Abstract
Background
Aedes aegypti is the main vector of the virus causing Dengue fever, a disease that has increased dramatically in importance in recent decades, affecting many tropical and sub-tropical areas of the globe. It is known that viruses and other parasites can potentially alter vector behavior. We investigated whether infection with Dengue virus modifies the behavior of Aedes aegypti females with respect to their activity level.
Methods/Principal Findings
We carried out intrathoracic Dengue 2 virus (DENV-2) infections in Aedes aegypti females and recorded their locomotor activity behavior. We observed an increase of up to ∼50% in the activity of infected mosquitoes compared to the uninfected controls.
Conclusions
Dengue infection alters mosquito locomotor activity behavior. We speculate that the higher levels of activity observed in infected Aedes aegypti females might involve the circadian clock. Further studies are needed to assess whether this behavioral change could have implications for the dynamics of Dengue virus transmission.
Introduction
Over the last decades, Dengue outbreaks have been a major public health concern in many parts of the World, where Dengue epidemics have been registered with a significant number of deaths [1]. There are four antigenically distinct RNA viruses that can cause the disease, and in Brazil, three Dengue serotypes (DENV-1; DENV-2 and DENV-3) have co-circulated in several areas and caused some severe Dengue epidemics [2].
Aedes aegypti (Diptera: Culicidae) is the urban vector of Yellow Fever and Dengue viruses. This diurnal mosquito is very anthropophilic and endophilic, being commonly found in urban and suburban areas, especially where house and human densities are high and where it seems to live longer and disperse to short distances (e.g. [3]–[5]).
It is known that parasites can alter vector behavior (reviewed by [6]–[8]) and a number of studies have reported behavioral changes in Ae. aegypti infected with pathogens and symbionts. For example, Rossignol et al [9] observed that Ae. aegypti females experimentally infected with an avian malaria parasite, Plasmodium gallinaceum, take more time to locate blood in guinea pigs than the uninfected ones. Rowland and Lindsay [10] studied the flight activity of females of this species infected with the filarial parasite Brugia pahangi and observed a decrease in flight capacity in heavily-infected mosquitoes under laboratory conditions. Recently, it has been shown that the infection by the symbiotic bacterium Wolbachia can also drastically alter this mosquito's behavior and physiology [11]–[14].
In the current study, we investigated whether infection with Dengue virus causes changes in the locomotor activity behavior of Ae. aegypti females under laboratory conditions.
Methods
Infection of Mosquitoes with the Dengue virus
The Paea strain of Ae. aegypti was used in all infection experiments. This laboratory strain is known to be highly susceptible to Dengue virus serotype 2 (DENV-2) infection [15]. Mosquito colony was reared according to procedures described in [16]. Males and females remained together and were fed with 15% sucrose solution since emergence.
Five-day-old female mosquitoes were individually infected by intrathoracic inoculation with 0.21 µl of L-15 (Leibovitz) Medium containing Dengue virus (DENV-2 strain FIOCRUZ-66985 [17]) in a concentration of 107 PFU using a Nanoject microinjector (Drummond Scientific). Control mosquitoes were intrathoracically inoculated with 0.21 µl of only L-15 (Leibovitz) Medium.
To verify the success of the experimental infections, the heads of a number of mosquitoes that were inoculated with virus and that were alive by the end of the locomotor activity experiments (around 8–10 days after inoculation), plus negative controls, were tested for Dengue infection via RT-PCR, as described below. The results indicated that over 95% (70/73) of the mosquitoes inoculated with the Dengue virus were infected (data not shown).
Detection of Dengue virus in mosquitoes
Mosquito heads were separated from bodies on a metal plate placed on dry ice and viral RNA was extracted from each head using the QIAmp Viral Mini Kit (Qiagen) according to the manufacturer's protocol. RT-PCR for detecting DENV2 was conducted in a PCR System 9700 GeneAmp (Applied Biosystems) using SuperScript™ One-Step RT-PCR with Platinum® Taq (Invitrogen) and Dengue virus consensus primers D1 and D2 [18], followed by a semi-nested PCR on the resulting product using Go Taq Green Master Mix (Promega) and primers D1 and TS2 [18]. PCR products were electrophoresed on 2.5% agarose gels. A band of 119 pb corresponding to DENV-2 could be seen under UV light in the infected mosquitoes.
Analysis of the locomotor activity behavior
The activity of infected and uninfected control Ae. aegypti females was recorded using a larger version of the Drosophila Activity Monitor (TriKinetics) as described in [19]. After inoculation with Dengue virus or L-15 medium, females were individually placed in glass tubes (1 cm×7 cm) with a cotton plug soaked in 15% sucrose solution and these tubes placed in the Activity Monitor inside a Precision Scientific Incubator Model 818 under a constant temperature of 25°C and a photoperiod of 12 hours of light and 12 hours of dark (LD 12∶12). For each mosquito, the total locomotor activity of 1 hour-intervals was recorded for about seven days after inoculation. The data of individuals that died during the experiments were excluded, and the data analysis was carried out comparing the activity data of infected and uninfected mosquitoes from the second to sixth day after inoculation.
Results
Table 1 shows the mean hourly locomotor activity of control and Dengue virus infected females in four different experiments. We observed that infected females of Ae. aegypti showed more activity than controls in all experiments. The relative increase in activity ranged from ∼10% to more than 50%. A two-way ANOVA indicated a significant difference in the activity between infected and uninfected control females (p<0.01). Although a significant difference in the overall activity levels was also observed between experiments (p<0.01), the interaction between experiments and infection was not significant (p = 0.82) indicating that the difference between infected and uninfected females was consistent.
Table 1. Activity increase in Dengue infected Aedes aegypti females.
| Experiment | Status | N | Mean activity per hour* (± SEM) | Relative increase of activity in infected mosquitoes (%) | |
| 1 | Control | 17 | 5.06±1.16(4.73±1.15) | 52.6 (48.0) | |
| Infected | 23 | 7.72±1.27(7.00±1.26) | |||
| 2 | Control | 53 | 11.22±1.45(10.52±1.49) | 30.8 (30.5) | |
| Infected | 74 | 14.68±1.50(13.73±1.49) | |||
| 3 | Control | 45 | 9.84±1.19(8.97±1.15) | 43.2 (45.3) | |
| Infected | 66 | 14.09±1.24(13.03±1.23) | (13.03±1.23) | ||
| 4 | Control | 70 | 11.88±1.01(10.95±1.00) | 13.3 (10.3) | |
| Infected | 83 | 13.46±1.13(12.39±1.06) |
*The numbers in parenthesis refer to the activity excluding the light-on/light-off transition.
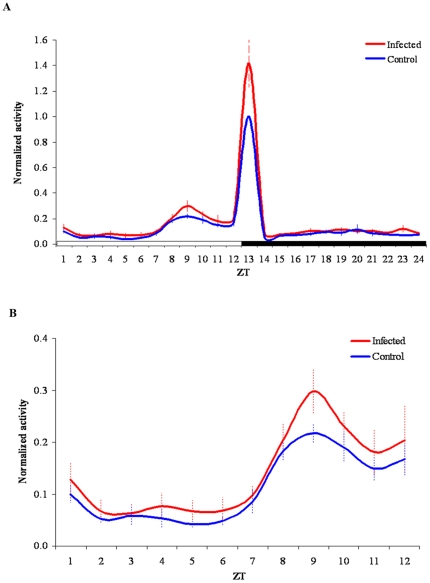
Figure 1 shows the normalized locomotor activity patterns of infected and control females during a full LD 12∶12 cycle (Fig. 1A) or during the photophase (Fig. 1B). As previously reported in the literature (reviewed in [20]), Ae. aegypti is a diurnal species showing higher activity levels towards the end of the photophase (“late afternoon”) and a characteristic startle response to the abrupt light-on/light-off transition [21]. The comparison of the normalized locomotor activity patterns of infected and uninfected females shows that Dengue infection causes an increase in activity throughout the 24 hour period. Although this effect is most dramatic during the light-on/light-off transition (Fig. 1A), an increase in activity is also seen throughout the day and night in infected females, especially during the “natural” activity peak occurring around ZT 9 and in the last hours of the photophase (Fig. 1B). In fact, this increase in activity associated with Dengue infection is still significant (p = 0.012) even when we exclude the light-on/light-off transition (Table 1). In summary, our results indicate that the locomotor activity of infected females is consistently increased when compared to that of uninfected females.
Figure 1. Locomotor activity of control (blue line) and infected (red line) Ae. aegypti females under LD 12∶12.
Lines represent the hourly mean activity (+/− SEM) of control and infected females in the four experiments, normalized to the peak of activity of each respective control. The grey shadow represents the dark phase. ZT is the Zeitgeber Time. Light turns on at ZT 0 and turns off at ZT 12. Panel (A) shows a full LD cycle while panel (B) shows only the photophase.
Discussion
Very little is known about the effects of viral infection on Aedes mosquitoes. Several authors have shown that Dengue virus exhibits a remarkable tropism for the mosquito nervous tissues. Linthicum et al [22] studied the tropism of DENV-3 in parenterally infected female Aedes aegypti mosquitoes using immunocytochemical methods and observed that the nervous tissues were among the first tissues to be infected. In fact, these authors suggested that the nervous system is the primary site of virus amplification in mosquitoes infected using this method [22]. Several years later, Salazar et al [23] corroborated these findings by showing that in mosquitoes orally infected with DENV-2, the nervous tissues are among the first to be infected, presenting detectable levels of viral antigens 5 days after an infective blood meal. Interestingly, these authors also showed that heads and salivary glands were the only tissues where viral antigens continued to accumulate throughout the 21 days observed in their study. All other mosquito infected tissues presented a decrease in the infection rate.
This remarkable tropism of Dengue virus for the insect nervous tissues led us to hypothesize that the infection might have some role in modulating the vector locomotor activity behavior, since it is known that activity rhythms in Drosophila and other Diptera are regulated by circadian clock neurons in the brain (reviewed in [24],[25]). In fact, our results show that although the daily activity patterns of DENV-2 infected and uninfected mosquitoes are similar, the total level of activity is clearly increased upon infection. This increase is most evident in the light-on/light-off transition (Fig. 1A), an observation that is particularly interesting considering that the visual system is also highly infected [22],[23]. However, it is important to mention that this effect is also clearly detected in the “natural” activity peak occurring during the last hours of the photophase (Fig. 1B), which is under circadian control [20],[21], indicating that a similar effect is likely to occur in nature.
Other authors have already observed alterations in Aedes behavior induced by virus infection. Grimstad et al [26] studied the feeding behavior of Ae. triseriatus females infected with La Crosse virus and reported that infected mosquitoes tend to probe more and engorge less than uninfected females. These results are in accordance with those obtained by Platt et al [27], who showed that the time required for feeding by DENV-3 infected mosquitoes was significantly longer than that required by uninfected mosquitoes. In contrast, Putnam and Scott [28] observed that DENV-2 infection did not alter Ae. aegypti female blood-feeding duration and efficiency in an uninfected host. An explanation for this difference might be that these authors infected mosquitoes with different Dengue virus (3 and 2, respectively) and that Putnam and Scott [28] fed mosquitoes 14 days after an intrathoracic infection while Platt et al [27] only observed significant differences in mosquitoes fed 5, 8 and 11 days after infection. In our study, we observed locomotor activity differences in DENV-2 infected mosquitoes 2 to 6 days after intrathoracic infection.
A considerable amount of information is currently available on the Aedes aegypti immune response to Dengue virus infection [29]–[31]. Several authors have shown an association between circadian rhythms and infection/immunity in insects (e.g. [32]–[34]). For example, Shirasu-Hiza et al [32] showed that Drosophila infected with bacterium exhibit disrupted circadian activity rhythms and that clock gene mutants are more susceptible to infection than wild-type flies. Also, Lee and Edery [33] showed that Drosophila's ability to fight infections is under circadian control and that flies are significantly more resistant to bacterium when infected in the middle of the night than during the day.
It has been shown that several genes from Aedes aegypti are up or down-regulated upon Dengue virus infection, and in DENV-2 infected mosquitoes at least one orthologue (AAEL012562) of a Drosophila gene involved in the control of circadian rhythms, Clock, has its expression nearly doubled after infection [29]. We believe this variation in a gene probably central to the control of mosquito circadian rhythms could also contribute to the observed changes in activity behavior and we are currently investigating whether Dengue virus infection alters the circadian expression patterns of other clock genes [21].
We are aware that our study suffers from possible caveats. For example, we see a large variation in behavioral effects of Dengue infection between experiments that we cannot explain at the moment. Nevertheless, our study shows that Dengue infection increases mosquito locomotor activity. Changes in vector behavior caused by infection can have potential epidemiological implications. Our results encourage further studies to assess whether increased locomotor activity could have an impact on virus transmission dynamics and Dengue epidemiology.
Acknowledgments
We thank Marcelo Quintela and Kathleen Gonçalves for technical assistance and Luciano Moreira, Pedro Oliveira and Denise Valle for reading earlier versions of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the Howard Hughes Medical Institute, CNPq, Faperj, and FIOCRUZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RM, de Araújo JM, Schatzmayr HG. Dengue viruses in Brazil, 1986–2006. Panam Salud Publica. 2007;22:358–363. doi: 10.1590/s1020-49892007001000009. [DOI] [PubMed] [Google Scholar]
- 3.Braks MAH, Honório NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- 4.Lima-Camara TN, Honório NA, Lourenço-de-Oliveira R. Freqüência e distribuição espacial de Aedes aegypti e Aedes albopictus (Diptera:Culicidae) no Rio de Janeiro, Brasil. Cad Saúde Públ. 2006;22:2079–2084. doi: 10.1590/s0102-311x2006001000013. [DOI] [PubMed] [Google Scholar]
- 5.David MR, Lourenço-de-Oliveira R, Maciel-de-Freitas R. Container productivity, daily survival and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Mem Inst Oswaldo Cruz. 2009;104:927–932. doi: 10.1590/s0074-02762009000600019. [DOI] [PubMed] [Google Scholar]
- 6.Moore J. Parasites and the behavior of biting flies. J Parasitol. 1993;79:1–16. [PubMed] [Google Scholar]
- 7.Hurd H. Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol. 2003;48:141–161. doi: 10.1146/annurev.ento.48.091801.112722. [DOI] [PubMed] [Google Scholar]
- 8.Schaub GA. Parasitogenic alterations of vector behaviour. Int J Med Microbiol. 2006;296(Suppl 40):37–40. doi: 10.1016/j.ijmm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Rossignol PA, Ribeiro JM, Spielman A. Increased intradermal probing time in sporozoite infected mosquitoes. Am J Trop Med Hyg. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 10.Rowland MW, Lindsay SW. The circadian flight activity of Aedes aegypti parasitized with the filarial nematode Brugia pahangi. Physiol Entomol. 1986;11:325–334. [Google Scholar]
- 11.Evans O, Caragata EP, McMeniman CJ, Woolfit M, Green DC, et al. Increased locomotor activity and metabolism of Aedes aegypti infected with a life-shortening strain of Wolbachia pipientis. J Exp Biol. 2009;212(Pt 10):1436–1441. doi: 10.1242/jeb.028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira LA, Saig E, Turley AP, Ribeiro JM, O'Neill SL, et al. Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl Trop Dis. 2009a;3:e568. doi: 10.1371/journal.pntd.0000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, et al. Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009b;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Turley AP, Moreira LA, O'Neill SL, McGraw EA. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl Trop Dis. 2009;3:e516. doi: 10.1371/journal.pntd.0000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vazeille-Falcoz M, Mousson L, Rodhain F, Chungue E, Failloux AB. Variation in oral susceptibility to dengue type 2 virus of populations of Aedes aegypti from the islands of Tahiti and Moorea, French Polynesia. Am J Trop Med Hyg. 1999;60:292–299. doi: 10.4269/ajtmh.1999.60.292. [DOI] [PubMed] [Google Scholar]
- 16.Lourenço-de-Oliveira R, Consoli RAGB. Principais mosquitos de importância sanitária no Brasil. 1994. 225 Ed. Fiocruz, Rio de Janeiro.
- 17.Miagostovich MP, Sequeira PC, Dos Santos FB, Maia A, Nogueira RM, et al. Molecular typing of dengue virus type 3 in Brazil. Rev Inst Med Trop Sao Paulo. 2003;45:17–21. doi: 10.1590/s0036-46652003000100004. [DOI] [PubMed] [Google Scholar]
- 18.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentile C, Meireles-Filho AC, Britto C, Lima JB, Valle D, et al. Cloning and daily expression of the timeless gene in Aedes aegypti (Diptera:Culicidae). Insect Biochem Mol Biol. 2006;36:878–884. doi: 10.1016/j.ibmb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Clements AN. The biology of mosquitoes. V 2. Sensory reception and behavior. UK: CABI publishing; 1999. 756 [Google Scholar]
- 21.Gentile C, Rivas GB, Meireles-Filho AC, Lima JB, Peixoto AA. Circadian expression of clock genes in two mosquito disease vectors: cry2 is different. J Biol Rhythms. 2009;24:444–451. doi: 10.1177/0748730409349169. [DOI] [PubMed] [Google Scholar]
- 22.Linthicum KJ, Platt K, Myint KS, Lerdthusnee K, Innis BL, et al. Dengue 3 virus distribution in the mosquito Aedes aegypti: an immunocytochemical study. Med Vet Entomol. 1996;10:87–92. doi: 10.1111/j.1365-2915.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 23.Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders DS. Insect Clocks. Amsterdam: Elsevier Science; 2002. 280 [Google Scholar]
- 25.Hall JC. Genetics and molecular biology of rhythms in Drosophila and other insects. Adv Genet. 2003;48:1–280. doi: 10.1016/s0065-2660(03)48000-0. [DOI] [PubMed] [Google Scholar]
- 26.Grimstad PR, Ross QE, Craig GB., Jr Aedes triseriatus (Diptera:Culicidae) and La Crosse virus II: Modification of mosquito feeding behavior by virus infection. J Med Entomol. 1980;17:1–7. doi: 10.1093/jmedent/17.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Platt KB, Linthicum KJ, Myint KS, Innis BL, Lerdthusnee K, et al. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- 28.Putnam JL, Scott TW. Blood-feeding behavior of dengue-2 virus-infected Aedes aegypti. Am J Trop Med Hyg. 1995;52:225–227. doi: 10.4269/ajtmh.1995.52.225. [DOI] [PubMed] [Google Scholar]
- 29.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sim S, Dimopoulos G. Dengue virus inhibits immune responses in Aedes aegypti cells. PLoS One. 2010;5:e10678. doi: 10.1371/journal.pone.0010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirasu-Hiza MM, Dionne MS, Pham LN, Ayres JS, Schneider DS. Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr Biol. 2007;17:R353–R355. doi: 10.1016/j.cub.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 33.Lee JE, Edery I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol. 2008;18:195–199. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo TH, Pike DH, Beizaeipour Z, Williams JA. Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFkappaB Relish. BMC Neurosci. 2010;11:17. doi: 10.1186/1471-2202-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]