Abstract
Infection with Edwardsiella tarda, a Gram-negative bacterium, causes high morbidity and mortality in both marine and freshwater fish. Outer membrane vesicles (OMVs) released from Gram-negative bacteria are known to play important roles in bacterial pathogenesis and host immune responses, but no such roles for E. tarda OMVs have yet been described. In the present study, we investigated the proteomic composition of OMVs and the immunostimulatory effect of OMVs in a natural host, as well as the efficacy of OMVs when used as a vaccine against E. tarda infection. A total of 74 proteins, from diverse subcellular fractions, were identified in OMVs. These included a variety of important virulence factors, such as hemolysin, OmpA, porin, GAPDH, EseB, EseC, EseD, EvpC, EvpP, lipoprotein, flagellin, and fimbrial protein. When OMVs were administrated to olive flounder, significant induction of mRNAs encoding IL-1β, IL-6, TNFα, and IFNγ was observed, compared with the levels seen in fish injected with formalin-killed E. tarda. In a vaccine trial, olive flounder given OMVs were more effectively protected (p<0.0001) than were control fish. Investigation of OMVs may be useful not only for understanding the pathogenesis of E. tarda but also in development of an effective vaccine against edwardsiellosis.
Introduction
Outer membrane vesicles (OMVs) are spherical blebs of average diameter 10–300 nm that are naturally released from Gram-negative bacteria into the environment [1]. Although the budding mechanisms are unclear, it has been shown that OMVs are continuously produced during growth of various Gram-negative bacteria including Escherichia coli, Helicobacter pylori, Neisseria meningitidis, Pseudoaltermonas antarctica, Pseudomonas aeruginosa, Shigella flexneri, and Vibrio cholerae [2]–[7]. Such vesicles are known to contain lipopolysaccharide (LPS), lipoproteins, outer membrane, periplasmic, and cytoplasmic proteins, DNA, and RNA [1], [8]–[10], and have been suggested to be involved in exclusion of competing bacteria, conveyance of proteins or genetic material to other bacteria, and presentation of virulence factors to the host [1].
Edwardsiella tarda is the causative agent of edwardsiellosis in a variety of cultured freshwater and marine fish, including channel catfish Ictalurus punctatus, olive flounder Paralichthys olivaceus, Japanese eel Anguilla japonica, red sea bream Pagrus major, mullet Mugil cephalus, and turbot Scophthalmus maximus [11]–[16]. Edwardsiellosis has been implicated in the mass mortality of olive flounder, which is the main mariculture species of South Korea. Typical clinical symptoms of E. tarda infection in olive flounder are exophthalmia, enlargement of the spleen, malodorous ascites, and rectal hernia [17].
A number of studies have shown that vaccination using outer membrane proteins results in development of protective effects against E. tarda infection [18]–[21]. In addition, the outer membrane proteins of E. tarda include several important virulence factors that play key roles in pathogenicity [17]. Virulence factors of E. tarda that have been investigated include dermatotoxin, hemolysins, catalase, outer membrane proteins, EseDs, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [19], [22]–[24].
Previous proteomic studies indicated that both outer membrane proteins and LPS in OMVs might play roles as pathogen-associated molecular patterns (PAMPs) delivered to the host innate immune system, and could thus elicit immune responses [25], [26]. Because OMVs have antigenic properties, such vesicles have been investigated as useful candidate vaccines against Gram-negative bacterial infections [27]–[29]. For example, a Neisseria meningitidis serogroup B vaccine was successfully developed using derived OMVs; 55 million doses have been administered to date [30].
However, none of OMV protein composition, antigenicity, or vaccine efficacy has been studied in bacteria pathogenic for fish, especially the bacterium E. tarda. Thus, we investigated the possibility of using OMVs released from E. tarda as a vaccine against edwardsiellosis, based on both a proteomic study and cytokine induction assays.
Results
TEM examination of OMVs
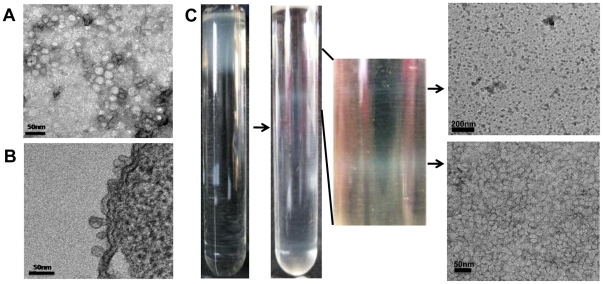
Numerous ovoid-to-round-shaped blebs were evident on the surface of ED45 cells when thin sections were stained to show OMVs (Figure 1-A). The supernatant concentrate contained OMVs that were round in shape, ranged from 10–40 nm in diameter, and contained electron-dense substances (Figure 1-B). Because cell debris and pili were observed upon negative staining, OMVs suspended in PBS were purified by discontinuous sucrose gradient centrifugation prior to protein analysis and in vivo immunogenicity testing. After centrifugation, two clear white bands were evident in the centrifugation tube, and the materials therein were examined by TEM (Figure 1-C). The upper band appeared to contain cell debris or aggregates, whereas the lower band was mainly OMVs, at a density of 1.185 g/ml. All further experiments were performed using these purified OMVs.
Figure 1. Transmission electron micrographs (TEMs) of outer membrane vesicles (OMVs) released from Edwardsiella tarda ED45.
(A) Thin-section TEM of OMVs released from an ED45 cell. Bar = 50 nm. (B) Negatively stained TEM of concentrated OMVs of ED45. Bar = 50 nm. (C) OMVs purified on a sucrose density gradient. The lower fraction is composed of OMVs.
Protein profiling of OMVs
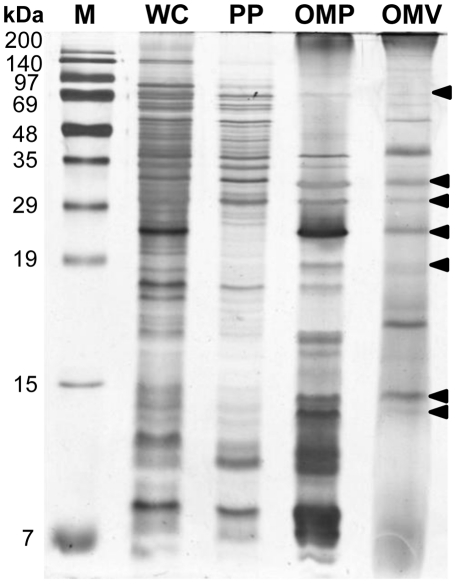
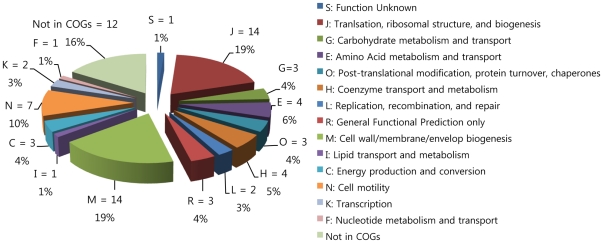
Purified OMVs were loaded onto 1D SDS-PAGE gels and proteins were visualized, after electrophoresis, using a silver staining method, and compared with proteins in WCL, PP, and OMP fractions. OMV proteins of molecular size 68, 31.5, 30, 24, 19, 14.5, and 14 kDa (Figure 2) were similar to those in the OMP fraction, but the 17-, 37-, and 54-kDa bands of OMVs were absent from the OMP sample. To identify the protein components of OMVs, the proteins were separated by 12.5% (w/v) SDS-PAGE and the gels were cut into 12 slices. Proteins in individual slices were analyzed by LC-ESI-MS/MS; acquired peptide mass data were analyzed using the MASCOT Daemon interface. A total of 74 proteins were identified in the E. tarda database (Table 1), and each slice contained at least 5 of these proteins (data not shown). Proteins were categorized into 15 different orthologous groups using the COG approach (Figure 3), indicating that the identified proteins were involved in both cellular processing and signaling (COG groups M, O, and N; 33% of proteins); information storage and processing (COG groups L, K, and J; 25% of proteins), and metabolism (COG groups I, H, G, F, E, and C; 21% of proteins). In total, 5% of proteins fell into poorly characterized categories (COG groups S and R), whereas 16% could not be identified in any COG grouping. The subcellular locations of the identified proteins were predicted using the PSORTb algorithm; this exercise suggested that 37 proteins were localized in the cytoplasmic space, 6 in the inner membrane, 16 in the outer membrane, and 9 in the extracellular space. The locations of six proteins could not be identified.
Figure 2. SDS-PAGE protein profiles of WCL, PPs, OMPs, and OMVs.
Arrowheads indicate OMV polypeptides with molecular weights the same as those of OMPs. M: protein marker lane; WCL: whole cell lysate, PP: periplasmic proteins; OMPs: outer membrane proteins; OMVs: outer membrane vesicles.
Table 1. Proteins of OMVs of Edwardsiella tarda identified by LC-ESI-MS/MS.
| Accession number | Description | Mascot score | PIa | MW (Da)b | Protein matches | Functional categoryc | Subcellular localizationd |
| gi|2244627 | Hemolysin | 288 | 5.99 | 165447 | 34 | R | outer membrane |
| gi|291091829 | Lipoprotein | 131 | 9.67 | 33041 | 10 | M | outer membrane |
| gi|267985457 | long-chain fatty acid transport protein | 429 | 6.45 | 47578 | 15 | I | outer membrane |
| gi|267984860 | murein lipoprotein | 224 | 8.93 | 8384 | 9 | M | outer membrane |
| gi|267985939 | nucleoside-specific channel-forming protein Tsx | 50 | 5.62 | 32786 | 2 | M | outer membrane |
| gi|267984912 | OmpA/MotB domain protein | 44 | 9.62 | 17648 | 9 | M | outer membrane |
| gi|25989456 | outer membrane protein | 575 | 5.27 | 47282 | 19 | M | outer membrane |
| gi|267984281 | outer membrane protein A | 1482 | 7.66 | 38034 | 105 | M | outer membrane |
| gi|253720368 | outer membrane protein A precursor | 1447 | 7.66 | 38048 | 104 | M | outer membrane |
| gi|291089413 | peptidoglycan-associated lipoprotein | 52 | 5.93 | 18793 | 10 | M | outer membrane |
| gi|267985578 | peptidoglycan-associated outer membrane lipoprotein | 89 | 5.92 | 18770 | 7 | M | outer membrane |
| gi|267983927 | putative hemolysin precursor | 288 | 5.9 | 167296 | 29 | R | outer membrane |
| gi|267983035 | putative outer membrane lipoprotein | 38 | 10.01 | 22250 | 2 | M | outer membrane |
| gi|267984253 | putative outer membrane porin F protein | 303 | 5.03 | 40058 | 11 | M | outer membrane |
| gi|73532672 | putative virulence-related membrane protein | 95 | 9.4 | 19858 | 5 | - | outer membrane |
| gi|267985681 | virulence-related outer membrane protein | 910 | 8.89 | 20476 | 34 | - | outer membrane |
| gi|291091795 | lysophospholipid transporter LplT | 36 | 9.08 | 34123 | 10 | R | cytoplasmic membrane |
| gi|291089401 | protein YdcF | 36 | 5.28 | 28546 | 4 | - | cytoplasmic membrane |
| gi|291089679 | putative inner membrane protein | 65 | 5.19 | 19700 | 2 | S | cytoplasmic membrane |
| gi|291090200 | transcriptional regulator, LysR family | 33 | 6.46 | 35207 | 4 | - | cytoplasmic membrane |
| gi|291089298 | twin arginine-targeting protein translocase TatA | 42 | 9.3 | 9330 | 21 | K | cytoplasmic membrane |
| gi|267985130 | ABC transporter-related protein | 32 | 9.43 | 28704 | 33 | N | cytoplasmic membrane |
| gi|267986212 | 30S ribosomal protein S13 | 146 | 10.58 | 13283 | 6 | J | cytoplasmic |
| gi|267985854 | 30S ribosomal protein S16 | 63 | 8.36 | 6755 | 4 | J | cytoplasmic |
| gi|267983753 | 30S ribosomal protein S2 | 37 | 6.61 | 26281 | 10 | J | cytoplasmic |
| gi|267986226 | 30S ribosomal protein S3 | 44 | 10.27 | 25980 | 9 | J | cytoplasmic |
| gi|267983384 | 30S ribosomal protein S6 | 51 | 5.29 | 15270 | 12 | J | cytoplasmic |
| gi|267986235 | 30S ribosomal protein S7 | 120 | 10.3 | 17609 | 12 | J | cytoplasmic |
| gi|267983534 | 30S ribosomal protein S9 | 96 | 10.94 | 14834 | 6 | J | cytoplasmic |
| gi|267983533 | 50S ribosomal protein L13 | 33 | 9.91 | 16049 | 15 | J | cytoplasmic |
| gi|291091309 | 6,7-dimethyl-8-ribityllumazine synthase | 76 | 5.32 | 16195 | 5 | H | cytoplasmic |
| gi|267983456 | altronate hydrolase | 31 | 5.78 | 54172 | 22 | G | cytoplasmic |
| gi|291091008 | asparagine–tRNA ligase | 39 | 5.16 | 52655 | 7 | J | cytoplasmic |
| gi|267984254 | asparaginyl-tRNA synthetase | 39 | 5.1 | 52605 | 7 | J | cytoplasmic |
| gi|291092134 | aspartate ammonia-lyase | 50 | 5.32 | 53103 | 7 | E | cytoplasmic |
| gi|291092137 | chaperonin GroL | 836 | 4.84 | 57456 | 65 | O | cytoplasmic |
| gi|267985752 | cobyrinic acid ac-diamide synthase | 32 | 5.12 | 30638 | 6 | H | cytoplasmic |
| gi|267983856 | conserved hypothetical protein | 36 | 10.34 | 13945 | 2 | - | cytoplasmic |
| gi|22121758 | Cpn60 | 54 | 4.46 | 19369 | 6 | O | cytoplasmic |
| gi|267986210 | DNA-directed RNA polymerase subunit alpha | 68 | 5.03 | 36725 | 12 | K | cytoplasmic |
| gi|55981975 | EseD | 166 | 5.34 | 21101 | 6 | - | cytoplasmic |
| gi|291089872 | formate acetyltransferase | 35 | 5.71 | 85456 | 13 | C | cytoplasmic |
| gi|267985188 | formate acetyltransferase 1 | 35 | 5.65 | 85471 | 13 | C | cytoplasmic |
| gi|222457929 | glyceraldehyde-3-phosphate dehydrogenase | 146 | 6.6 | 35684 | 11 | G | cytoplasmic |
| gi|224382175 | heat shock protein 60 | 54 | 4.41 | 17870 | 5 | O | cytoplasmic |
| gi|267985045 | hypothetical protein ETAE_2037 | 104 | 9.3 | 20384 | 10 | - | cytoplasmic |
| gi|291092198 | lysine decarboxylase | 70 | 5.63 | 81254 | 15 | E | cytoplasmic |
| gi|267983773 | lysine decarboxylase 1 | 327 | 5.53 | 81313 | 33 | E | cytoplasmic |
| gi|291091450 | lysine decarboxylase, inducible | 235 | 5.53 | 81368 | 34 | E | cytoplasmic |
| gi|291089681 | O-succinylbenzoate-CoA ligase | 39 | 8.62 | 50218 | 7 | IQ | cytoplasmic |
| gi|267985017 | polypeptide-transport-associated domain protein | 396 | 6.17 | 27889 | 24 | - | cytoplasmic |
| gi|267983427 | polyribonucleotide nucleotidyltransferase | 130 | 5.2 | 76425 | 7 | J | cytoplasmic |
| gi|267985748 | putative integrase | 32 | 9.73 | 44853 | 8 | L | cytoplasmic |
| gi|267983991 | riboflavin synthase beta-chain | 76 | 5.65 | 16208 | 8 | H | cytoplasmic |
| gi|267984414 | ribose-phosphate pyrophosphokinase | 143 | 5.25 | 34557 | 9 | FE | cytoplasmic |
| gi|291088840 | ribosomal protein S19 | 42 | 10.42 | 10380 | 6 | J | cytoplasmic |
| gi|291091495 | ribosomal protein S2 | 37 | 6.33 | 26830 | 13 | J | cytoplasmic |
| gi|291089791 | site-specific recombinase, phage integrase family | 32 | 9.7 | 44682 | 9 | L | cytoplasmic |
| gi|73532652 | type III secretion system effector protein D | 53 | 5.11 | 13695 | 4 | N | cytoplasmic |
| gi|74474901 | antigenic protein Et 46 | 55 | 5.2 | 43774 | 19 | H | extracellular |
| gi|256599595 | Chain A, Structure Of A Type Six Secretion System Protein | 290 | - | 18043 | 16 | - | extracellular |
| gi|61743025 | EseC | 597 | 6.15 | 53717 | 42 | O | extracellular |
| gi|40287638 | EvpC | 308 | 5.71 | 18143 | 19 | K | extracellular |
| gi|38016008 | fimbrial protein | 281 | 9.14 | 38109 | 12 | - | extracellular |
| gi|117307392 | flagella (r) hook associated protein HAP2 | 95 | 6.62 | 50267 | 33 | C | extracellular |
| gi|24306148 | Flagellin | 55 | 4.88 | 43722 | 18 | C | extracellular |
| gi|27808146 | major fimbrial subunit protein | 2000 | 7.71 | 18473 | 78 | G | extracellular |
| gi|267983886 | type III secretion system effector protein C | 597 | 6.08 | 50911 | 37 | O | extracellular |
| gi|61743023 | EseB | 287 | 5.51 | 21777 | 12 | - | unknown |
| gi|237861343 | EvpP | 165 | 9.38 | 20991 | 11 | E | unknown |
| gi|291092160 | N-acetylmuramoyl-L-alanine amidase | 47 | 10.81 | 60479 | 15 | E | unknown |
| gi|267985878 | outer membrane lipoprotein | 217 | 9.56 | 33180 | 11 | E | unknown |
| gi|291089780 | pseudouridine synthase, RluA family | 35 | 9.11 | 7339 | 4 | IQ | unknown |
| gi|267983367 | putative N-acetylmuramoyl-L-alanine amidase | 47 | 10.56 | 59093 | 12 | - | unknown |
aTheoretical protein charge.
bTheoretical protein molecular mass.
cFunctional categorization based on COGs. The abbreviations are shown in Figure 3. ‘-’ indicates ‘Not in COGs’.
dSubcellular localization was predicted using the PSORTb v3.0 algorithm.
Figure 3. Functional classification of OMVs according to COG functional categories.
The pie chart shows the numbers and percentages of identified proteins in each COG grouping. Individual protein assignment to COGs is shown in Table 1.
Immune responses in olive flounder
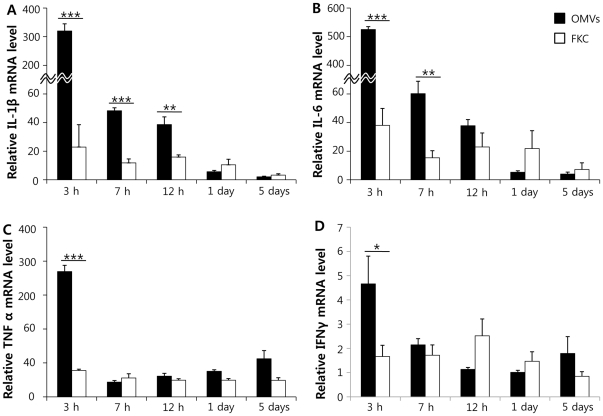
Based on the proteomic data, QRT-PCR was conducted to measure mRNA expression levels of IL-1β, IL-6, TNFα, and IFNγ in olive flounder injected with FKC or OMVs, to investigate whether OMVs could elicit host immune responses. After intraperitoneal injection of OMVs or FKC, kidneys of injected fish were sampled at 0, 3, 7, and 12 hpi; and 1 and 5 dpi (Figure 4). Fish injected with OMVs showed increased cytokine levels compared with those measured at 0 hours. IL-1β and IL-6 expression levels were induced 320- and 515-fold at 3 hpi, and these levels were maintained to 5 dpi. TNFα and IFNγ synthesis levels increased 4.8- and 7.6-fold at 3 hpi, compared with zero time measurements. Fish injected with FKC showed differences in IL-1β and IL-6 expression levels (compared with controls) at early time points, but neither the TNFα nor IFNγ synthesis level was distinct from that of 0-hour control values. When expression levels of IL-1β, IL-6, TNFα, and IFNγ in fish injected with OMVs were compared with those in fish injected with FKC, the cytokine levels of OMV-injected fish were significantly higher, at early time points, than in fish injected with FKC. The level of IL-1β expression in fish injected with OMVs differed significantly from that in fish injected with FKC, at 3, 7, and 12 hpi, whereas IL-6 levels were significantly different at 3 and 7 hpi. TNFα and IFNγ expression levels in fish injected with OMVs differed, at 3 hpi, from those in fish injected with FKC.
Figure 4. Relative induction of IL-1β (A), IL-6 (B), TNFα (C), and IFNγ (D) in olive flounder injected with OMVs or FKC, estimated using quantitative real-time PCR.
OMV: group injected with outer membrane vesicles; FKC: group injected with formalin-killed ED45. *P<0.05, **P<0.01, and ***P<0.001. Bars indicate standard deviations. N = 4.
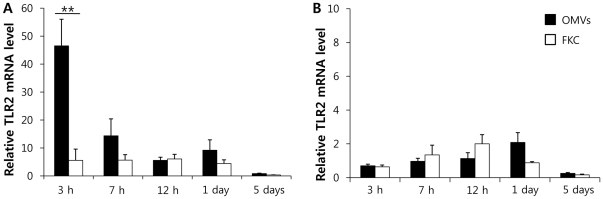
mRNA TLR22 (toll-like receptor 22) and TLR2 expression levels, which are pattern recognition receptors (PRRs) in olive flounder, were measured to determine whether the innate immune response could be elicited by OMVs [31]. TLR22 levels in fish injected with OMVs were significantly higher than in fish injected with FKC. However, TLR2 levels did not differ (Figure 5).
Figure 5. Relative induction of TLR22 (A) and TLR2 (B) in olive flounder injected with OMVs or FKC, estimated using quantitative real-time PCR.
OMV: group injected with outer membrane vesicles; FKC: group injected with formalin-killed ED45. **P<0.01. Bars indicate standard deviations. N = 4.
Protective effects of OMV injection into olive flounder
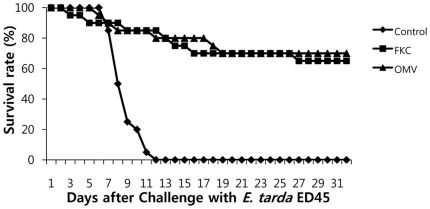
OMVs were administered to olive flounder as a candidate vaccine against E. tarda infection; control fish were injected with PBS (Figure 6). Fish injected with FKC were used as positive controls. After acclimatization of fish for 28 days, 1.1×104 CFU/ml ED45 was used as an intraperitoneal challenge, and survival rates were recorded over 31 days. Control fish died from 6 dpi and all fish were dead at 11 dpi. OMV-vaccinated fish showed some mortality at 5 dpi; however, 70% of fish survived until 31 dpi. FKC-injected fish had a survival rate of 65%. OMV showed an RPS of 70%; this differed significantly from that of control (PBS) (p<0.0001).
Figure 6. Survival rates of olive flounder challenged with ED45 four weeks after immunization.
Control: PBS-injected group; FKC: group injected with formalin-killed ED45; OMV: group injected with outer membrane vesicles.
Discussion
Several Gram-negative bacteria produce OMVs [1]. In the present study, E. tarda was also shown to produce round OMVs, 10–40 nm in diameter, when grown in a liquid medium. Preparation of pure OMVs from bacterial supernatants is essential when studying the roles played by OMVs in both bacteria and their hosts [9], [10]. Recently, pure OMVs from bacterial supernatants have been obtained by filtration followed by density gradient ultracentrifugation [8], [25]. In the present study, homogenously sized E. tarda OMVs were separated from contaminants using such methods. Flagella, pili, and aggregated proteins were present in OMV samples before sucrose density gradient purification, but were removed during this step.
Two representative proteomic analysis methods have been suggested for the study of OMVs. Of these, the first is two-dimensional electrophoresis (2-DE) followed by matrix-associated laser desorption/ionization time-of-flight (MALDI-TOF) spectrometry, and the other is 1D SDS-PAGE followed by LC-ESI-MS/MS [10], [25], [26]. 2-DE is most commonly used to establish proteomic maps of bacteria, but limitations of the technique include poor separation of high-molecular weight and hydrophobic proteins [32]. However, 1D SDS-PAGE in combination with LC-ESI-MS/MS was effective in analysis of hydrophobic outer membrane proteins contained in OMVs [6]. In the present work, a total of 74 proteins were identified, 16 of which were characteristic in the outer membrane, as indicated by the PSORTb algorithm. However, a proteomic survey of the E. tarda outer membrane identified only 21 proteins by 2-DE analysis, and only 1 protein was confined to this membrane [33]. Therefore, 1D SDS-PAGE coupled with LC-ESI-MS/MS is more sensitive when used to analyze proteins of outer membranes or OMVs.
Some reports have suggested that OMV proteomes consist mainly of outer membrane and periplasmic proteins [34], but other proteomic studies found that OMVs contained proteins of various origin, including all of cytoplasmic, inner membrane, outer membrane, and periplasmic proteins [8], [25], [35]. By 1D SDS-PAGE, a 17-kDa band evident in OMVs could not be observed in outer membrane or periplasmic protein fractions. The PSORTb algorithm also indicated that proteins of OMVs originated not only from the outer membrane but also from cytoplasmic and extracellular compartments. These findings, together with those of previous proteomic studies, indicate that the OMV proteome includes proteins varying in subcellular origin, and other components.
LC-ESI-MS/MS analysis of OMVs showed that several proteins involved in pathogenesis, including hemolysin, outer membrane protein A (OmpA), GAPDH, EseB, EseC, EseD, EvpC, EvpP, lipoprotein, flagellin, and fimbrial protein, were present. Hemolysin is known to be required for cellular invasion and cytotoxicity [22]; hemolysin-negative E. tarda mutants could not invade human epithelial cell lines [36]. An ompA-negative E. coli strain invaded brain microvascular endothelial cells only with difficulty [37], [38]. GAPDH in the outer membrane is highly antigenic, and is considered to be a strong vaccine candidate to counter not only Gram-negative but also Gram-positive bacterial infections [19], [39]. EseB, EseC, EseD, EvpC, and EvpP are essential for E. tarda pathogenesis; these proteins are located on the cell surface and are responsible for pore formation [21], [40], [41]. Thus, OMVs released from E. tarda contain numerous virulence factors that may be important both for bacterial survival and to enhance immunogenicity in the natural host.
QRT-PCR demonstrated that the genes encoding all of IL-1β, IL-6, TNFα, and IFNγ were induced in fish injected with OMVs of E. tarda, compared with fish injected with FKC, especially at early post-injection time points. Similarly, mice injected with OMVs of Bordetella pertussis showed upregulation of mRNAs encoding IL-6 and TNFα, compared with the levels seen in animals injected with formalin-killed B. pertussis [27]. It is known that the primary host immune response is mediated by the proinflammatory cytokines IL-1β, IL-6, and TNFα [42]. Expression of such cytokines following injection of E. tarda OMVs indicates that the OMVs may initiate a proinflammatory cytokine cascade, including both recruitment and activation of macrophages and stimulation of an adaptive immune response. IFNγ, a Th1 cytokine, is also upregulated in fish injected with OMVs, and is known to enhance cell-mediated immunity and antigen presentation to macrophages [42]. Such observations may indicate that OMVs trigger an elevated host immune response, thus provoking host adaptive immunity, even though protein levels in OMVs are lower than those in FKC.
Innate immunity is an important first line of defense against invading pathogens, and recognition of PAMPs is achieved principally by PRRs [43]. In the present study, virulence factors, including lipoprotein, flagellin, and peptidoglycan, contained within OMVs of E. tarda, may have played roles as PAMPs, thus interacting with the innate immune system. In addition, OMVs are known to possess LPS and bacterial DNA that function as ligands for host PRRs [44], [45]. OMVs have been reported to be recognized by TLR2 and TLR9, both of which participate in IL-6 production via myeloid differentiation factor 88 (MyD88)-dependent pathways [45]. In the present study, TLR2 was not induced after injection with OMVs (compared with what was seen in positive control fish), whereas, in contrast, the TLR22 level was significantly increased. Therefore, the PRRs of olive flounder also participate in recognition of OMVs but the teleost detection mechanism for OMVs may differ from that of mammals.
The proteomic data, and the demonstrated immunostimulatory effects of OMVs from E. tarda, showed that OMVs exhibited high protective efficacy against E. tarda infection of olive flounder, with an RPS of 70% (p<0.0001). Other reports have described successful inhibition of E. tarda infection in laboratory experiments using various antigen preparations including formalin-killed and ghost cells, recombinant proteins, and outer membrane fractions [18]–[21]. However, these advances have not been translated into successful commercial vaccines. Although OMVs were no more effective as a vaccine than was FKC, vaccines of the latter type sometimes function poorly [46]. The OMV work of the present study offers a new insight into vaccine formulation. Thus, an acellular vaccine, or the use of acellular materials as a vaccine adjuvant, may be helpful in development of an effective vaccine protecting against edwardsiellosis in fish.
In conclusion, OMVs naturally released from E. tarda contained various important virulence factors originating from diverse subcellular locations. Such vesicles were able to induce synthesis of several proinflammatory cytokines, and may stimulate the host innate immune system, thus serving as PAMPs. To demonstrate vaccine efficacy, we injected olive flounder with OMVs, and found that such fish were protected to a significantly higher extent than were control fish. The present study on OMVs may lead to development of more effective vaccines, and may enhance our understanding of the roles of OMVs in hosts.
Materials and Methods
Bacterial strain and growth conditions
ED45, a virulent E. tarda strain isolated from the spleen of infected olive flounder, was used in the present study; the LD50 value was 1.2×103 CFU/ml (data not shown). ED45 was cultured on Tryptone Soya Agar (TSA; Oxoid, Hampshire, England) or in Tryptone Soya Broth (TSB; Oxoid), supplemented with 2% (w/v) NaCl (termed the TSA-2 and TSB-2 media, respectively). ED45 was grown on TSA-2 at 25°C for 36 h; a single colony was inoculated into 25 ml TSB-2, and the culture grown with shaking to a cell density of 1×109 CFU/ml. Next, 20 ml of this culture was added to 4 l TSB-2 and growth proceeded in a shaking incubator at 25°C for 15 h. After centrifugation at 5,000×g for 20 min, the supernatant was used for isolation of OMVs.
OMV preparation
OMVs were collected and purified from the supernatant as described previously, with several modifications [25], [47]. The supernatant containing OMVs were filtered through a 0.45 µm pore-sized hollow fiber cartridge and concentrated using a hollow cartridge 100 kDa in size cutoff, employing a Quixstand benchtop system (GE Healthcare, Uppsala, Sweden). The concentrated supernatant was filtered through a 0.2 µm pore-sized vacuum filter to eliminate all remaining ED45 cells. Subsequently, OMVs were pelleted by ultracentrifugation at 150,000×g for 3 h at 4°C in an XL-90 ultracentrifuge running a 70Ti rotor (Beckman Coulter, Palo Alto, CA).
Pelleted OMVs were resuspended in phosphate-buffered saline (PBS; pH 7.2), layered onto a 30–60% (w/v) discontinuous sucrose gradient, and centrifuged at 200,000×g for 20 h at 4°C in a XL-90 ultracentrifuge running an SW41 Ti rotor, to obtain purified OMVs. Two clear bands were visualized, and the sucrose density in the vicinity of each band was measured by refractometry (Figure 1). After washing material from both bands (collected separately) with a 20-fold dilution of PBS, the materials were centrifuged at 150,000×g for 3 h at 4°C in a XL-90 ultracentrifuge. The final pellets were resuspended in PBS and protein concentrations were determined using a non-interfering assay (G-Biosciences, St. Louis, MO). The final preparations were stored at −80°C prior to use.
Transmission electron microscopy (TEM)
ED45 cells were grown in TSB-2 at 25°C until an OD600 value of 1.0 was attained, centrifuged at 5,000×g for 30 min, and washed three times with PBS prior to preparation of ultrathin sections. After centrifugation at 5,000×g for 20 min, pellets were pre-fixed in 2% (v/v) paraformaldehyde for 3 h, washed, and fixed in 4% (w/v) osmium tetroxide for 2 h. After washing with PBS, the fixed pellets were dehydrated in a series of 70–100% (v/v) ethanol baths and embedded in epoxy resin. Sections were cut using a diamond blade (Diatome, Biel, Switzerland) fitted to an ULTRACUT UCT (Leica, Vienna, Austria), mounted on carbon-coated copper grids, and stained with 3% (w/v) uranyl acetate and lead citrate. To visualize OMVs after negative staining, samples were placed on 400- mesh carbon-coated grids for 2 min, washed with deionized sterile water (dd H20), and negatively stained with 3% (w/v) uranyl acetate for 30 sec. All TEM images were acquired using a Technai 12 (FEI, Hillsboro, OR) operating at an acceleration voltage of 120 kV.
Preparation of whole cell lysates, periplasmic proteins, and outer membrane proteins
Periplasmic proteins (PPs) and outer membrane proteins (OMPs) were purified as described previously [48]. Whole cell lysates (WCLs) of ED45 were obtained from cells grown in TSB-2 at 25°C, pelleted at 5,000×g for 30 min, and washed with PBS. Pelleted cell density was adjusted to 4 g/ml in 20% (w/v) sucrose dissolved in 20 mM Tris-HCl (pH 8.0), also with 0.1 M EDTA and lysozyme (600 µg/g cells). After incubation on ice for 40 min, 0.5 M MgCl2 (0.16 ml/g cells) was added and spheroplasts were removed by centrifugation (9,500×g for 20 min). The supernatant containing PPs was stored at −80°C until use. Spheroplasts were resuspended in ice-cold 10 mM Tris-HCl (pH 8.0) and sonicated for purification of OMPs. Following centrifugation at 8,000×g for 5 min to remove cells, the supernatant was centrifuged at 40,000×g for 1 h to pellet cell membrane material. Pellets were washed in 10 mM Tris-HCl (pH 8.0), resuspended in dd H20, and freeze-thawed. The membranes were incubated in 0.5% (w/v) Sarkosyl (sodium N-lauroylsarcosinate; Sigma, St. Louis, MO) at 25°C for 20 min and OMP pellets were purified by centrifugation at 40,000×g for 1 h. Purified OMPs were resuspended in 10 mM Tris-HCl (pH 8.0) and stored at −80°C until use. Protein concentration was determined using a non-interfering protein assay (G-Biosciences, Hercules, CA).
Electrophoresis and in-gel digestion
SDS-PAGE was performed using a 15% (w/v) acrylamide separating gel, according to the method of Laemmli [49]. Briefly, each resuspended WCL, PP, OMP, and OMV protein sample was mixed with 5×sample buffer (5∶1 v/w ratio of buffer to sample). The buffer contained 60 mM Tris-HCl, 25% (v/v) glycerol, 2% (w/v) SDS, 14.4 mM β-mercaptoethanol, and 0.1% (w/v) bromophenol blue. Samples were boiled for 10 min, cooled on ice, and centrifuged at 16,000×g for 20 min. Supernatants were subjected to SDS-PAGE analysis. Five microgram amounts of WCL, PP, OMP, and OMV proteins were loaded and proteins were detected by silver staining [50].
To achieve in-gel digestion, 20 µg amounts of OMVs were electrophoresed on a 12.5% (w/v) separating gel and stained with Bio-safe Coomassie G-250 (Bio-Rad, Hercules, CA). After cutting the gel into 12 slices, each slice was destained with a 500 µl amount of 40% (v/v) ethanol in 75 mM ammonium bicarbonate (ABC) and treated with DTT (0.0039 g dithiothreitol in 5 ml 25 mM ABC) and IAA (0.0509 g iodoacetamide in 5 ml 25mM ABC) solutions. Each gel slice was dehydrated using 300 µl acetonitrile (ACN) for 30 min at 37°C, dried, and used for in-gel digestion employing 20 ng/ml of sequencing-grade modified trypsin (Promega, Madison, WI) at 37°C overnight. Tryptic peptides were extracted into 30 µl 0.1% (v/v) formic acid and the solutions were sonicated for 10 min.
LC-ESI-MS/MS and data analysis
LC-ESI-MS/MS (liquid chromatography electrospray ionization tandem mass spectrometry) analysis was carried out using a Thermo Finnigan Proteome X workstation with an LTQ linear ion trap and a MS apparatus equipped with NSI sources (Thermo Electron, San Jose, CA). Twelve microliter amounts of peptide mixtures were injected and loaded onto peptide trap cartridges (Agilent, Palo Alto, CA). Trapped peptides were eluted onto a 10 cm-long reverse-phase PicoFrit column packed in-house with 5 µm C18 resin of pore size 300 Å, and the peptides were next separated on an RP column using gradient elution. The mobile phase solutions were H2O and ACN, both containing 0.1% (v/v) formic acid, and delivered at a constant flow rate of 0.2 µl/min. The gradient commenced with 2.0% (v/v) ACN, rising linearly to 60% (v/v) ACN over 50 min, next increasing to 80% (v/v) ACN over the next 5 min, with a change to 100% H2O for the final 15 min. A data-dependent acquisition mode (m/z 300–1,800) was enabled, and each survey MS scan was followed by five MS/MS scans with the 30 sec dynamic exclusion option set. The spray voltage was 1.9 kV and the ion transfer tube temperature 195°C. The normalized collision energy was set to 35%.
The mzData file of tandem mass spectra was used to search the E. tarda database of NCBI downloaded on 9 July 2010, using the Mascot Deamon interface (Version 2.2.2, Matrix Science Inc., London, UK) supported by the Korea Basic Science Institute (KBSI; Yusung-gu, Daejeon, Korea). The peptide tolerance of the parent ion was adjusted to be 1 Da and the MS/MS tolerance was 0.8 Da. During analysis, carbamidomethyl (C) modification was fixed whereas oxidation modification (M) was not. One missed cleavage was permitted, and peptide charges were set at 2+ and 3+. Individual ion scores of more than 15 (p<0.05) were considered reliable and were subsequently used.
Functional annotations of OMV proteins were performed using the “clusters of orthologous groups” (COGs) functional classification (http://www.ncbi.nih.gov/COG) [51]. The subcellular localization of identified proteins was predicted using PSORTb version 3.0 (http://www.psort.org) [52].
Fish, and immunization with OMVs or FKC
Naïve olive flounders (with an approximate weight of 55 g) were purchased from a commercial fish farm in Korea and acclimatized for 2 weeks at 21°C in aerated seawater. Formalin-killed E. tarda cells (FKC) were prepared by addition of 1% (v/v) formalin to an ED45 culture, followed by adjustment to an OD600 value of 1.0 using PBS. OMVs were prepared as described above and used after confirmation that no colonies formed when OMVs were plated onto TSA-2. Aliquots (0.1 ml) of FKC (1.2×108 CFU/ml, 344 µg), or 10 µg amounts of OMVs, were intraperitoneally injected into olive flounder. The administred dosage was determined based on previous studies on immunization with OMVs originating from several gram negative bacteria [25], [27], [53], [54]. To evaluate expression of immune response-related molecules in fish, the kidneys of immunized olive flounder were dissected 0, 3, 7, and 12 hours post-infection (hpi), and 1.5 days post-infection (dpi), and stored in “RNA-later” solution (Ambion, Austin, TX) until all samples were available (N = 4).
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Gyeongsang National University, Jinju, Republic of Korea (Approval Number: GNU-LA-17).
Quantitative real-time PCR
Total RNA was isolated from kidney samples using the TRIzol reagent (Invitrogen, Scotland, UK) according to the manufacturer's protocol. After purification of 1 µg aliquots of mRNA using deoxyribonuclease I (Invitrogen), cDNAs were synthesized with the assistance of a high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster city, CA) according to the manufacturer's protocol. cDNA samples were diluted 1∶4 (v/v) into nuclease-free water prior to amplification using quantitative real-time PCR (QRT-PCR). Primers were designed employing the Primer Express software of Real-Time PCR version 3.0 (Applied Biosystems). Primer names, sequences, and GenBank accession numbers are listed in Table 2. QRT-PCR was achieved using StepOne-Plus Real-Time PCR (Applied Biosystems) with a Fast-Start Universal SYBR Green Master Mix (Roche, Indianapolis, IN), according to the manufacturers' instructions. The amplification steps consisted of initiation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 sec each followed by annealing at 60°C for 1 min, with next a melting curve analysis step featuring 1 cycle at 95°C for 15 sec, a hold at 60°C for 1 min, and a further hold at 95°C for 15 sec, to confirm that only a single amplicon was present. All reactions were performed in duplicate using olive flounder β-actin mRNA as an internal control. The relative standard curve method was applied to calculate standard errors of the mean (SEM). In statistical analysis, Student's t-test was used to determine differences between tests using FKC and OMV; p<0.05 was viewed as significant.
Table 2. Oligonucleotide sequences used in the present study.
| Gene | Accession number | Primer name | Sequences (5′→3′) |
| β-actin | AU050773 | WCUP090-F | CTGCCTTCACCTCCAAGAAG |
| WCUP091-R | CTCCATGTCATCCCAGTTGGT | ||
| IL-1β | AB070835 | WCUP279-F | ATGGAATCCAAGATGGAATGC |
| WCUP280-R | TTAACTCTGATGATGGATGTT | ||
| IL-6 | DQ884914 | WCUP281-F | CCTGCACACCTACATGGTTCT |
| WCUP282-R | TTGGGCATCTCTCTTTTACGA | ||
| TNFα | AB040448 | WCUP294-F | GTCCTGGCGTTTTCTTGGTA |
| WCUP295-R | CGTCCTCCTGACTCTTCTGG | ||
| IFNγ | AB435093 | WCUP284-F | TGCAAGGATGAACAAAACCA |
| WCUP058-R | AGAACTCGCCTCCTCGTACA | ||
| TLR22 | AB109394 | WCUP784-F | CTTGGCTTTGCTCTTTGACACA |
| WCUP785-R | GGGCAGCAGCTCTCTCACA | ||
| TLR2 | AB109393 | WCUP781-F | CCACCGTCAGCGTCATAGAGA |
| WCUP780-R | TTTTTCCCACCTGCCTTCAC |
Fish vaccination and challenge
Before vaccination, 10 naïve olive flounders in each group were intraperitoneally injected with ED45 (1.2×104–109 CFU/ml) and mortality was recorded over the next 28 days. A group of fish injected with 1.2×104 CFU/ml of ED45 showed 70% mortality, and this dose was thus chosen as the challenge (data not shown). Twenty fish were vaccinated with 0.1 ml amounts of FKC (1.2×108 CFU/ml; 344 µg) or 10 µg amounts of OMV, whereas fish injected with PBS served as controls. After acclimatization for 28 days, fish were challenged with 100 µl amounts of ED45 (1.1×104 CFU/ml) by intraperitoneal injection, and mortality was recorded over the following 31 days. Vaccine efficacy was calculated as relative percentage survival (RPS = [1 minus vaccine group mortality/control group mortality]×100) [55]. In statistical analysis, Fisher's exact test was applied to compare survival differences between vaccinated and control groups at the p<0.0001 level.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a grant (no. R32-10253) from the World Class University Program funded by the Ministry of Education, Science and Technology, of South Korea. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 2.Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol. 1999;181:4725–4733. doi: 10.1128/jb.181.16.4725-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devoe IW, Gilchrist JE. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973;138:1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoekstra D, van der Laan JW, de Leij L, Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976;455:889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- 5.Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, et al. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. uptake of released toxin and vesicles by gastric epithelium. J Pathol. 1999;188:220–226. doi: 10.1002/(SICI)1096-9896(199906)188:2<220::AID-PATH307>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Kadurugamuwa JL, Beveridge TJ. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology. 1999;145:2051–2060. doi: 10.1099/13500872-145-8-2051. [DOI] [PubMed] [Google Scholar]
- 7.Kondo K, Takade A, Amako K. Release of the outer membrane vesicles from Vibrio cholerae and Vibrio parahaemolyticus. Microbiol Immunol. 1993;37:149–152. doi: 10.1111/j.1348-0421.1993.tb03192.x. [DOI] [PubMed] [Google Scholar]
- 8.Nevot M, Deroncelé V, Messner P, Guinea J, Mercadé E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ Microbiol. 2006;8:1523–1533. doi: 10.1111/j.1462-2920.2006.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/s0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- 10.Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microb Infect. 2006;8:2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer FP, Bullock GL. Edwardsiella tarda, a new pathogen of channel catfish (Ictalurus punctatus). Appl Microbiol. 1973;25:155–156. doi: 10.1128/am.25.1.155-156.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egusa S. Some bacterial diseases of freshwater fishes in Japan. Fish Pathol. 1976;10:103–114. [Google Scholar]
- 13.Nakatsugawa T. Edwardsiella tarda isolated from cultured young flounder. Fish Pathol. 1983;18:99–101. [Google Scholar]
- 14.Kusuda R, Toyoshima T, Iwamura Y, Sako H. Edwardsiella tarda from an epizootic of mullets (Mugil cephalus) in okitsu bay. Bull Jap Soc Sci Fish. 1976;42:271–275. [Google Scholar]
- 15.Nougayrede PH, Vuillaume A, Vigneulle M, Faivre B, Luengo S, et al. First isolation of Edwardsiella tarda from diseased turbot (Scophthalmus maximus) reared in a sea farm in the bay of biscay. EAFP Bulletin. 1994;14:128–129. [Google Scholar]
- 16.Herman RL, Bullock GL. Pathology caused by the bacterium Edwardsiella tarda in striped bass. Trans Am Fish Soc1. 1986;15:232–235. [Google Scholar]
- 17.Mohanty BR, Sahoo PK. Edwardsiellosis in fish: A brief review. J Biosci. 2007;32:1331–1344. doi: 10.1007/s12038-007-0143-8. [DOI] [PubMed] [Google Scholar]
- 18.Castro N, Toranzo AE, Núņez S, Magariņos B. Development of an effective Edwardsiella tarda vaccine for cultured turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2008;25:208–212. doi: 10.1016/j.fsi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Kawai K, Liu Y, Ohnishi K, Oshima S. A conserved 37 kDa outer membrane protein of Edwardsiella tarda is an effective vaccine candidate. Vaccine. 2004;22:3411–3418. doi: 10.1016/j.vaccine.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Kwon SR, Nam YK, Kim SK, Kim KH. Protection of tilapia (Oreochromis mosambicus) from edwardsiellosis by vaccination with Edwardsiella tarda ghosts. Fish Shellfish Immunol. 2006;20:621–626. doi: 10.1016/j.fsi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Mo ZL, Xiao P, Li J, Zou YX, et al. EseD, a putative T3SS translocon component of Edwardsiella tarda, contributes to virulence in fish and is a candidate for vaccine development. Mar Biotechnol. 2010 doi: 10.1007/s10126-009-9255-5. doi: 10.1007/s10126-009-9255-5. [DOI] [PubMed] [Google Scholar]
- 22.Janda JM, Abbott SL. Expression of an iron-regulated hemolysin by Edwardsiella tarda. FEMS Microbiol Lett. 1993;111:275–280. doi: 10.1111/j.1574-6968.1993.tb06398.x. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasa Rao PS, Yamada Y, Leung KY. A major catalase (KatB) that is required for resistance to H2O2 and phagocyte-mediated killing in Edwardsiella tarda. Microbiology. 2003;149:2635–2644. doi: 10.1099/mic.0.26478-0. [DOI] [PubMed] [Google Scholar]
- 24.Ullah MA, Arai T. Pathological activities of the naturally occurring strains of Edwardsiella tarda. Fish Pathol. 1983;18:65–70. [Google Scholar]
- 25.Lee EY, Bang JY, Park GW, Choi DS, Kang JS, et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 2007;7:3143–3153. doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- 26.Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, et al. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J Biol Chem. 2005;280:38383–38394. doi: 10.1074/jbc.M508063200. [DOI] [PubMed] [Google Scholar]
- 27.Roberts R, Moreno G, Bottero D, Gaillard ME, Fingermann M, et al. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine. 2008;26:4639–4646. doi: 10.1016/j.vaccine.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009;77:472–484. doi: 10.1128/IAI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Dobbelsteen GPJM, van Dijken HH, Pillai S, van Alphen L. Immunogenicity of a combination vaccine containing pneumococcal conjugates and meningococcal PorA OMVs. Vaccine. 2007;25:2491–2496. doi: 10.1016/j.vaccine.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Holst J, Martin D, Arnold R, Huergo CC, Oster P, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27:B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 31.Hirono I, Takami M, Miyata M, Miyazaki T, Han HJ, et al. Characterization of gene structure and expression of two toll-like receptors from Japanese flounder, Paralichthys olivaceus. Immunogenetics. 2004;56:38–46. doi: 10.1007/s00251-004-0657-2. [DOI] [PubMed] [Google Scholar]
- 32.Wu CC, Yates JR. The application of mass spectrometry to membrane proteomics. Nat Biotechnol. 2003;21:262–267. doi: 10.1038/nbt0303-262. [DOI] [PubMed] [Google Scholar]
- 33.Kumar G, Sharma P, Rathore G, Bisht D, Sengupta U. Proteomic analysis of outer membrane proteins of Edwardsiella tarda. J Appl Microbiol. 2010;108:2214–2221. doi: 10.1111/j.1365-2672.2009.04627.x. [DOI] [PubMed] [Google Scholar]
- 34.Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000;275:12489–12496. doi: 10.1074/jbc.275.17.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galka F, Wai SN, Kusch H, Engelmann S, Hecker M, et al. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect Immun. 2008;76:1825–1836. doi: 10.1128/IAI.01396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss EJ, Ghori N, Falkow S. An Edwardsiella tarda strain containing a mutation in a gene with homology to shlB and hpmB is defective for entry into epithelial cells in culture. Infect Immun. 1997;65:3924–3932. doi: 10.1128/iai.65.9.3924-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasadarao NV, Wass CA, Kim KS. Endothelial cell GlcNAc beta 1-4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasadarao NV, Wass CA, Weiser JN, Stins MF, Huang SH, et al. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolton A, Song XM, Willson P, Fontaine MC, Potter AA, et al. Use of the surface proteins GapC and mig of Streptococcus dysgalactiae as potential protective antigens against bovine mastitis. Can J Microbiol. 2004;50:423–432. doi: 10.1139/w04-016. [DOI] [PubMed] [Google Scholar]
- 40.Rao PSS, Yamada Y, Tan YP, Leung KY. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol Microbiol. 2004;53:573–586. doi: 10.1111/j.1365-2958.2004.04123.x. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol. 2007;66:1192–1206. doi: 10.1111/j.1365-2958.2007.05993.x. [DOI] [PubMed] [Google Scholar]
- 42.Whyte SK. The innate immune response of finfish-A review of current knowledge. Fish Shellfish Immunol. 2007;23:1127–1151. doi: 10.1016/j.fsi.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Alaniz RC, Deatherage BL, Lara JC, Cookson BT. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 45.Jendholm J, Mörgelin M, Månsson A, Larsson C, Cardell LO, et al. B cell activation by outer membrane Vesicles—A novel virulence mechanism. PLoS Pathog. 6:e1000724. doi: 10.1371/journal.ppat.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mekuchi T, Kiyokawa T, Honda K, Nakai T, Muroga K. Vaccination trials in the Japanese flounder against edwardsiellosis. Fish Pathol. 1995;30:251–256. [Google Scholar]
- 47.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem. 2004;279:2069–2076. doi: 10.1074/jbc.M307628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 50.Shin GW, Palaksha KJ, Yang HH, Shin YS, Kim YR, et al. Partial two-dimensional gel electrophoresis (2-DE) maps of Streptococcus iniae ATCC29178 and Lactococcus garvieae KG9408. Dis Aquat Organ. 2006;70:71–79. doi: 10.3354/dao070071. [DOI] [PubMed] [Google Scholar]
- 51.Tatusov RL, Natale DA, Garkavtsev IV, Tatusova TA, Shankavaram UT, et al. The COG database: New developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001;29:22–28. doi: 10.1093/nar/29.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.González S, Caballero E, Soria Y, Cobas K, Granadillo M, et al. Immunization with Neisseria meningitidis outer membrane vesicles prevents bacteremia in neonatal mice. Vaccine. 2006;24:1633–1643. doi: 10.1016/j.vaccine.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 54.Aoki M, Kondo M, Nakatsuka Y, Kawai K, Oshima SI. Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine. 2007;25:561–569. doi: 10.1016/j.vaccine.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 55.Amend DF. Potency testing of fish vaccines. Dev Biol Stand. 1981;49:447–454. [Google Scholar]