Protistol. 2004;4:265–281. [Google Scholar]
- 25.Bernhard D, Stechmann A, Foissner W, Ammermann D, Hehn M, et al. Phylogenetic relationships within the class Spirotrichea (Ciliophora) inferred from small subunit rRNA gene sequences. Mol Phylogenet Evol. 2001;21:86–92. doi: 10.1006/mpev.2001.0997. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt EA, Muller KM, Cannone J, Hogan DJ, Gutell R, et al. Phylogenetic relationships among 28 spirotrichous ciliates documented by rDNA. Mol Phylogenet Evol. 2003;29:258–267. doi: 10.1016/s1055-7903(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 27.Paiva TS, Borges BN, Harada ML, Silva-Neto ID. Comparative phylogenetic study of Stichotrichia (Alveolata: Ciliophora: Spirotrichea) based on 18S-rDNA sequences. Genet Mol Res. 2009;8:223–246. doi: 10.4238/vol8-1gmr529. [DOI] [PubMed] [Google Scholar]
- 28.Dalby AB, Prescott DM. The scrambled actin I gene in Uroleptus pisces. Chromosoma. 2004;112:247–254. doi: 10.1007/s00412-003-0270-4. [DOI] [PubMed] [Google Scholar]
- 29.Snoeyenbos-West OL, Salcedo T, McManus GB, Katz LA. Insights into the diversity of choreotrich and oligotrich ciliates (Class: Spirotrichea) based on genealogical analyses of multiple loci. Int J Syst Evol Microbiol. 2002;52:1901–13. doi: 10.1099/00207713-52-5-1901. [DOI] [PubMed] [Google Scholar]
- 30.Leigh JW, Susko E, Baumgartner M, Roger AJ. Testing congruence in phylogenomic analysis. Syst Biol. 2008;57:104–115. doi: 10.1080/10635150801910436. [DOI] [PubMed] [Google Scholar]
- 31.Rokas A, Krüger D, Carroll SB. Animal evolution and the molecular signature of radiations compressed in time. Science. 2005;310:1933–1938. doi: 10.1126/science.1116759. [DOI] [PubMed] [Google Scholar]
- 32.Philippe H, Lartillot N, Brinkmann H. Multigene analyses of bilaterian animals corroborate the monophyly of ecdysozoa, lophotrochozoa, and protostomia. Mol Biol Evol. 2005;22:1246–1253. doi: 10.1093/molbev/msi111. [DOI] [PubMed] [Google Scholar]
- 33.James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, et al. Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 34.Brochier C, Forterre P, Gribaldo S. An emerging phylogenetic core of archaea: phylogenies of transcription and translation machineries converge following addition of new genome sequences. BMC Evol Biol. 2005;5:36. doi: 10.1186/1471-2148-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levasseur C, Lapointe FJ. War and peace in phylogenetics: A rejoinder on total evidence and consensus. Syst Biol. 2001;50:881–891. doi: 10.1080/106351501753462858. [DOI] [PubMed] [Google Scholar]
- 36.Chippindale PT, Wiens JJ. Weighting, Partitioning, and Combining Characters in Phylogenetic Analysis. Syst Biol. 1994;43:278–287. [Google Scholar]
- 37.Barker FK, Lutzoni F. The utility of the incongruence length difference test. Syst Biol. 2002;51:625–637. doi: 10.1080/10635150290102302. [DOI] [PubMed] [Google Scholar]
- 38.Hogan DJ, Hewitt EA, Orr KE, Prescott DM, Muller KM. Evolution of IESs and scrambling in the actin I gene in hypotrichous ciliates. Proc Natl Acad Sci U S A. 2001;98:15101–6. doi: 10.1073/pnas.011578598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croft KE, Dalby AB, Hogan DJ, Orr KE, Hewitt EA, et al. Macronuclear molecules encoding actins in spirotrichs. J Mol Evol. 2003;56:341–350. doi: 10.1007/s00239-002-2405-2. [DOI] [PubMed] [Google Scholar]
- 40.Yi Z, Lin X, Warren A, Al-Rasheid K, Song W. Molecular phylogeny of Nothoholosticha (Protozoa, Ciliophora, Urostylida) and systematic relationships of the Holosticha-complex. Syst Biodiv. 2010;8:149–155. [Google Scholar]
- 41.Coleman AW. Paramecium aurelia revisited. J Eukaryot Microbiol. 2005;52:68–77. doi: 10.1111/j.1550-7408.2005.3327r.x. [DOI] [PubMed] [Google Scholar]
- 42.Yi Z, Chen Z, Warren A, Roberts D, Al-Rasheid K, et al. Molecular phylogeny of Pseudokeronopsis (Protozoa, Ciliophora, Urostylida), with reconsideration of three closely related species at inter- and intra-specific levels inferred from the small subunit ribosomal RNA gene and the ITS1-5.8S-ITS2 region sequences. J Zool. 2008;275:268–275. [Google Scholar]
- 43.Yi Z, Clamp J, Al-Rasheid K, Al-Khedhairy A, Chen Z, et al. Evolutionary relationship and species separation of four morphologically similar stichotrichous ciliates (Protozoa, Ciliophora). Prog Nat Sci. 2009;19:581–586. [Google Scholar]
- 44.Foissner W, Stoeck T. Morphology, ontogenesis and molecular phylogeny of Neokeronopsis (Afrokeronopsis) aurea nov. subgen., nov. spec. (Ciliophora: Hypotricha), a new African flagship ciliate confirms the CEUU hypothesis. Acta Protozool. 2008;47:1–33. [PMC free article] [PubMed] [Google Scholar]
- 45.Berger H. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monogr Biol. 1999;78:1–1080. [Google Scholar]
- 46.Li L, Song W, Al-Rasheid K, Hu X, Al-Quraishy SA. Redescription of a poorly known marine ciliate, Leptoamphisiella vermis Gruber, 1888 n. g., n. comb. (Ciliophora, Stichotrichia, Pseudoamphisiellidae), from the Yellow Sea, China. J Eukaryot Microbiol. 2007;54:527–534. doi: 10.1111/j.1550-7408.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 47.Shao C, Hu X, Warren A, Al-Rasheid K, Al-Quraishy SA, et al. Morphogenesis in the marine spirotrichous ciliate Apokeronopsis crassa (Claparéde & Lachmann, 1858) n. comb. (Ciliophora: Stichotrichia), with the establishment of a new genus, Apokeronopsis n. g., and redefinition of the genus Thigmokeronopsis. J Eukaryot Microbiol. 2007;54:392–401. doi: 10.1111/j.1550-7408.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 48.Miao M, Shao C, Song W. Evolution of discocephalid ciliates: all molecular, morphological and ontogenetic data support a sister group of discocephalids and pseudoamphisiellids (Protozoa, Ciliophora), with establishment of a new suborder Pseudoamphisiellina subord. n. Sci China Ser C-Life Sci. 2011;54 doi: 10.1007/s11427-011-4192-8. in press. [DOI] [PubMed] [Google Scholar]
- 49.Song W, Warren A, Hu X. Morhology and morphogenesis of Pseudoamphisiella lacazei (Maupas, 1883) Song, 1996 with suggestion of establishment of a new family Pseudoamphisiellidae nov. fam. (Ciliophora, Hypotrichida). Arch Protistenkd. 1997;147:265–276. [Google Scholar]
- 50.Hu X, Song W, Warren A. Orbservations on the morphology and morphogenesis of a new marine urostylid ciliate, Parabirojimia similis nov. gen., nov. spec. (Protozoa, Ciliophora, Hypotrichida). Eur J Protistol. 2002;38:351–364. [Google Scholar]
- 51.Shi X, Song W, Shi X. Systematic revision of the hypotrichous ciliates. In: Song W, editor. (1999) Progress in Protozoology Qingdao: Qingdao Ocean University Press; 1999. pp. 77–154. (in Chinese) [Google Scholar]
- 52.Jerka-Dziadosz M, Janus I. Localization of primordia during cortical development in Keronopsis rubra (Ehrbg., 1838) (Hypotrichida). Acta Protozool. 1972;10:249–268. [Google Scholar]
- 53.Borror AC. Tidal marsh ciliates (Protozoa): morphology, ecology, systematics. Acta Protozool. 1972;31:29–73. [Google Scholar]
- 54.Oberschmidleitner R, Aescht E. Taxonomische Untersuchungen über einige Ciliaten (Ciliophora, Protozoa) aus Belebtschlämmen oberösterreichischer Kläranlagen. Beitr Naturk Oberöster. 1996;3:3–30. [Google Scholar]
- 55.Shao C, Song W, Li L, Warren A, Hu X. Morphological and morphogenetic redescriptions of the stichotrich ciliate Diaxonella trimarginata Jankowski, 1979 (Ciliophora, Stichotrichia, Urostylida). Eur J Protistol. 2007;46:25–39. [Google Scholar]
- 56.Kaltenbach A. Ökologische Untersuchungen an Donauciliaten. Wass Abwass Wien. 1960;1960:151–174. [Google Scholar]
- 57.Hu X, Hu X, Al-Rasheid K, Song W. Reconsideration on the phylogenetic position of Epiclintes (ciliophora, stichotrichia) based on SS rRNA gene sequence and morphogenetic data. Acta Protozool. 2009;48:203–211. [Google Scholar]
- 58.Li L, Song W, Shin MK, Warren A, Al-Rasheid K, et al. Reconsideration of the phylogenetic positions of three stichotrichous genera Holosticha, Anteholosticha and Pseudokeronopsis (Spirotrichea: Ciliophora) inferred from complete SSU rRNA gene sequences. Prog Nat Sci. 2009;19:769–773. [Google Scholar]
- 59.Berger H. Redefinition of Holosticha Wrzesniowski, 1877 (Ciliophora, Hypotricha). Eur J Protistol. 2003;39:373–379. [Google Scholar]
- 60.Li L, Song W, Warren A, Al-Rasheid K, Roberts D, et al. Morphology and morphogenesis of a new marine ciliate, Apokeronopsis bergeri nov. spec. (Ciliophora, Hypotrichida), from the Yellow Sea, China. Eur J Protistol. 2008;44:208–219. doi: 10.1016/j.ejop.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Li Z, Hu X, Kusuoka Y. Morphology, morphogenesis and gene sequence of a freshwater ciliate, Pseudourostyla cristata (Ciliophora, Urostyloidea) from the ancient Lake Biwa, Japan. Eur J Protistol. 2010;46:43–60. doi: 10.1016/j.ejop.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Kahl A. Urtiere oder Protozoa. I: Wimpertiere oder Ciliata (Infusoria), 3. Spirotricha. Tierwelt Dtl. 1932;25:399–650. [Google Scholar]
- 63.Bamforth SS. Anatomy and ecology of hypotrichous protozoa. The Official Publication of the Association of Southeastern Biologists. 1962;9:34. [Google Scholar]
- 64.Corliss JO. The ciliated protozoa: characterization, classification, and guide to the literature. In: Kerkut GA, editor. (1961) International series of monographs on pure and applied biology, Zoology division Oxford, London, New York, Paris.: Pergamon Press; 1961. 310 [Google Scholar]
- 65.Calkins GN. The biology of the Protozoa. Philadelphia, New York: Lea & Febiger; 1926. [Google Scholar]
- 66.Borror AC. Revision of the order Hypotrichida (Ciliophora, Protozoa). J Protozool. 1972;19:1–23. doi: 10.1111/j.1550-7408.1972.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 67.Eigner P. Divisional morphogenesis in Uroleptus caudatus (Stokes, 1886), and the relationship between the Urostylidae and the Parakahliellidae, Oxytrichidae, and Orthoamphisiellidae on the basis of morphogenetic processes (Ciliophora, Hypotrichida). J Eukaryot Microbiol. 2001;48:70–9. doi: 10.1111/j.1550-7408.2001.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 68.Hemberger H. Revision der Ordnumg Hypotrichida Stein (Ciliophora, Protozoa) an Hand von Protargolpräparaten und Morphogenesedarstellungen. Diss Univ Bonn 1982 [Google Scholar]
- 69.Fauré-Fremiet E. Remarques sur la morphologie comparée et la systématique des ciliata Hypotrichida. C. r. hebd. Séanc Acad Sci Paris. 1961;252:3515–3519. [Google Scholar]
- 70.Corliss JO. Annotated assignment of families and genera to the orders and classes currently compreising the Corlissian scheme of higher the phylum Ciliophora. Trans Amer Micros Soc. 1977;96:104–140. [Google Scholar]
- 71.Tuffrau M. Proposition d'une classification nouvelle de I'Ordre Hypotrichida (Protozoa, Ciliophora), fondée sur quelques données récentes. Ann Sci nat Zool. 1987;8:111–117. [Google Scholar]
- 72.Carey PG. Marine interstitial ciliates. An illustrated key. London, New York, Tokyo, Melbourne, Madras: Chapman and Hall; 1992. [Google Scholar]
- 73.Tuffrau M. Une nouvelle famille d'hypotriches, Kahliellidae n. fam., et ses consequences dans la repartition des Stichotrichina. Trans Amer Micros Soc. 1979;98:521–528. [Google Scholar]
- 74.Shi X, Song W, Shi X. Morphogenetic modes of hypotrichous ciliates. In: Se al., editor. (1999) Progress in Protozoology: Qingdao Ocean University Press; 1999. pp. 189–210. [Google Scholar]
- 75.Shi X. Systematic revision of the order Hypotrichida I. Protohypotrichina and Stichotrichina (Ciliophora). Acta Zootaxon Sin. 1999;24:241–264. [Google Scholar]
- 76.Dini F, Lucchesi P, Macchioni G. Protozoa. In: Minelli A, Ruffo S, La Posta S, editors. (1995) Checklist delle specie della fauna italiana Bologna: Calderini; 1995. [Google Scholar]
- 77.Wicklow BJ, Borror AC. Ultrastructure and morphogenesis of the marine epibenthic ciliate Epiclintes ambiguus (Epiclintidae, n. fam.; Ciliophora). Eur J Protistol. 1990;26:182–194. doi: 10.1016/S0932-4739(11)80113-6. [DOI] [PubMed] [Google Scholar]
- 78.Wicklow BJ. Morphogenetic and ultrastructural investigation in the Hypotrichida (Ciliophora, Protozoa): implications in ciliate evolution. Diss Abstr Internat. 1983;43B:2135. [Google Scholar]
- 79.Sung G, Sung J, Hywel-Jones N, Spatafora J. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol Phylogenet Evol. 2007;44:1204–1223. doi: 10.1016/j.ympev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 80.Struck T, Purschke G, Halanych K. Phylogeny of Eunicida (Annelida) and exploring data congruence using a partition addition bootstrap alteration (PABA) approach. Syst Biol. 2006;55:1–20. doi: 10.1080/10635150500354910. [DOI] [PubMed] [Google Scholar]
- 81.Shao C, Miao M, Li L, Song W, Al-Rasheid KA, et al. Morphogenesis and morphological redescription of a poorly known ciliate Apokeronopsis ovalis (Kahl, 1932) nov. comb. (Ciliophora: Urostylida). Acta Protozool. 2009;47:363–376. [Google Scholar]
- 82.Berger H. Monograph of the Amphisiellidae and Trachelostylidae (Ciliophora, Hypotricha). Monographiae Biol. 2008;88:1–737. [Google Scholar]
- 83.Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 84.Shang H. Molecular phylogeny of Scuticociliatia (Ciliophora, Oligohymenophorea), 2004, Ocean University of China: Qingdao.
- 85.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rijk PD, Wachter RD. RnaViz, a program for the visualisation of RNA secondary structure. Nucleic Acids Res. 1997;25:4679–4684. doi: 10.1093/nar/25.22.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siebert S, Backofen R. MARNA: multiple alignment and consensus structure prediction of RNAs based on sequence structure comparisons. Bioinformatics. 2005;21:3352–3359. doi: 10.1093/bioinformatics/bti550. [DOI] [PubMed] [Google Scholar]
- 88.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 89.Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 90.Nylander JA. 2004. MrModeltest v2: Evolutionary Biology Centre, Uppsala University.
- 91.Guindon S, Gascuel O. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 92.Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 93.Page RDM. TREEVIEW: An application to view phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 94.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 95.Farris JS, Källersjö M, Kluge AG, Bult C. Testing significance of incongruence. Cladistics. 1994;10:315–319. [Google Scholar]
- 96.Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16:1114–1116. [Google Scholar]
- 97.Nygren A, Sundberg P. Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida). Mol Phylogenet Evol. 2003;29:235–249. doi: 10.1016/s1055-7903(03)00095-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ITS2 region secondary structures of 21 sequenced urostylid species.
(TIF)
Evolutionary Similarities among Dataset 6 and 7, and Expressed as Percentages.
(DOC)
Evolutionary Similarities among Dataset 9, and Expressed as Percentages.
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 1 (See Figure 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 2 (See Fig. 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 3 (See Fig. 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 4 (See Fig. 4 ).
(DOC)
Alteration of Bootstrap Support δ Depending on the Order a Particular Partition Is Added Shown for Node 5 (See Fig. 4 ).
(DOC)