Abstract
Treatment of tumor-bearing mice with a stimulatory Ab to glucocorticoid-induced TNFR family-related receptor (GITR) has previously been shown to elicit protective T cell responses against poorly immunogenic tumors. However, the role of GITR stimulation on CD8 T cells and the nature of tumor rejection Ags have yet to be determined. In this study, we show that a stimulatory mAb to GITR (clone DTA-1) acts directly on CD8 T cells, but not on CD4+CD25+ regulatory T (Treg) cells, in B16 tumor-bearing mice to induce concomitant immunity against secondary B16 tumors, as well as protective memory following surgical excision of the primary tumor. Melanoma growth itself induced GITR expression on tumor-specific CD8 T cells, providing a mechanism whereby these cells may respond to stimulatory anti-GITR. Unexpectedly, in contrast to Treg cell depletion therapy with anti-CD4, GITR stimulation induced very weak CD8 T cell responses to melanocyte differentiation Ags expressed by the tumor, and did not induce autoimmune vitiligo. Accordingly, GITR-stimulated hosts that were primed with B16 melanoma rejected B16, but not the unrelated JBRH melanoma, indicating that tumor rejection Ags are tumor-specific rather than shared. In support of this, we show that GITR stimulation induces CD8 T cell responses to a tumor-specific Ag, and that these responses are of higher functional avidity compared with those induced by Treg cell depletion. We conclude that stimulation of GITR on effector CD8 T cells results in high-avidity T cell responses to tumor-specific Ags, thereby inducing potent antitumor immunity in the absence of auto-immunity.
A major challenge of cancer immunotherapy has been the generation of antitumor immunity in the absence of autoimmunity. In addition to self-Ags, tumors express unique Ags that are derived from mutated proteins (1–3) and can serve as potent tumor-rejection Ags (4–6). Therapies that stimulate immunity against such tumor-specific Ags, rather than self-Ags, may provide more potent and durable antitumor immunity without eliciting autoimmunity (3, 6, 7). However, autoimmunity has been unavoidable when T cell responses are globally and nonspecifically induced by therapies such as CTLA-4 blockade and regulatory T (Treg) cell depletion (8–10).
The glucocorticoid-induced TNFR family-related receptor (GITR) is a member of the costimulatory TNFR subfamily that is constitutively expressed on Treg cells and upregulated by CD8 and CD4 effector T cells upon activation (11, 12). Treatment with agonistic anti-GITR (clone DTA-1) has been shown to induce rejection of highly immunogenic tumors (4, 13), but it is markedly less effective against poorly immunogenic tumors. We have previously shown that GITR stimulation in mice bearing progressive B16 melanoma tumors induces concomitant immunity, which is evidenced by the rejection of a secondary B16 tumor inoculated at a distal site (14). Early and frequent or high-dose administration of anti-GITR has also been shown to induce rejection of primary B16 tumors (14–16). GITR delivers a costimulatory signal to effector T cells, resulting in enhanced survival, proliferation, cytokine production, and resistance to Treg cell-mediated suppression (17–19). Stimulation of GITR also reduces Treg cell accumulation and stability within B16 tumors (16). However, direct evidence is still lacking as to whether CD8 T cells require GITR stimulation for effective and systemic antitumor immunity.
Similar to anti-GITR therapy, we have previously shown that total depletion of Treg cells by treatment with an Ab to CD4 induces concomitant immunity against B16 melanoma (14). Anti-CD4 treatment breaks CD8 T cell tolerance to melanoma differentiation Ags, resulting in protective immunity that is directed against shared melanoma Ags (14). Accordingly, CD4-depleted hosts develop autoimmune vitiligo following surgical excision of primary B16 melanoma tumors (20). Treatments with agonistic anti-GITR can induce or exacerbate autoimmunity (11, 15, 21–23), and GITR-stimulated hosts that reject primary B16 tumors have been shown to mount T cell responses against multiple melanocyte differentation Ags (15). However, it remains unknown whether similar T cell specificities govern tumor rejection and autoimmunity in GITR-stimulated and Treg cell-depleted hosts.
The main goal of the present studies was to define the mechanism whereby GITR stimulation induces systemic protection against poorly immunogenic melanoma. We demonstrate that, in contrast to CD4-depletion therapy, GITR stimulation directly on CD8 T cells in melanoma-bearing hosts drives protective and high-avidity T cell responses to tumor-specific Ags. These studies reveal how GITR stimulatory therapy is capable of driving potent and durable antitumor immunity in the absence of autoimmunity.
Materials and Methods
Mice and cell lines
Experiments were performed in accordance with the Institutional Animal Care and Use Guidelines at Dartmouth Medical School. Six to 8-wk-old C57BL/6J mice were obtained from Charles River Laboratories (Wilmington, MA). RAG-1−/− mice and Ly5.2+ OT-1 mice were purchased from The Jackson Laboratory (Bar Harbor, ME), GITR−/− mice on a C57BL/6 background were a gift from Randy Noelle (Dartmouth Medical School) and used with permission from P. Pandolfi (Memorial Sloan-Kettering Cancer Center), and pmel-1 TCR transgenic mice were a gift from Nicholas Restifo (National Cancer Institute). These strains were bred at Dartmouth Medical School. Male and female mice were used at 6–12 wk of age. The B16-F10 mouse melanoma cell line was originally obtained from Isaiah Fidler (MD Anderson Cancer Center) and passaged intradermally in C57BL/6 mice seven times to ensure reproducible growth. The B16-OVA (MO4) and JBRH melanoma cell lines were provided by Alan Houghton (Memorial Sloan-Kettering Cancer Center). Cell lines were screened for pathogens by IMPACT at the University of Missouri. Cells were cultured in RPMI 1640 containing 7.5% FBS, and inoculated into mice only if viability was >96%.
Tumor models
Tumors were generated by inoculation of 1.2 × 105 live cells intradermally, or 1 × 106 cells where noted. Where indicated, cells were mixed with growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) prior to injection to enable the isolation and analysis of tumor-infiltrating lymphocytes. For concomitant immunity experiments, mice were inoculated with primary tumors in the right flank and challenged with secondary tumors in the left flank 6 d later. For postsurgical immunity experiments, primary tumors in the right flank were surgically excised with negative boundaries on day 12, and secondary tumors were inoculated in the left flank either 1 or 30 d post-surgery. Tumor diameters were measured thrice weekly. Only mice that developed primary tumors (>95%) were used as recipients of secondary tumor challenge. Tumor metastasis was not observed with this B16 subline, and mice with recurrent primary tumors following surgery (<5%) were removed from these studies.
Autoimmune depigmentation, observed as the outgrowth of white fur, was assessed thrice weekly following surgery. Depigmentation was considered “local” if it was confined to the flank from which the primary tumor had been excised, or “disseminated” if it was observed on the primary tumor flank as well as other locations (e.g., the other flank, back, trunk).
mAbs and peptides
Ab-producing hybridoma cell lines were obtained from American Type Culture Collection (Manassas, VA). Anti-CD4 (GK1.5), anti-CD8 (clone 2.43), and anti-NK1.1 (clone PK136) were produced as bioreactor supernatants and administered at doses of 250 μg i.p. Purified anti-GITR (clone DTA-1) was purchased from BioXCell (West Lebanon, NH) and administered at doses of 500 μg (i.p.). Peptides (>80% purity) were obtained from New England Peptide (Gardner, MA): TRP-2/DCT180–188 (SVYDFFVWL), gp10025–33 (EGSRNQDWL), and OVA257–264 (SIIN-FEKL).
Reconstitution of RAG−/− hosts
To prepare cells for adoptive transfer, RBC-lysed splenocytes from donor wild-type C57BL/6 mice were treated to deplete CD8 T cells with mAb clone 2.43 (anti-CD8) and complement, or treated to deplete CD4+CD25+ Treg cells by incubation with anti-CD25 magnetic beads (Miltenyi Biotec, Auburn, CA) and subsequent passage through a MACS magnetic column. Cell preparations were then reconstituted with either CD8+ or CD4+CD25+ T cells that had been magnetically purified from spleens of naive GITR−/− mice. Groups of recipient RAG−/− mice received either total wild-type splenocytes, wild-type splenocytes containing GITR−/− CD8 T cells, or wild-type splenocytes containing GITR−/− Treg cells. Concomitant immunity was assessed beginning 1 wk later, by administering primary tumors, anti-GITR, and then secondary tumors, as described above.
IFN-γ ELISPOT (MabTech, Mariemont, OH) was performed as previously described (14, 24). Briefly, CD8+ effector T cells were magnetically purified from pooled spleens or inguinal lymph nodes of six mice per group. Alternatively, CD8 T cells were purified from spleens of individual mice. Purified CD8 T cells were plated at a 10:1 ratio with peptide-pulsed (1 μg/ml, or indicated concentrations) EL-4 thymoma cells (American Type Culture Collection) as targets. Plates were incubated for 20 h at 37°C prior to development using aminoethylcarbazole.
Monitoring transgenic T cell responses
CD8+ T cells were magnetically purified from naive Thy1.1+ TCR transgenic pmel-1 mice (25) or Ly5.2+ TCR transgenic OT-1 mice and adoptively transferred into C57BL/6 mice (1 × 104 or 1 × 106 cells/mouse, as indicated in figure legends). Cells were later detected in various tissues by flow cytometry, after staining with Abs to CD8, Thy1.1, Ly5.2, and CD44 (eBioscience, San Diego, CA) as well as GITR (Miltenyi Biotec). Data were acquired on a FACSCalibur (BD Biosciences) and analyzed using FlowJo software (version 8.1; Tree Star, Ashland, OR).
Vaccination
Mice were vaccinated in the footpad with 10 μg OVA257 peptide emulsified in TiterMax Classic adjuvant (Sigma-Aldrich, St. Louis, MO). Mice were injected i.p. with 200 μg GK1.5 at time of vaccination, 200 μg LTF2 (isotype control mAb) at time of vaccination, or 250 μg DTA-1 both 1 d after and 3 d after vaccination. Draining inguinal and popliteal lymph nodes were harvested 5 d after vaccination and restimulated overnight with OVA257 in the presence of brefeldin A. Cells were stained intracellularly with an Ab to IFN-γ, and cytokine production in the Ag-experienced CD3+ CD8+CD44hiCD62Llow population was assessed by flow cytometry as described above.
Statistical analysis
To detect differences in tumor-free survival, log-rank analyses of Kaplan–Meier data were conducted using GraphPad Prism 4 (GraphPad Software, San Diego, CA). Statistical differences in the ELISPOT assay or flow cytometry were determined by a Student two-tailed t test.
Results
Anti-GITR induces concomitant and postsurgical immunity against B16 melanoma by direct stimulation of CD8 T cells but not Treg cells
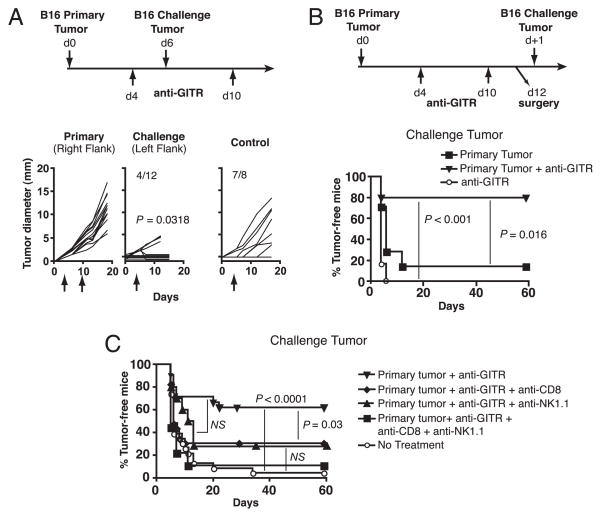
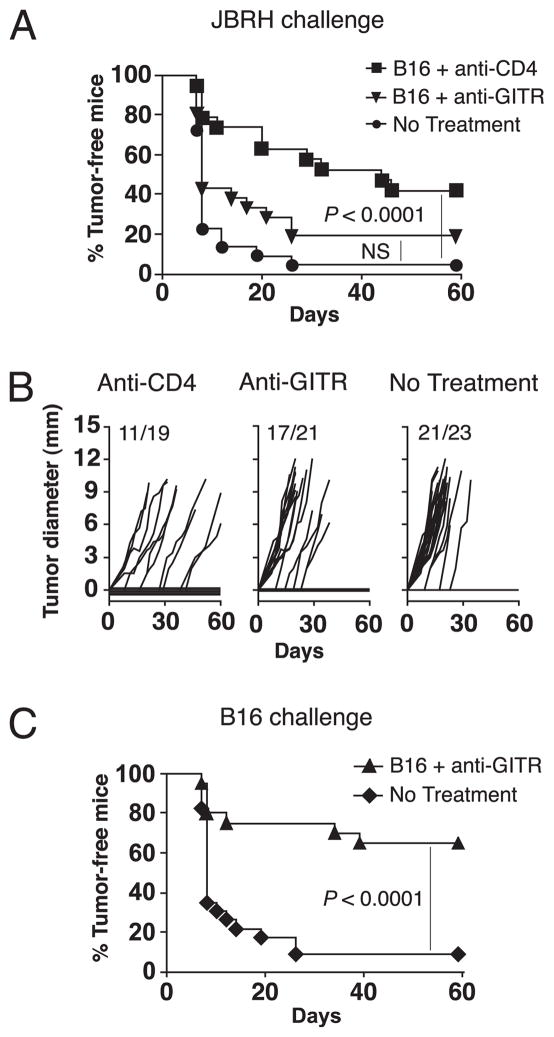
We have previously established that the poorly immunogenic B16 melanoma fails to induce spontaneous concomitant immunity against secondary B16 tumors (14). However, concomitant immunity was induced by treating mice with agonistic anti-GITR mAb 1 d after primary tumor inoculation (14). To extend these studies we asked whether concomitant immunity could still be induced if GITR stimulation was delayed until palpable primary tumors were found. Mice were inoculated in the right flank with B16 cells and treated with 500 μg anti-GITR beginning on day 4, and again on day 10. They were then challenged with 1 × 105 B16 cells in the left flank on day 6 (Fig. 1A). While all primary tumors grew progressively, concomitant immunity was evidenced by the rejection of secondary tumors (Fig. 1A). Control mice that received anti-GITR in the absence of a primary tumor were not protected, indicating a requirement for the first tumor in priming this response (Fig. 1A). Concomitant immunity was very robust, as evidenced by the rejection of a secondary inoculum of B16 cells 10 times the lethal dose (Supplemental Fig. 1). Thus, while anti-GITR treatment on days 4 and 10 was ineffective at inducing rejection of primary B16 tumors, it resulted in a highly protective response against secondary tumors at a distal site.
FIGURE 1.
GITR stimulation induces concomitant and postsurgical immunity against B16 melanoma. A, Concomitant immunity. Mice were treated according to the timeline. Growth curves of primary and challenge tumors, as well as control tumors (mice receiving anti-GITR but no primary tumor), are depicted, with tumor incidence represented as numerical fractions. Significance was determined by comparing growth of challenge and control tumors. B, Postsurgical immunity. Mice were treated according to the timeline and as specified in the legend. Incidence of challenge tumors is depicted. C, CD8 T cells are required for postsurgical immunity. Mice were treated according to the timeline in B, and as specified in the legend. Anti-CD8 and anti-NK1.1 treatments were administered starting 1 d before surgery and weekly thereafter. A and B each involved 10–16 mice/group and were performed twice with similar results. C depicts combined data from two separate experiments. NS, p > 0.05.
We have also shown that the more clinically relevant phenomenon of postsurgical immunity can be induced by depletion of Treg cells with anti-CD4 (20). To determine whether postsurgical immunity could be induced by GITR stimulation, mice were treated with anti-GITR during primary tumor growth, primary tumors were excised on day 12, and then challenge tumors were inoculated 1 d later (Fig. 1B). Anti-GITR-treated, tumor-excised mice developed robust postsurgical immunity, whereas mice receiving anti-GITR and sham surgery, or primary tumors and surgery alone, were not protected (Fig. 1B). Thus, GITR stimulation of mice bearing established B16 tumors induces robust concomitant and postsurgical immunity.
To determine the mechanism whereby GITR stimulation provides secondary tumor protection, CD8+ and/or NK1.1+ cells were depleted by mAbs beginning 1 d prior to surgery. Upon depletion of CD8+ cells, the proportion of mice rejecting secondary tumors decreased substantially (Fig. 1C). Depletion of NK1.1+ cells (NK and NKT cells) also reduced tumor protection, although this difference did not reach statistical significance. However, codepletion of CD8+ and NK1.1+ cells completely abrogated protection (Fig. 1C). Therefore, tumor protection is mediated by CD8 T cell cells and also likely by NK1.1+ cells.
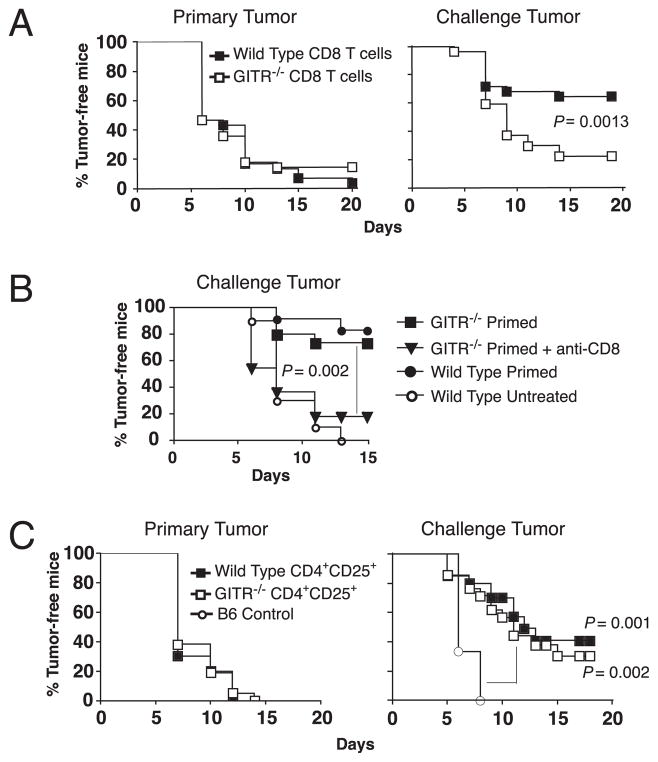
To test the specific role of GITR on CD8 effector T cells we employed a model of concomitant immunity in T cell-reconstituted RAG−/− mice (14). RAG−/− mice were reconstituted with wild-type splenocytes containing CD8 T cells from either wild-type or GITR−/− mice, and concomitant immunity was then evaluated in these mice. As expected, primary tumors grew progressively in all reconstituted mice despite anti-GITR treatment (Fig. 2A, left). GITR-stimulated mice with wild-type CD8 T cells also exhibited concomitant immunity against secondary tumors (Fig. 2A, right). However, GITR-stimulated mice that had GITR−/− CD8 T cells demonstrated significantly reduced concomitant immunity (Fig. 2A, right). Thus, GITR responsiveness in CD8 T cells is required for optimal concomitant immunity against B16 tumors.
FIGURE 2.
Stimulation of GITR on CD8 effector T cells is required for optimal concomitant tumor immunity. A, RAG−/− mice were adoptively transferred with total naive splenocytes containing either wild-type or GITR−/− CD8 T cells and then inoculated with B16 tumors and treated with anti-GITR according to the timeline in Fig. 1A. Primary and challenge tumor incidence is depicted. Data are combined from two separate experiments involving 12–15 mice/group. B, GITR−/− CD8 T cells are functional. Wild-type or GITR−/− mice were “primed” by inoculation of primary B16 tumors on day 0 and treatment with anti-CD4 on days 4 and 10. Incidence of challenge tumors, inoculated on day 6, is depicted. Anti-CD8 treatment was administered starting 1 d before challenge and weekly thereafter. C, RAG−/− mice were reconstituted with naive wild-type splenocytes containing CD4+CD25+ Treg cells from either wild-type or GITR−/− mice and then inoculated with B16 tumors and treated with anti-GITR according to the timeline in Fig. 1A. B6 control mice only received challenge tumors. Primary and challenge tumor incidence is depicted. Data are combined from two separate experiments involving 10–24 mice/group.
Because GITR−/− CD8 T cells may have had inherent defects related to reduced costimulatory signals (26), we also tested whether these cells were capable of mediating concomitant immunity. Concomitant immunity was primed directly in GITR−/− or wild-type mice by inoculation with B16 tumors and treatment with anti-CD4 to deplete Treg cells. Upon challenge with B16 melanoma on day 6, GITR−/− mice mounted robust concomitant immunity that was equivalent to wild-type mice and was mediated by CD8 T cells (Fig. 2B). Thus, GITR−/− CD8 T cells are capable of mediating B16 tumor rejection in the absence of Treg cells. These studies collectively show that stimulation through GITR directly on CD8 T cells induces protective antitumor immunity.
GITR stimulation directly on Treg cells has recently been shown to reduce Treg cell stability and accumulation in B16 tumors, leading to decreased growth of B16 primary tumors (16). To investigate whether GITR stimulation on Treg cells is important for the induction of concomitant immunity, RAG−/− mice were reconstituted with splenocytes containing CD4+CD25+ Treg cells from wild-type or GITR−/− mice and then inoculated with B16 tumors. As expected, primary tumors grew progressively in all T cell-reconstituted RAG−/− hosts despite anti-GITR treatment and despite the presence or absence of GITR on Treg cells (Fig. 2C, left). Furthermore, GITR stimulation induced concomitant immunity against secondary tumors in ~40% of mice possessing wild-type Treg cells (Fig. 2C, left). Interestingly, no decrease in tumor protection was observed in mice possessing GITR−/− Treg cells (Fig. 2C, right). Thus stimulation of GITR directly on Treg cells was not required for concomitant immunity. Rather, CD8 T cells were the major targets of anti-GITR therapy.
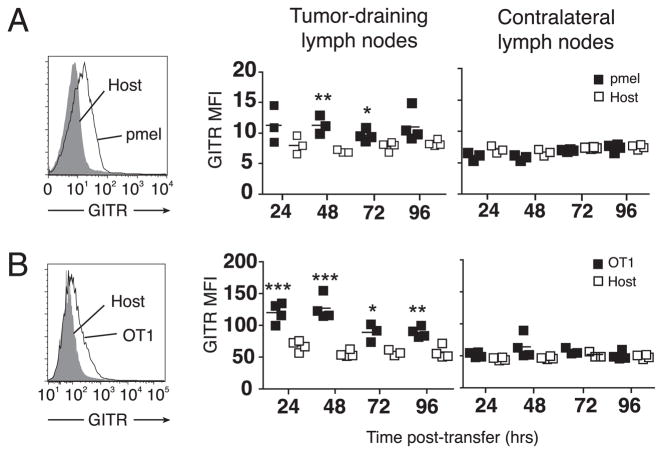
Because naive T cells express very low levels of GITR (11, 17), it was unclear how GITR could be stimulated on tumor-specific CD8 T cells without preactivation. One possibility was that antigenic stimuli provided during the course of normal tumor growth were sufficient for GITR expression on tumor-specific T cells. To test this idea, mice bearing B16 tumors were adoptively transferred with naive gp10025–33-specific pmel T cells (25). In B16 tumor-draining lymph nodes, pmel cells, but not total host CD8+ T cells, upregulated GITR within 48 h and maintained GITR expression for 72 h (Fig. 3A, left). Differences in GITR expression were no longer significant 96 h posttransfer. This was a local phenomenon, as it was only observed in tumor-draining lymph nodes (Fig. 3A, right). Similarly, we found that transgenic OT-1 cells in tumor-draining lymph nodes began to express GITR within the first 24 h of exposure to B16 tumors expressing OVA, and continuing for at least 96 h (Fig. 3B). The increase in GITR expression over host T cells was roughly similar for pmel and OT-1 cells. These studies collectively show that growth of B16 melanoma is sufficient for the induction of GITR on CD8 T cells specific for tumor-expressed self- and foreign Ags, and that anti-GITR can act directly on these CD8 T cell effectors.
FIGURE 3.
Tumor growth induces GITR expression on tumor Ag-specific CD8 T cells. Mice were inoculated with tumors and, when tumors were 6–8 mm in diameter, were adoptively transferred with 106 naive, congenically marked transgenic T cells. A, Mice received pmel T cells and wild-type B16 tumors. B, Mice received OT-1 T cells and B16-OVA tumors. For A and B, flow cytometry was performed to detect GITR expression on transferred T cells in lymph nodes at the indicated time points. Histograms (left) depict GITR expression on congenically marked transgenic T cells versus host CD8+ cells in tumor-draining lymph nodes of representative mice. Graphs (right) depict summarized data, with symbols representing individual mice and horizontal lines representing averages. Asterisks represent statistical differences between mean fluorescence intensity (MFI) of GITR expression on transgenic T cells (pmel or OT-1) as compared with host CD8 T cells: *p < 0.05; **p < 0.01; ***p < 0.001. Experiments were repeated at least twice with similar results.
GITR stimulation induces a lower level of T cell self-reactivity compared with Treg cell depletion
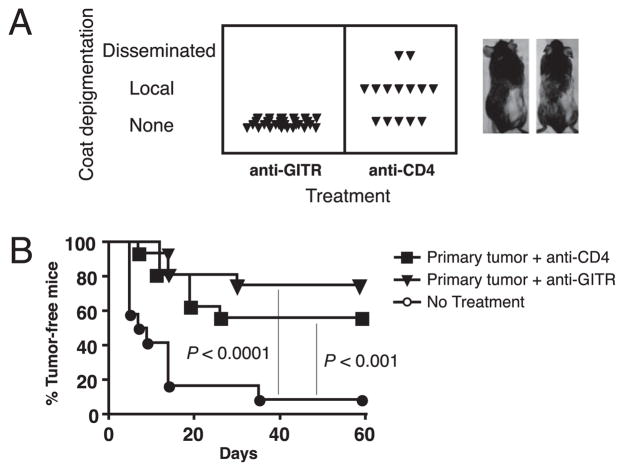
We have shown that CD4 T cell depletion during primary B16 melanoma growth induces CD8 T cell-mediated tumor immunity, as well as autoimmune vitiligo following surgical tumor excision (20). To compare CD4 depletion and GITR stimulation with regard to melanocyte-specific autoimmunity, we monitored GITR-stimulated, B16 tumor-excised mice for the development of vitiligo. We found no evidence of vitiligo in mice that had been treated with anti-GITR, despite the appearance of both local (at the surgery site) and disseminated vitiligo in control anti-CD4-treated mice (Fig. 4A). In a total of five experiments involving >50 mice, postsurgical vitiligo was never observed in GITR-stimulated mice.
FIGURE 4.
GITR stimulation induces protective postsurgical memory in the absence of autoimmunity. Mice received primary B16 tumors, anti-GITR, or anti-CD4 treatment on days 4 and 10 and surgery to remove primary tumors on day 12. A, Incidence and level of autoimmune de-pigmentation 30 d post-surgery. Images depict representative mice treated with anti-CD4 that developed local (left) or disseminated (right) de-pigmentation. Experiment was performed five times with similar results. B, Mice were challenge with B16 melanoma 30 d after surgery and incidence of challenge tumors is depicted. Experiment involved 10–16 mice/group.
Because the lack of melanocyte-specific autoimmunity in GITR-stimulated mice could have been attributed to a weaker or more short-lived antitumor response, we compared long-lived tumor protection induced by anti-GITR versus anti-CD4. Following surgery, mice were rested for 30 d to enable the development of T cell memory and then challenged with B16 cells. GITR stimulation induced similar, if not slightly better, long-lived tumor protection compared with CD4 depletion (Fig. 4B). Thus, tumor immunity induced by GITR stimulation is both potent and long-lived and proceeds in the total absence of vitiligo.
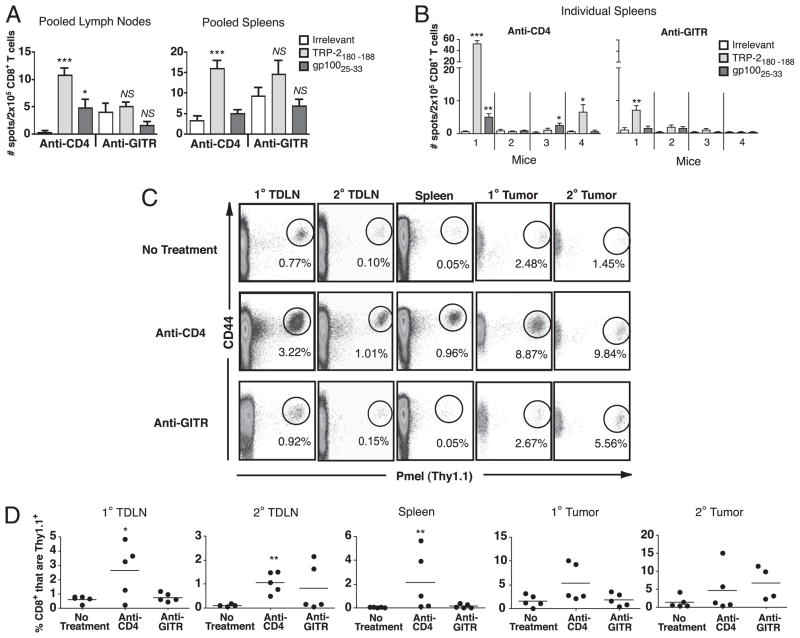
Another possible explanation for the lack of vitiligo in hosts treated with anti-GITR could have been the absence of functional CD8 T cell responses to shared melanoma/melanocyte Ags. CD8 T cell responses to melanoma differentiation Ags TRP-2, TRP-1, and gp100 have previously been demonstrated in anti-GITR-treated mice that were actively rejecting, or had previously rejected, B16 tumors (15). To analyze CD8 T cell responses in GITR-stimulated mice during tumor progression, we assayed responses against TRP-2180–188 and gp10025–33 epitopes by IFN-γ ELISPOT on day 15 of primary tumor growth. As we have previously reported (14, 20), in CD4-depleted mice we detected responses to both TRP-2 and gp100 in pooled lymph nodes and spleens (Fig. 5A). In contrast, there were no significant responses to gp100 or TRP-2 in either tissue of anti-GITR-treated mice (Fig. 5A). Because these responses were small, CD8 T cells from individual mice were also analyzed. For anti-CD4 treatment, three out of four mice exhibited significant responses to TRP-2 and/or gp100 epitopes (Fig. 5B). In contrast, only one out of four mice treated with anti-GITR demonstrated a significant response to TRP-2, and this response was small. Thus, we conclude that GITR stimulation induces weaker responses to melanocyte differentiation Ags compared with anti-CD4 treatment.
FIGURE 5.
GITR stimulation during melanoma progression induces weak CD8 T cell responses to Ags. A and B, Mice received primary and challenge B16 tumors on days 0 and 6 and received either anti-GITR or anti-CD4 on days 4 and 10. On day 15, IFN-γ ELISPOT was performed on CD8+ T cells (six mice per group) from (A) pooled lymph nodes and spleens or (B) spleens taken from individual mice. EL4 cells pulsed with peptides described in the legend were used as targets. Data represent averages of four replicate wells per sample; error bars represent standard deviations. *p < 0.05; **p < 0.01; ***p < 0.001 compared with irrelevant (OVA) peptide. C and D, Mice received 106 naive Thy1.1+ pmel T cells on day −1 and primary B16 tumors in Matrigel on days 0 and 6 either alone (no treatment) or with anti-CD4 or anti-GITR on days 4 and 10. Flow cytometry was performed on day 12 to detect pmel cells. C, Representative data from one mouse in each treatment group; gated on live CD8+ T cells. D, Summary of data; y-axis represents percentages of pmel cells (Thy1.1+) among total CD8+ T cells in live lymphocyte gate. Each point represents a single mouse, and horizontal lines represent averages. *p < 0.05; **p < 0.01, relative to no treatment. Each experiment was performed at least twice with similar results.
To more sensitively monitor systemic T cell responses to a tumor/self-Ag, we adoptively transferred mice with naive pmel T cells 1 d before primary tumor inoculation and tracked these cells by flow cytometry. B16 cells were inoculated in Matrigel to enable analysis of T cell infiltration into tumors. As we have previously shown (20), CD4 T cell depletion induced expansion of gp100-specific T cells in tumor-draining lymph nodes and spleens (Fig. 5C). In contrast, GITR stimulation did not significantly increase T cell responses in any tissue analyzed on day 12. A similar lack of response was observed on day 21 (data not shown). There was a trend to increased infiltration of pmel T cells into rejecting secondary tumors, although this difference was not significant. These data support the conclusion that systemic CD8 T cell responses to melanoma-expressed self-Ags are not induced by GITR stimulation. Because pmel T cells upregulated GITR in response to B16 tumor growth (Fig. 3A), this suggests that the lack of T cell responsiveness was not due to a lack of GITR expression.
Protective immunity induced by GITR stimulation is directed against tumor-specific Ags rather than shared tumor/self-Ags
Because GITR-stimulated mice reject secondary B16 tumors despite the absence of detectable T cell responses to melanocyte Ags, we hypothesized that tumor rejection Ags may be tumor-specific rather than shared. We have previously demonstrated that CD4-depleted, B16 tumor-bearing mice develop concomitant immunity against the unrelated JBRH melanoma (14). To test whether GITR stimulation also induced protective immunity against shared melanoma Ags, mice were inoculated with B16 primary tumors, treated with anti-GITR, and then challenged with JBRH melanoma 1 d following surgery. Control anti-CD4-treated mice were protected against JBRH tumors, whereas GITR-stimulated mice demonstrated no significant cross-protection (Fig. 6A). Kinetics of JBRH tumor growth were also reduced in anti-CD4-treated mice, but similar in GITR-stimulated and untreated mice (Fig. 6B). In the same experiment, anti-GITR induced robust protection against B16 challenge tumors, confirming that effective priming had occurred (Fig. 6C). This demonstrates that protective immunity in GITR-stimulated mice is preferentially directed against tumor-specific Ags, rather than shared melanoma Ags.
FIGURE 6.
Tumor rejection Ags in GITR-stimulated hosts are tumor-specific rather than shared. Mice received primary B16 tumors on day 0, anti-GITR or anti-CD4 treatment on days 4 and 10, and surgery to remove primary tumors on day 12. A, Incidence of JBRH challenge tumors inoculated 1 d post-surgery. B, Growth kinetics of JBRH challenge tumors; numerical fractions represent tumor incidence. C, Incidence of B16 challenge tumors inoculated 1 d post-surgery. Data represent two combined experiments, where each experiment involved 8–12 mice/group. NS, p > 0.05.
GITR stimulation results in higher avidity CD8 T cell responses to tumor-specific Ags, as compared with Treg cell depletion
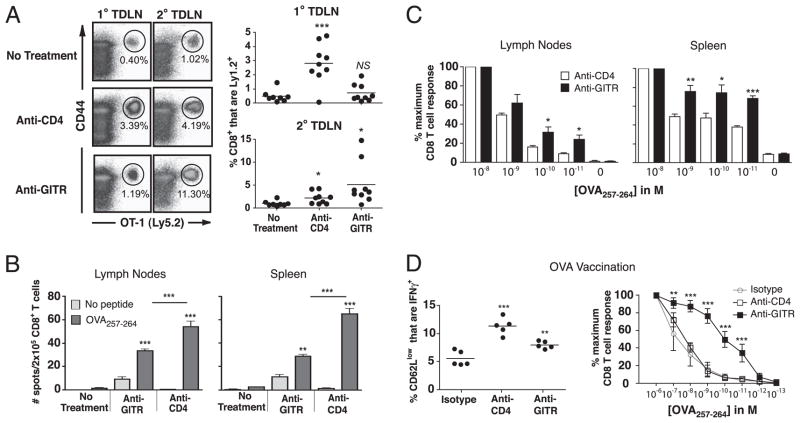
To directly test whether CD8 T cell responses induced by GITR stimulation were skewed toward the recognition tumor-specific Ags, we employed B16 tumors expressing OVA as a model tumor-expressed neo-Ag. We had found that B16-OVA tumors induced GITR expression on adoptively transferred OT-1 cells (Fig. 3B), and therefore we first analyzed whether GITR stimulation in the presence of B16-OVA tumors could drive the priming of OT-1 T cells. B16-OVA tumor growth alone induced some T cell priming, indicated by the appearance of an Ag-experienced (CD44hi) OT-1 population in primary and secondary tumor-draining lymph nodes. As observed for pmel cells, OT-1 T cell priming was significantly enhanced by treatment with anti-CD4 (Fig. 7A). Importantly, GITR stimulation also enhanced priming of OT-1 T cells as evidenced by large CD44hi OT-1 T cell responses in secondary tumor draining-lymph nodes. Therefore, whereas day 4 and 10 anti-GITR treatment drives no measurable expansion of gp100-specific pmel T cells in response to wild-type B16 tumors (Fig. 5C), it drives robust expansion of OT-1 T cells in response to B16 tumors expressing the neo-Ag OVA.
FIGURE 7.
GITR stimulation induces high-avidity T cell responses to a tumor-specific Ag. A, Mice received 106 Ly5.2+ OT-1 T cells on day −1 and primary B16-OVA on days 0 and 6 either alone (no treatment) or with anti-CD4 or anti-GITR on days 4 and 10. Flow cytometry was performed on day 12 to detect OT-1 cells. Left, Representative data from one mouse in each treatment group; gated on live CD8+ T cells. Right, Summary of data; y-axis represents percentages of Ly5.2+ OT-1 cells among total CD8+ T cells in live lymphocyte gate. Symbols represent individual mice, horizontal lines represent averages, and asterisks represent statistical significance relative to untreated control. Data represent two combined experiments. B and C, Mice were inoculated with B16-OVA tumors on days 0 and 6 and were left untreated or were treated with anti-GITR or anti-CD4 on days 4 and 10. On day 15, IFN-γ ELISPOT was performed on purified CD8 T cells from pooled spleens (six mice per group). EL4 cells pulsed with no peptide or with OVA were used as targets, as specified in the legend. B, GITR stimulation increases T cell functional avidity. The percentage of CD8 T cells secreting IFN-γ at decreasing peptide concentrations, normalized relative to the maximal response, is shown. Statistical differences (asterisks) were calculated between anti-CD4 and anti-GITR groups. D, Mice were vaccinated in the footpad with 10 μg OVA257 peptide emulsified in TiterMax and treated with anti-CD4 (day 0), LTF2 isotype control mAb (day 0), or anti-GITR (days 1 and 3). On day 5, cells pooled from lymph nodes were restimulated with the specified concentration of OVA peptide and the percentage of Ag-experienced CD8 T cells secreting IFN-γ was determined by flow cytometry. The y-axis represents the percentage of CD3+CD8+ CD44hiCD62Llow cells secreting IFN-γ when restimulated with 1 μM OVA peptide. Left, Each point represents a single mouse, horizontal lines represent averages, and asterisks represent statistical significance relative to isotype control. Right, Data were normalized relative to the maximal response at the highest peptide concentration. Symbols represent the average of five mice per group, and error bars represent standard deviations. Each experiment was conducted at least twice with similar results. *p < 0.05; **p < 0.01; ***p < 0.001.
We also analyzed endogenous T cell responses to tumor-expressed OVA by ELISPOT. B16-OVA tumor growth alone (no treatment) was insufficient for the priming of OVA-specific CD8 T cells. However, GITR stimulation elicited systemic priming of T cell responses to tumor-expressed OVA in both pooled lymph nodes and spleens (Fig. 7B). In comparison, anti-CD4 treatment also induced CD8 T cell responses to OVA, and these responses were of significantly greater magnitude than those induced by anti-GITR (Fig. 7B, Supplemental Fig. 2). Therefore, we assessed whether polyclonal T cell responses to OVA might exhibit differences in functional avidity. Cells were restimulated with decreasing concentrations of OVA peptide, and IFN-γ production was again assessed by ELISPOT. Compared with CD4-depleted mice, OVA-specific T cells from lymph nodes and spleens of GITR-stimulated mice were capable of secreting IFN-γ at lower peptide concentrations, indicating that a higher avidity T cell response had been primed (Fig. 7C, Supplemental Fig. 2). Therefore, despite the smaller magnitude of OVA-specific T cells in GITR-stimulated mice, the avidity of these cells was significantly greater than those induced by CD4 depletion.
To further extend and confirm this finding, naive mice were vaccinated with OVA peptide emulsified in TiterMax adjuvant and treated systemically with isotype control Ab, anti-CD4, or anti-GITR. Both anti-CD4 and anti-GITR significantly increased the magnitude of the IFN-γ–producing, OVA-specific CD8 T cell response by a similar magnitude (Fig. 7D). However, GITR stimulation increased the functional avidity of the response by almost three orders of magnitude (Fig. 7D, Supplemental Fig. 2). Taken together, these experiments show that GITR stimulation skews the avidity of polyclonal CD8 T cell responses, resulting in high-avidity responses against tumor-specific Ags.
Discussion
Since the initial discovery that GITR stimulation drives T cell immunity (11), agonistic anti-GITR has been used extensively for tumor immunotherapy, both as a monotherapy (13–15) and in conjunction with vaccines (21, 27–29). GITR stimulation has been shown to drive potent CD8 T cell-mediated tumor protection (15, 30), and tumor immunity has been shown to proceed in the absence of overt autoimmunity (13, 31). However, two key questions have remained unanswered; namely, on what cell population must GITR be stimulated, and what Ags mediate tumor rejection? The present studies reveal that tumor rejection Ags in GITR-stimulated hosts are tumor-specific, and that stimulation of GITR directly on CD8 T cells preferentially drives high-avidity responses to putative neo-Ags. Our work highlights anti-GITR as a nonspecific immune stimulatory therapy that naturally skews protective T cell immunity to tumor-specific Ags.
We were initially surprised by the lack of CD8 T cell responses to melanocyte differentiation Ags in GITR-stimulated, B16 tumor-bearing mice. Even very sensitive monitoring of transgenic gp100-specific cells produced no evidence of local or systemic T cell priming. This is in contrast to published studies showing that CD8 T cell responses to TRP-2, TRP-1, and gp100 develop in B16 tumor-inoculated mice that are treated with anti-GITR on days 1, 3, and 9 and are therefore actively rejecting the melanoma (15). It was also recently shown that GITR stimulation results in increased pmel T cell activation within rejecting B16 primary melanomas, although pmel T cell population size did not increase (16). One major difference in the present studies is the fact that primary B16 tumors were not rejected. This may suggest that active tumor rejection is required for the priming of T cells against self-Ags (i.e., via epitope spreading). Also, because anti-GITR therapy was delayed until palpable primary tumors were found, tumor-induced immune suppression could play a role. Regardless, when hosts bear small, established B16 tumors, we find that GITR stimulation is ineffective at breaking tolerance to melanocyte Ags.
Despite the lack of responses to self-Ags, GITR stimulation clearly induced a CD8 T cell response to B16 tumor-expressed OVA. Preferential recognition of tumor-specific Ags is also supported by a study showing that GITR stimulation leads to efficient regression of B16 tumors only when they express mutated TRP-1 containing multiple high-affinity MHC class I binding epitopes (4). Furthermore, GITR stimulation coupled with vaccines against xenogeneic human TRP-2 (21), xenogeneic Her-2/neu (27), or mutated ERK (28) has been shown to elicit protective CTL responses, whereas responses were not induced with a vaccine encoding wild-type mouse TRP-2 (21). These studies collectively suggest that non-self tumor Ags may be the primary targets of T cell responses induced by GITR stimulation. We demonstrate this by showing that unshared, tumor-specific Ags govern B16 secondary tumor rejection in GITR-stimulated hosts. The identity of these rejection Ags remains unknown, as B16 is characteristically non-immunogenic. However, the ability to skew protective immunity toward tumor-specific Ags on such a weakly immunogenic tumor was not observed by global depletion of regulatory T cells (with anti-CD4), which induced protective immunity against shared melanoma Ags as well. Whether this property is peculiar to anti-GITR remains to be seen. Exploration of T cell specificity induced by agonistic Abs to TNFR family-related receptors such as OX-40 and 4-1BB, which exhibit similar T cell expression patterns, is warranted.
We also report for the first time, to our knowledge, that compared with Treg cell depletion, GITR stimulation increases the functional avidity of CD8 T cell responses. We favor a model whereby TCR stimuli above a threshold are required for effective GITR costimulation of T cells, leading to the selective expansion of T cells with higher affinity TCRs, and thus to polyclonal responses with higher functional avidities. This is reminiscent of CTLA-4 blockade, which has been shown to increase the functional avidity of CD8 T cell responses by preferentially affecting T cells that receive stronger TCR signals (32–34). In contrast, global elimination of Treg cell suppression would be expected to rescue T cell responses regardless of TCR affinity. Our finding that tumor growth is sufficient to induce GITR expression on gp100-specific CD8 T cells, but that anti-GITR still has no effect on this population, further suggests that a strong TCR signal is required in addition to GITR signaling. Indeed, we show that GITR stimulation induces expansion of tumor-primed OT-1 cells, which possess a high-affinity TCR. Furthermore, because it has been shown that GITR stimulation on CD8 T cells enables them to overcome Treg cell-mediated suppression (28, 35), GITR stimulation may preferentially enable those T cells that receive stronger TCR signals to bypass suppression, leaving T cells with lower affinity TCRs subject to Treg cell control. Preferential expansion of T cell clones with high-affinity TCRs would also support our finding that GITR does not induce appreciable T cell responses to self-Ags, as most high-affinity clones against self-Ags have already been deleted in the thymus.
While these studies identify a major role for GITR stimulation on CD8 T cells, the possibility that GITR stimulation influences other effector cell subsets still remains possible. NK1.1+ cells are known to express GITR (36, 37) and appear to be involved in early post-surgical protection against B16. There may also be a role for CD4 effector and/or helper T cells, as previously shown in GITR-stimulated mice bearing B16 or CT-26 tumors (15, 38). GITR stimulation can directly expand CD4 T cells (39), and it has been shown to enhance the function of adoptively transferred tumor-specific CD4 T cells (40). Future studies will be required to compare the functional and phenotypic characteristics of CD8 memory T cell responses induced by anti-CD4 versus anti-GITR. A more classical and durable memory T cell response may develop in GITR-stimulated hosts due to the presence of CD4 T cell help and the non-self nature of tumor rejection Ags.
Despite a clear influence of anti-GITR on CD8 T cells, our work demonstrates that stimulation of GITR on Treg cells does not contribute to concomitant tumor immunity. This was surprising in light of recent reports that GITR stimulation reduces Treg cell accumulation in primary tumors (16, 35, 41), and that high-dose administration of anti-GITR acts directly on Treg cells to control growth of B16 primary tumors (16). Seeming disparities between these and the present studies may underscore differences in mechanisms governing primary tumor rejection and systemic tumor control. Whereas perturbation of Treg cell populations within the primary tumor microenvironment may relieve suppression locally, we find that GITR stimulation on Treg cells is insufficient for priming systemic T cell responses that provide distal tumor protection.
Continued growth of primary tumors remains an intriguing phenomenon of concomitant immunity, which may be due to a shortcoming in the T cell response primed by the first tumor, or the primary tumor’s large size and established microenvironment. Indeed, studies published by ourselves and others have shown that early, frequent, and/or high-dose administration of anti-GITR can induce primary B16 tumor regressions in a larger proportion of mice (14–16). This suggests that T cells that provide concomitant immunity are on the verge of causing primary tumor rejection. Interestingly, the fact that very early GITR stimulation induces B16 primary tumor rejection (14, 15) is consistent with our observation that tumor-specific CD8 T cells upregulate GITR within 24–48 h of exposure to the tumor.
Perhaps the most important therapeutic advantage of GITR stimulatory therapy is the induction of antitumor immunity in the absence of autoimmunity. Continuous GITR stimulation has been linked to the exacerbation of autoimmune conditions, including gastritis (11), experimental autoimmune encephalomyelitis (22), and collagen-induced arthritis (23). Despite this, the absence of auto-immunity (13), or very mild localized vitiligo in a small fraction of mice (15), has been reported when early GITR stimulation induces tumor rejection. Our studies demonstrate that melanocyte-specific autoimmunity can be avoided entirely by limited therapeutic administration of agonistic anti-GITR. This is in clear contrast to CD4 Treg cell depletion, which elicits pronounced postsurgical vitiligo.
The effectiveness of GITR stimulation in humans has yet to be demonstrated. Human GITR ligand is costimulatory for CD4 and CD8 T cells and can drive Ag-specific T cell responses in vitro (42), although GITR/GITR-L interactions have been shown to suppress human NK cell responses to tumor cells (43, 44). The present studies of concomitant and postsurgical immunity demonstrate that GITR stimulation during primary tumor growth could be sufficient for the treatment of minimal residual disease, or the prevention of tumor metastasis and recurrence. GITR stimulatory therapy clearly warrants further investigation as a method for generating protective, high-avidity T cell responses to tumor-specific Ags in the absence of autoimmunity.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (Grants R01 CA120777 and P20 RR 16437 COBRE to M.J.T., and R21CA127037-01A1 to J.A.G.-P.), the Melanoma Research Foundation (New Investigator Award to M.J.T.), the American Cancer Society–Illinois Division (Young Investigator Award Grant 07-20 to J.A.G.-P.), the American Cancer Society (Grant ACSLIB112496-RSG to J.A.G.-P.), and the Cancer Research Foundation (Young Investigator Award to J.A.G.-P.). A.L.C. was supported by a Rosaline Borison Fellowship (Dartmouth Medical School), and J.A.O. was supported by a National Institutes of Health T32 grant.
We thank Laurie Horne for assistance with breeding mice. We also thank Brent Berwin and Kate Byrne for guidance in preparing this manuscript.
Abbreviations used in this paper
- GITR
glucocorticoid-induced TNFR family-related receptor
- MFI
mean fluorescence intensity
- Treg
regulatory T
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 2.Monach PA, Meredith SC, Siegel CT, Schreiber H. A unique tumor antigen produced by a single amino acid substitution. Immunity. 1995;2:45–59. doi: 10.1016/1074-7613(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 3.Neller MA, López JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol. 2008;20:286–295. doi: 10.1016/j.smim.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Duan F, Lin Y, Liu C, Engelhorn ME, Cohen AD, Curran M, Sakaguchi S, Merghoub T, Terzulli S, Wolchok JD, Houghton AN. Immune rejection of mouse tumors expressing mutated self. Cancer Res. 2009;69:3545–3553. doi: 10.1158/0008-5472.CAN-08-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 6.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Semin Immunol. 2008;20:276–285. doi: 10.1016/j.smim.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchi H, Stan R, Turk MJ, Engelhorn ME, Rizzuto GA, Goldberg SM, Wolchok JD, Houghton AN. Unraveling the complex relationship between cancer immunity and autoimmunity: lessons from melanoma and vitiligo. Adv Immunol. 2006;90:215–241. doi: 10.1016/S0065-2776(06)90006-6. [DOI] [PubMed] [Google Scholar]
- 8.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Auto-immunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P, Rudensky A, Sparwasser T. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol. 2009;183:7631–7634. doi: 10.4049/jimmunol.0804308. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 12.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 13.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, Miles RC, Sakaguchi S, Houghton AN, van den Brink MR. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 18.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 19.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Côté AL, de Vries VC, Usherwood EJ, Turk MJ. Induction of postsurgical tumor immunity and T-cell memory by a poorly immunogenic tumor. Cancer Res. 2007;67:6468–6476. doi: 10.1158/0008-5472.CAN-07-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8+ T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- 23.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, Liew FY. Glucocorticoid-induced TNFR family-related protein (GITR) activation exacerbates murine asthma and collagen-induced arthritis. Eur J Immunol. 2005;35:3581–3590. doi: 10.1002/eji.200535421. [DOI] [PubMed] [Google Scholar]
- 24.Scheibenbogen C, Lee KH, Stevanovic S, Witzens M, Willhauck M, Waldmann V, Naeher H, Rammensee HG, Keilholz U. Analysis of the T cell response to tumor and viral peptide antigens by an IFNγ-ELISPOT assay. Int J Cancer. 1997;71:932–936. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C. Glucocorticoid-induced TNFR-related protein lowers the threshold of CD28 costimulation in CD8+ T cells. J Immunol. 2007;179:5916–5926. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- 27.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa H, Kato T, Hirayama M, Orito Y, Sato E, Harada N, Gnjatic S, Old LJ, Shiku H. Regulatory T cell-resistant CD8+ T cells induced by glucocorticoid-induced tumor necrosis factor receptor signaling. Cancer Res. 2008;68:5948–5954. doi: 10.1158/0008-5472.CAN-07-5839. [DOI] [PubMed] [Google Scholar]
- 29.Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S, Lustgarten J. Systemic targeting of CpG-ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res. 2008;68:7530–7540. doi: 10.1158/0008-5472.CAN-08-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piao J, Kamimura Y, Iwai H, Cao Y, Kikuchi K, Hashiguchi M, Masunaga T, Jiang H, Tamura K, Sakaguchi S, Azuma M. Enhancement of T-cell-mediated anti-tumour immunity via the ectopically expressed glucocorticoid-induced tumour necrosis factor receptor-related receptor ligand (GITRL) on tumours. Immunology. 2009;127:489–499. doi: 10.1111/j.1365-2567.2008.03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boczkowski D, Lee J, Pruitt S, Nair S. Dendritic cells engineered to secrete anti-GITR antibodies are effective adjuvants to dendritic cell-based immunotherapy. Cancer Gene Ther. 2009;209:900–911. doi: 10.1038/cgt.2009.39. [DOI] [PubMed] [Google Scholar]
- 32.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 33.Gregor PD, Wolchok JD, Ferrone CR, Buchinshky H, Guevara-Patiño JA, Perales MA, Mortazavi F, Bacich D, Heston W, Latouche JB, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–1708. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsui J, Nishikawa H, Muraoka D, Wang L, Noguchi T, Sato E, Kondo S, Allison JP, Sakaguchi S, Old LJ, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 36.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Ndhlovu LC, Takahashi T, Takeda K, Ikarashi Y, Kikuchi T, Murata K, Pandolfi PP, Riccardi C, Ono M, et al. Co-inhibitory roles for glucocorticoid-induced TNF receptor in CD1d-dependent natural killer T cells. Eur J Immunol. 2008;38:2229–2240. doi: 10.1002/eji.200838167. [DOI] [PubMed] [Google Scholar]
- 38.Zhou P, L’italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179:7365–7375. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 39.van Olffen RW, Koning N, van Gisbergen KP, Wensveen FM, Hoek RM, Boon L, Hamann J, van Lier RA, Nolte MA. GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo. J Immunol. 2009;182:7490–7500. doi: 10.4049/jimmunol.0802751. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Tian S, Falo LD, Jr, Sakaguchi S, You Z. Therapeutic immunity by adoptive tumor-primed CD4+ T-cell transfer in combination with in vivo GITR ligation. Mol Ther. 2009;17:1274–1281. doi: 10.1038/mt.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuyaerts S, Van Meirvenne S, Bonehill A, Heirman C, Corthals J, Waldmann H, Breckpot K, Thielemans K, Aerts JL. Expression of human GITRL on myeloid dendritic cells enhances their immunostimulatory function but does not abrogate the suppressive effect of CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;82:93–105. doi: 10.1189/jlb.0906568. [DOI] [PubMed] [Google Scholar]
- 43.Baltz KM, Krusch M, Bringmann A, Brossart P, Mayer F, Kloss M, Baessler T, Kumbier I, Peterfi A, Kupka S, et al. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. FASEB J. 2007;21:2442–2454. doi: 10.1096/fj.06-7724com. [DOI] [PubMed] [Google Scholar]
- 44.Baltz KM, Krusch M, Baessler T, Schmiedel BJ, Bringmann A, Brossart P, Salih HR. Neutralization of tumor-derived soluble glucocorticoid-induced TNFR-related protein ligand increases NK cell anti-tumor reactivity. Blood. 2008;112:3735–3743. doi: 10.1182/blood-2008-03-143016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.