Abstract
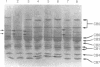
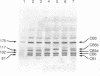
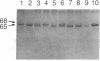
The structural changes in four genetic variants of human serum albumin were analyzed by tandem high-pressure liquid chromatography (HPLC) of the tryptic peptides, HPLC mapping and isoelectric focusing of the CNBr fragments, and amino acid sequence analysis of the purified peptides. Lysine-372 of normal (common) albumin A was changed to glutamic acid both in albumin Naskapi, a widespread polymorphic variant of North American Indians, and in albumin Mersin found in Eti Turks. The two variants also exhibited anomalous migration in NaDodSO4/PAGE, which is attributed to a conformational change. The identity of albumins Naskapi and Mersin may have originated through descent from a common mid-Asiatic founder of the two migrating ethnic groups, or it may represent identical but independent mutations of the albumin gene. In albumin Adana, from Eti Turks, the substitution site was not identified but was localized to the region from positions 447 through 548. The substitution of aspartic acid-550 by glycine was found in albumin Mexico-2 from four individuals of the Pima tribe. Although only single-point substitutions have been found in these and in certain other genetic variants of human albumin, five differences exist in the amino acid sequences inferred from cDNA sequences by workers in three other laboratories. However, our results on albumin A and on 14 different genetic variants accord with the amino acid sequence of albumin deduced from the genomic sequence. The apparent amino acid substitutions inferred from comparison of individual cDNA sequences probably reflect artifacts in cloning or in cDNA sequence analysis rather than polymorphism of the coding sections of the albumin gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. S., Martin J. R., Melartin L. Alloalbuminemia. Albumin Naskapi in Indians of the Ungava. JAMA. 1968 Jan 15;203(3):180–185. doi: 10.1001/jama.203.3.180. [DOI] [PubMed] [Google Scholar]
- Brennan S. O., Carrell R. W. A circulating variant of human proalbumin. Nature. 1978 Aug 31;274(5674):908–909. doi: 10.1038/274908a0. [DOI] [PubMed] [Google Scholar]
- Brennan S. O. The molecular abnormality of albumin Parklands: 365 Asp----His. Biochim Biophys Acta. 1985 Aug 23;830(3):320–324. doi: 10.1016/0167-4838(85)90289-4. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Law S. W., Dennison O. E. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Natl Acad Sci U S A. 1982 Jan;79(1):71–75. doi: 10.1073/pnas.79.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehske K. J., Müller W. E., Wollert U. The location of drug binding sites in human serum albumin. Biochem Pharmacol. 1981 Apr 1;30(7):687–692. doi: 10.1016/0006-2952(81)90151-9. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Wolf S. I., Ozdemir Y., Yüregir G. T., Isbir T., Blumberg B. S. Albumin Naskapi variant in North American Indians and Eti Turks. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5480–5482. doi: 10.1073/pnas.77.9.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin S. G., Wolf S. I., Zweidler A., Blumberg B. S. Localization of the amino acid substitution site in a new variant of human serum albumin, albumin Mexico-2. Proc Natl Acad Sci U S A. 1980 May;77(5):2505–2509. doi: 10.1073/pnas.77.5.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano M., Minchiotti L., Ferri G., Iadarola P., Zapponi M. C., Fine J. M. Structural characterization of the human albumin variant "Pollibauer". Rev Fr Transfus Immunohematol. 1984 Oct;27(5):597–602. doi: 10.1016/s0338-4535(84)80080-x. [DOI] [PubMed] [Google Scholar]
- Galliano M., Minchiotti L., Iadarola P., Zapponi M. C., Ferri G., Castellani A. A. Structural characterization of a chain termination mutant of human serum albumin. J Biol Chem. 1986 Mar 25;261(9):4283–4287. [PubMed] [Google Scholar]
- Hawkins J. W., Dugaiczyk A. The human serum albumin gene: structure of a unique locus. Gene. 1982 Jul-Aug;19(1):55–58. doi: 10.1016/0378-1119(82)90188-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Adelman J., Bock S. C., Franke A. E., Houck C. M., Najarian R. C., Seeburg P. H., Wion K. L. The sequence of human serum albumin cDNA and its expression in E. coli. Nucleic Acids Res. 1981 Nov 25;9(22):6103–6114. doi: 10.1093/nar/9.22.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloun B., Morávek L., Kostka V. Complete amino acid sequence of human serum albumin. FEBS Lett. 1975 Oct 15;58(1):134–137. doi: 10.1016/0014-5793(75)80242-0. [DOI] [PubMed] [Google Scholar]
- Minghetti P. P., Ruffner D. E., Kuang W. J., Dennison O. E., Hawkins J. W., Beattie W. G., Dugaiczyk A. Molecular structure of the human albumin gene is revealed by nucleotide sequence within q11-22 of chromosome 4. J Biol Chem. 1986 May 25;261(15):6747–6757. [PubMed] [Google Scholar]
- Murray J. C., Mills K. A., Demopulos C. M., Hornung S., Motulsky A. G. Linkage disequilibrium and evolutionary relationships of DNA variants (restriction enzyme fragment length polymorphisms) at the serum albumin locus. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3486–3490. doi: 10.1073/pnas.81.11.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Polesky H. F., Rokala D. A., Burch T. A. Serum albumin polymorphism in Indians of the southwestern United States. Nature. 1968 Oct 12;220(5163):175–176. doi: 10.1038/220175a0. [DOI] [PubMed] [Google Scholar]
- Schell L. M., Agarwal S. S., Blumberg B. S., Levy H., Bennett P. H., Laughlin W. S., Martin J. P. Distribution of albumin variants Naskapi amd Mexico among Aleuts, Frobisher Bay Eskimos, and Micmac, Naskapi, Mohawk, Omaha, and Apache Indians. Am J Phys Anthropol. 1978 Jul;49(1):111–117. doi: 10.1002/ajpa.1330490117. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Ishioka N., Blumberg B. S., Putnam F. W. Application of an automated tandem high-performance liquid chromatographic system to peptide mapping of genetic variants of human serum albumin. J Chromatogr. 1986 May 30;359:181–191. doi: 10.1016/0021-9673(86)80072-3. [DOI] [PubMed] [Google Scholar]
- Tárnoky A. L. Genetic and drug-induced variation in serum albumin. Adv Clin Chem. 1980;21:101–146. doi: 10.1016/s0065-2423(08)60087-6. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Leavitt J., Kakunaga T., Weber K. Coexpression of a mutant beta-actin and the two normal beta- and gamma-cytoplasmic actins in a stably transformed human cell line. Cell. 1980 Dec;22(3):893–899. doi: 10.1016/0092-8674(80)90566-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weitkamp L. R., McDermid E. M., Neel J. V., Fine J. M., Petrini C., Bonazzi L., Ortali V., Porta F., Tanis R., Harris D. J. Additional data on the population distribution of human serum albumin genes; three new variants. Ann Hum Genet. 1973 Oct;37(2):219–226. doi: 10.1111/j.1469-1809.1973.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Wilding G., Blumberg E. S., Vesell E. S. Reduced warfarin binding of albumin variants. Science. 1977 Mar 11;195(4282):991–994. doi: 10.1126/science.841323. [DOI] [PubMed] [Google Scholar]
- Winter W. P., Weitkamp L. R., Rucknagel D. L. Amino acid substitution in two identical inherited human serum albumin variants: albumin Oliphant and albumin Ann Arbor. Biochemistry. 1972 Feb 29;11(5):889–896. doi: 10.1021/bi00755a031. [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Liang Z. Q., Wu H., Wang L. F. The gene frequency of serum albumin variants in Chinese and the electrophoretic characterization of several serum albumin variants. Sci Sin. 1981 Nov;24(11):1597–1602. [PubMed] [Google Scholar]
- de Souza S. L., Frain M., Mornet E., Sala-Trépat J. M., Lucotte G. Polymorphisms of human albumin gene after DNA restriction by HaeIII endonuclease. Hum Genet. 1984;67(1):48–51. doi: 10.1007/BF00270557. [DOI] [PubMed] [Google Scholar]