Abstract
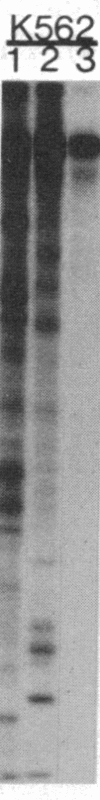
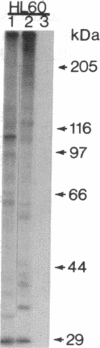
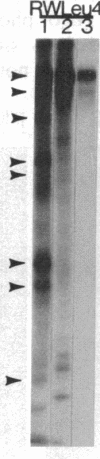
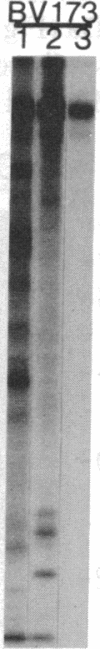
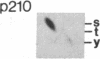
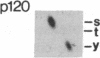
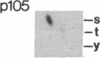
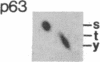
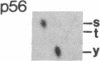
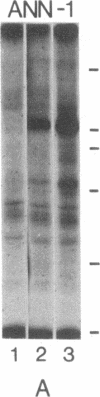
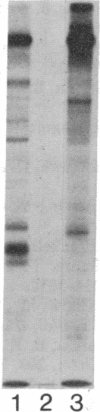
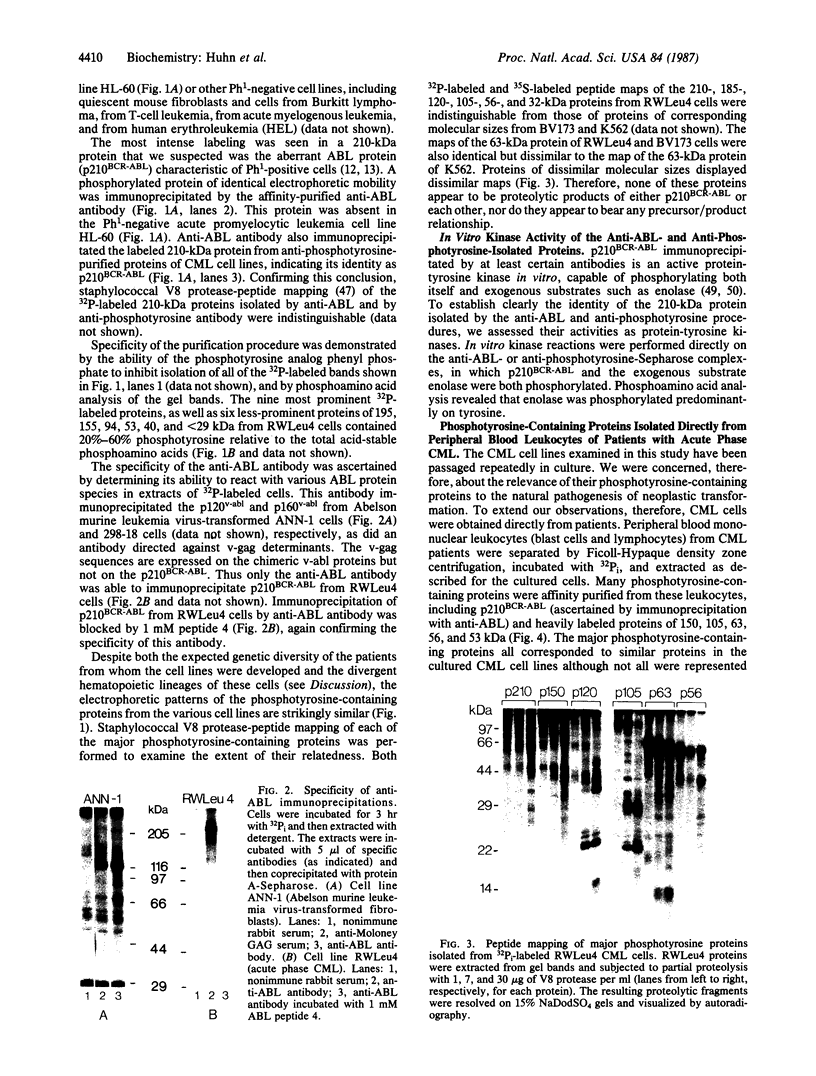
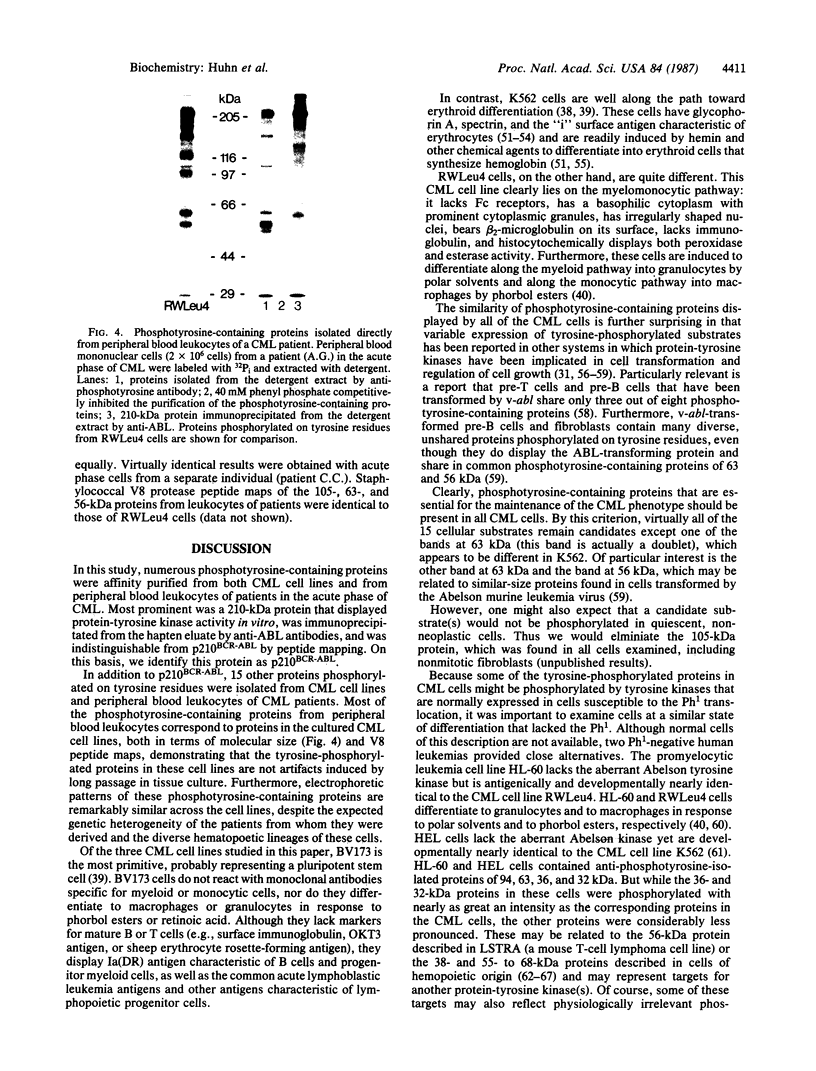
An aberrant p210BCR-ABL protein that possesses constitutive protein-tyrosine kinase activity is presumed to be involved in the development of the neoplastic phenotype in chronic myelogenous leukemia (CML). Using a highly specific antibody against phosphotyrosine, we have isolated the tyrosine-phosphorylated p210BCR-ABL and several other proteins containing phosphotyrosine from a variety of CML cell lines. p210BCR-ABL isolated by the monoclonal anti-phosphotyrosine antibody possessed protein-tyrosine kinase activity in vitro comparable to that of the p210BCR-ABL isolated by antibody to a specific peptide sequence in the ABL protein-tyrosine kinase. Other prominent proteins containing phosphorylated tyrosine residues were observed at 185, 150, 120, 105, 63, 56, 36, and 32 kDa, and less prominent proteins were observed at 195, 155, 94, 53, 40, and less than 29 kDa. Staphylococcal V8 peptide mapping indicated that proteins of similar molecular weights were highly homologous to each other across cell lines, despite the diverse hematopoietic lineages of these cells and the genetic heterogeneity of the patients from whom the CML cell lines were derived. Phosphopeptide mapping also revealed that these proteins were distinct from each other as well as from p210BCR-ABL. Because virtually identical phosphotyrosine-containing proteins were found in peripheral blood leukocytes taken directly from CML patients, these proteins are not an artifact of long-term tissue culture but appear to be an integral part of the CML phenotype.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Jokinen M., Gahmberg C. G. Induction of erythroid differentiation in the human leukaemia cell line K562. Nature. 1979 Mar 22;278(5702):364–365. doi: 10.1038/278364a0. [DOI] [PubMed] [Google Scholar]
- Andersson L. C., Nilsson K., Gahmberg C. G. K562--a human erythroleukemic cell line. Int J Cancer. 1979 Feb;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- Antler A. M., Greenberg M. E., Edelman G. M., Hanafusa H. Increased phosphorylation of tyrosine in vinculin does not occur upon transformation by some avian sarcoma viruses. Mol Cell Biol. 1985 Jan;5(1):263–267. doi: 10.1128/mcb.5.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram C. R., de Klein A., Hagemeijer A., van Agthoven T., Geurts van Kessel A., Bootsma D., Grosveld G., Ferguson-Smith M. A., Davies T., Stone M. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- Benz E. J., Jr, Murnane M. J., Tonkonow B. L., Berman B. W., Mazur E. M., Cavallesco C., Jenko T., Snyder E. L., Forget B. G., Hoffman R. Embryonic-fetal erythroid characteristics of a human leukemic cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3509–3513. doi: 10.1073/pnas.77.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Gale R. P., Steiner-Saltz D., Berrebi A., Aghai E., Januszewicz E. Altered transcription of an oncogene in chronic myeloid leukaemia. Lancet. 1984 Mar 17;1(8377):593–595. doi: 10.1016/s0140-6736(84)90997-8. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champlin R. E., Golde D. W. Chronic myelogenous leukemia: recent advances. Blood. 1985 May;65(5):1039–1047. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Kubonishi I., Miyoshi I., Groudine M. T. Altered transcription of the c-abl oncogene in K-562 and other chronic myelogenous leukemia cells. Science. 1984 Jul 6;225(4657):72–74. doi: 10.1126/science.6587568. [DOI] [PubMed] [Google Scholar]
- Comoglio P. M., Di Renzo M. F., Tarone G., Giancotti F. G., Naldini L., Marchisio P. C. Detection of phosphotyrosine-containing proteins in the detergent-insoluble fraction of RSV-transformed fibroblasts by azobenzene phosphonate antibodies. EMBO J. 1984 Mar;3(3):483–489. doi: 10.1002/j.1460-2075.1984.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp H. S., Austin K. S., Buessow S. C., Dy R., Gillespie G. Y. Membranes from T and B lymphocytes have different patterns of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2347–2351. doi: 10.1073/pnas.81.8.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Ferrero D., Pessano S., Pagliardi G. L., Rovera G. Induction of differentiation of human myeloid leukemias: surface changes probed with monoclonal antibodies. Blood. 1983 Jan;61(1):171–179. [PubMed] [Google Scholar]
- Foulkes J. G., Chow M., Gorka C., Frackelton A. R., Jr, Baltimore D. Purification and characterization of a protein-tyrosine kinase encoded by the Abelson murine leukemia virus. J Biol Chem. 1985 Jul 5;260(13):8070–8077. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Friedman B., Frackelton A. R., Jr, Ross A. H., Connors J. M., Fujiki H., Sugimura T., Rosner M. R. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 May;81(10):3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Witte O. N., Gilboa E., Rosenberg N., Baltimore D. Genome structure of Abelson murine leukemia virus variants: proviruses in fibroblasts and lymphoid cells. J Virol. 1981 May;38(2):460–468. doi: 10.1128/jvi.38.2.460-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Mathey-Prevot B., Feldman R. A., Hanafusa H. Mutants of Fujinami sarcoma virus which are temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1981 Apr;38(1):347–355. doi: 10.1128/jvi.38.1.347-355.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. L., Low P. S., Geahlen R. L. T and B lymphocytes express distinct tyrosine protein kinases. J Biol Chem. 1984 Aug 10;259(15):9348–9350. [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen M., Gahmberg C. G., Andersson L. C. Biosynthesis of the major human red cell sialoglycoprotein, glycophorin A, in a continuous cell line. Nature. 1979 Jun 14;279(5714):604–607. doi: 10.1038/279604a0. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Singer J. W., Collins S. J., Witte O. N. Cell lines and clinical isolates derived from Ph1-positive chronic myelogenous leukemia patients express c-abl proteins with a common structural alteration. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1810–1814. doi: 10.1073/pnas.82.6.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Detection of c-abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol. 1985 Nov;5(11):3116–3123. doi: 10.1128/mcb.5.11.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratsune H., Owada K., Machii T., Nishimori Y., Ueda E., Tokumine Y., Tagawa S., Taniguchi N., Fujio H., Kitani T. Association of specific tyrosine phosphorylation with stages of B-cell differentiation in human lymphoid leukemias. Biochem Biophys Res Commun. 1985 Dec 17;133(2):598–607. doi: 10.1016/0006-291x(85)90947-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C., HUNGERFORD D. A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960 Jul;25:85–109. [PubMed] [Google Scholar]
- Naldini L., Stacchini A., Cirillo D. M., Aglietta M., Gavosto F., Comoglio P. M. Phosphotyrosine antibodies identify the p210c-abl tyrosine kinase and proteins phosphorylated on tyrosine in human chronic myelogenous leukemia cells. Mol Cell Biol. 1986 May;6(5):1803–1811. doi: 10.1128/mcb.6.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. E., Landreth G. E., Goldschmidt-Clermont P. J., Tung H. E., Galbraith R. M. Enhanced tyrosine phosphorylation in B lymphocytes upon complexing of membrane immunoglobulin. Biochem Biophys Res Commun. 1984 Dec 28;125(3):859–866. doi: 10.1016/0006-291x(84)91362-7. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Huang J. S., Deuel T. F. Platelet-derived growth factor stimulates tyrosine-specific protein kinase activity in Swiss mouse 3T3 cell membranes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4303–4307. doi: 10.1073/pnas.79.14.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R., Ohtsuka M., Watanabe Y. Complement-mediated lysis of K562 human leukemic cells by antibodies to phosphotyrosine and identification of cell surface proteins phosphorylated on tyrosine. Cancer Res. 1986 May;46(5):2507–2510. [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A., White M. F., Kahn C. R. Predominance of tyrosine phosphorylation of insulin receptors during the initial response of intact cells to insulin. J Biol Chem. 1985 Jun 10;260(11):7131–7136. [PubMed] [Google Scholar]
- Pegoraro L., Matera L., Ritz J., Levis A., Palumbo A., Biagini G. Establishment of a Ph1-positive human cell line (BV173). J Natl Cancer Inst. 1983 Mar;70(3):447–453. [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Baltimore D. The minimum transforming region of v-abl is the segment encoding protein-tyrosine kinase. J Virol. 1985 Apr;54(1):114–122. doi: 10.1128/jvi.54.1.114-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. H., Baltimore D., Eisen H. N. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981 Dec 17;294(5842):654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Saggioro D., Ferracini R., Di Renzo M. F., Naldini L., Chieco-Bianchi L., Comoglio P. M. Protein phosphorylation at tyrosine residues in v-abl transformed mouse lymphocytes and fibroblasts. Int J Cancer. 1986 Apr 15;37(4):623–628. doi: 10.1002/ijc.2910370424. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Cooper J. A. Some lymphoid cell lines transformed by Abelson murine leukemia virus lack a major 36,000-dalton tyrosine protein kinase substrate. Mol Cell Biol. 1983 Jan;3(1):56–63. doi: 10.1128/mcb.3.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., McGrath J. P., Levinson A. D. A mutation at the ATP-binding site of pp60v-src abolishes kinase activity, transformation, and tumorigenicity. Mol Cell Biol. 1985 Jul;5(7):1772–1779. doi: 10.1128/mcb.5.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam K., Heisterkamp N., Grosveld G., de Klein A., Verma R. S., Coleman M., Dosik H., Groffen J. Evidence of a new chimeric bcr/c-abl mRNA in patients with chronic myelocytic leukemia and the Philadelphia chromosome. N Engl J Med. 1985 Dec 5;313(23):1429–1433. doi: 10.1056/NEJM198512053132301. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Wickremasinghe R. G., Piga A., Mire A. R., Taheri M. R., Yaxley J. C., Hoffbrand A. V. Tyrosine protein kinases and their substrates in human leukemia cells. Leuk Res. 1985;9(12):1443–1450. doi: 10.1016/0145-2126(85)90034-7. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]