Abstract
Background
Self-referential processing (i.e. linking internal and external stimuli to one’s own self) has received scant attention thus far in schizophrenia. This type of processing is a key component of social cognition and thought to be important for adaptive social functioning. Memory studies in healthy subjects have shown that stimuli processed with reference to the self are better remembered than stimuli processed in other semantic forms. It is not known whether schizophrenia patients benefit from such a memory boost for self-referenced information.
Methods
Twenty-five schizophrenia patients and 22 controls were assessed with a self-referential recognition memory paradigm. During an encoding phase, participants rated personality adjectives in each of three conditions: (1) structural features (uppercase or lowercase letters?); (2) social desirability (is adjective socially desirable or not?); or (3) self-referential (does adjective describe me or not?). Recognition memory for these personality adjectives was then tested during an unexpected yes-no recognition test.
Results
Patients and controls were comparable in memory performance for the structural (p = 0.12) and social desirability (p = 0.30) conditions. In contrast, patients showed significantly reduced recognition sensitivity compared to controls for the self-referential condition (p = 0.03).
Discussion
Compared to healthy controls, patients with schizophrenia did not benefit from a memory boost for self-referenced information. Such impaired self-referential memory may be associated with abnormal function of the medial prefrontal cortex. The inability to enhance memory for personally relevant information may partly explain poor social functioning in schizophrenia patients.
Keywords: self-referential processing, recognition memory, social cognition, schizophrenia
INTRODUCTION
Self-referential processing can be defined as the mental process of associating internal and external stimuli to one’s self . Objects and events analyzed in relation with the self usually gain privileged emotional and cognitive processing . This phenomenon is particularly well reflected in memory studies showing that stimuli (typically personality trait adjectives) processed with reference to the self generally are better remembered than stimuli processed in other semantic terms . This is referred to as the self-reference memory (SRM) effect, which is thought to reflect deeper encoding of information related to a cognitively rich mental representation of the self-concept .
In general, self-referential processing is thought to be crucial for adaptive functioning in the social environment. Indeed, a clear understanding of one’s own traits, abilities, and attitudes is needed for a person to evaluate his/her own role in a social context, and compare him/herself to others . It has been suggested that the ability to understand emotional states in others requires self-reflection as a basis for the interpretation of their experience . As such, self-referential processing is necessary both for relating, and making a distinction between, the self and others, and is considered to be a core component of social cognition . The intrinsic relationship between social cognition and self-referential processing is supported by findings from functional magnetic resonance imaging (fMRI) studies. A network composed of cortical midline structures (i.e. medial prefrontal cortex (mPFC), anterior cingulate cortex, precuneus) has been associated with judgments about one’s own states and traits, as well as how we use social cognition to understand other people (e.g., mentalizing about thoughts, intentions and feelings of others) . These midline structures also appear to be critical for linking self with others; for example, using the self as the basis for perceiving others and thinking about the self from the perspective of others.
There are only a few studies on self-referential processing in schizophrenia and research thus far has focused on the experience of agency and self-recognition. For instance, schizophrenia patients have a tendency to misidentify their own voices as alien and difficulties in discriminating self-generated tactile sensations from those generated by others, though they show normal visual self-recognition . Another possible manifestation of impaired self-referential processing in schizophrenia may be disturbances in self-related memory processes. Although episodic memory impairment in schizophrenia is well documented, its relation with self-referential processing has received scant attention thus far. One study identified source memory deficits for self-generated information in schizophrenia, but used a source monitoring paradigm that did not directly assess the memory bias for self-referenced information .
Whereas healthy adults routinely show a memory boost for information processed in a self-referential manner, it is not known if schizophrenia patients do. Literature on emotional memory has shown that schizophrenia patients are usually impaired in the emotional enhancement of recognition memory . Given that the SRM effect consistently observed in healthy controls may be partly explained by increased emotional processing of self-reference information, one may predict that schizophrenia patients will not show such an effect. In this initial study, we investigated the SRM effect in outpatients with schizophrenia and healthy controls using an incidental memory task in which trait adjectives initially are encoded in terms of their structural features, social desirability, or relevance for self. We hypothesized that patients with schizophrenia would be comparable to controls in their memory for words encoded by structural features or social desirability; however, they would differ from controls by not showing a typical pattern of enhanced recognition for adjectives processed in reference to self.
METHODS
Participants
Twenty-five patients with schizophrenia and twenty-two healthy controls were recruited from a larger NIMH study of early visual processing in schizophrenia. Schizophrenia patients were 18–60 years of age and recruited from outpatient clinics at the VA Greater Los Angeles Healthcare System (VAGLAHS) and through local board and care facilities. Patients were clinically stable, medicated, and received the Structural Clinical Interview for DSV-IV Axis I Disorders (SCID-I) to confirm diagnosis of schizophrenia. Exclusion criteria for patients were: (1) substance abuse or dependence in the last 6 months, (2) mental retardation, (3) history of loss of consciousness > 1 hour, (4) identifiable neurological disorder, and (5) not sufficiently fluent in English to comprehend instructions. We assessed clinical symptoms using the expanded Brief Psychiatric Rating Scale (BPRS) and examined the BPRS total score, as well as BPRS mean subscores for positive symptoms (thinking disturbance factor), negative symptoms (withdrawal-retardation factor), and depression/anxiety .
Normal control participants were recruited through flyers posted in the local community, newspaper advertisements, and website postings. Exclusion criteria for control participants were: (1) history of schizophrenia or other psychotic disorder, bipolar disorder, recurrent depression, history of substance dependence, or any substance abuse in the last 6 months based on the SCID, (2) avoidant, paranoid, schizoid, or schizotypal personality disorders based on the SCID-II, (3) schizophrenia or other psychotic disorder in a first-degree relative, (4) significant neurological disorder or head injury, and (5) not sufficiently fluent in English to comprehend instructions. Efforts were made to group match controls and patients based on sex, age, and parental education.
SCID and BPRS interviewers were thoroughly trained to a minimum kappa of 0.75 through the Treatment Unit of the Desert Pacific Mental Illness Research, Education, and Clinical Center, and participated in continuous quality assurance procedures. Participants were evaluated for the capacity to give informed consent and provided written informed consent after all procedures were fully explained, according to procedures approved by the institutional review boards at UCLA and VAGLAHS.
Procedure
Stimuli
We selected 156 different personality-trait adjectives from a validated list . Six lists of 26 adjectives were constructed for the self-referential memory task. Within each of the 6 lists, half of the words were positive (i.e. high desirability) and half were negative (i.e. low desirability). The average number of letters and syllables per word was comparable between valences and across the six lists.
Self-referential memory task
The self-referential memory task consisted of two phases: encoding and recognition. The encoding phase included trials drawn from three separate conditions: structural, social desirability, and self-referential. In the structural processing condition, participants judged whether the letters of the word were presented in uppercase or lowercase. In the social desirability condition, participants judged whether the trait was socially desirable for other people in general. In the self-referential condition, participants judged whether each personality trait described them. In all conditions, participants responded with a two-alternative forced-choice button press (i.e. “uppercase” or “lowercase” for structural; “desirable” or “not desirable” for social desirability; and “me” or “not me” for self-referential condition). Trials were presented in an intermixed and randomized fashion; not in blocks of the same trial type. Participants were not explicitly asked to remember the word adjectives.
Four versions of the task were developed, using different combinations of words, and each subject received only one version. For each version, three lists of words (total of 78) were randomly assigned to the encoding phase (one list each for structural, social desirability, and self-referential conditions), and three lists were used as new words during recognition phase. Half of the word adjectives were presented in uppercase and half in lowercase; half were positive and half were negative valence.
During encoding, each trial consisted of a fixation crosshair displayed for 500 ms, followed by the condition instructions (i.e. “self”, “social desirability”, “letter case”) displayed for 2000 ms, followed by the target adjective for another 2000 ms. Instructions remained in the upper part of the screen during the presentation of the target adjective to reduce memory demands. After the trait adjective, subjects made their choice. Participants were encouraged to respond as soon as they knew the answer. When they made their response, another fixation point was presented for 500 msec. Both response and reaction time were recorded.
The encoding phase was followed after a 15 min delay by an unexpected recognition task. Subjects were instructed to discriminate between 78 old and 78 new adjectives presented in a pseudorandom order. Each adjective was presented individually with a two-alternative forced-choice response (i.e. “old” or “new”). There was no time limit for the response. The task was generated using E-Prime software (Psychology Software Tools) and performed on a desktop PC computer.
Statistical analyses
For the encoding phase, mean response time was calculated for each task condition. For the structural condition, we calculated the letter case judgment accuracy (i.e. either lowercase or uppercase). For the social desirability condition, we calculated the desirability classification accuracy (i.e. either high or low social desirability) for each adjective relative to available norms . Similarly, for the self-referential condition, we calculated the mean proportion of self-attribution for positive and negative adjectives separately.
For the recognition phase, we first calculated the hit rate (i.e. proportion of old adjectives correctly recognized) for each condition and each valence separately, and we calculated a false alarm rate (i.e. proportion of new adjectives incorrectly identified as old adjectives) for each valence. Calculation of separate false alarm rates for each condition was not possible because new words did not belong to a condition. To obtain a quantitative measure of correct performance, we used d-prime (d′). This index of sensitivity is used in signal detection theory and is calculated based on hit rate and false-alarm rate, but is independent of response bias. A higher d′ indicates that the signal can be more readily detected. We then used d′ to calculate for each participant the magnitude of the SRM bias (d′ self-referential minus d′ social desirability).
Statistical tests were performed using SPSS version 18.0 for Windows. We compared demographic data between groups using Student t tests for independent samples, except for the sex variable, for which we used χ2. We performed a repeated measures analysis of variance (ANOVA) with group as a between-subject factor and encoding condition (structural, social desirability, and self-referential) as a within-subject factor, to compare patients and controls on response time. A student t test for independent samples was used to compare groups for the letter case judgment accuracy (structural condition). Repeated measures ANOVAs with group as a between-subject factor and valence (positive or negative) as a within-subject factor were also conducted to compare groups for desirability classification accuracy and mean proportion of self-attribution. For the recognition phase, a 2 × 3 × 2 repeated measures ANOVA was performed with group as a between-subject factor and encoding condition and valence as within-subject factors to compare groups on sensitivity (d′). The mean magnitude of the SRM bias was compared between groups using a Student t test for independent samples.
RESULTS
Table 1 provides demographic and clinical data for participants. The groups were well-matched for age, parental education and sex ratio. Patients were chronically ill with mild to moderate symptom levels.
Table 1.
Demographic and clinical data
| Characteristic | Group; mean (SD) |
|||
|---|---|---|---|---|
| Schizophrenia, n = 25 | Control, n = 22 | Statistical test | p value | |
| Demographic | ||||
| Age, yr | 45.9 (11.9) | 44.5 (8.6) | t45 = −0.43 | 0.67 |
| Education, yr | 13.0 (1.3) | 14.9 (1.8) | t45 = 4.07 | < 0.001 |
| Parental Education, yr | 11.9 (2.5) | 13.3 (3.0) | t45 = 1.70 | 0.10 |
| Sex, male:female | 20:5 | 17:5 | χ2 = 0.05 | 0.82 |
| Ethnicity | ||||
| African American | 9 | 5 | ||
| Asian | 0 | 3 | ||
| Hispanic | 10 | 2 | ||
| White | 4 | 12 | ||
| Other | 2 | 0 | ||
| Clinical | ||||
| BPRS1 total score | 40.6 (7.5) | |||
| BPRS positive2 | 2.0 (1.0) | |||
| BPRS negative2 | 1.9 (0.8) | |||
| BPRS depression/anxiety2 | 1.9 (0.6) | |||
| Age of onset, yr | 23.7 (6.5) | |||
| Number of hospitalizations | 2.3 (1.3) | |||
BPRS = Brief Psychiatric Rating Scale;
Mean score of each BPRS subscale was calculated by dividing the total score by the number of items included in the subscale.
Encoding phase
Table 2 shows the data for the encoding phase. Response time analysis showed a significant main effect of group (F1,45 = 9.16, p = 0.004) and a significant main effect of condition (F2,90 = 3.97, p = 0.02) but no significant group by condition interaction (F2,90 = 0.49, p = 0.62). Patients were slower overall than controls to rate adjectives, and all participants were slower to classify the desirability of an adjective (social desirability) compared to identifying the case of letters (pairwise comparison, Bonferroni corrected: p = 0.005).
Table 2.
Behavioral data for the encoding phase
| Data | Group; mean (SD) |
|
|---|---|---|
| Schizophrenia, n = 25 | Control, n = 22 | |
| Response time (msec) | ||
| Structural | 1903 (851) | 1249 (510) |
| Social desirability | 2133 (959) | 1506 (691) |
| Self-referential | 2132 (1211) | 1343 (628) |
| Accuracy in identifying of the case of letters – structural condition (%) | ||
| 80 (22) | 94 (14) | |
| Desirability classification accuracy – social desirability condition (%) | ||
| Negative | 83 (19) | 93 (6) |
| Positive | 83 (21) | 97 (5) |
| Self attribution – self-referential condition (%) | ||
| Negative | 35 (24) | 23 (17) |
| Positive | 72 (21) | 90 (6) |
For the structural condition, patients were less accurate than controls in identifying the case of letters (80% versus 94% for patients and controls, respectively, t = 2.58, df = 45, p = 0.01). Regarding desirability classification accuracy for the social desirability condition, the ANOVA revealed a significant main effect of group (F1,45 = 9.21, p = 0.004), but neither a significant main effect of valence (F1,45 = 1.16, p = 0.29) nor a group by valence interaction (F1,45 = 0.73, p = 0.40). Patients were less accurate than controls in classifying the adjectives as positive or negative, but accuracy was generally high (> 80% in both groups).
Self attribution of adjectives for the self-referential condition showed a non-significant main effect of group (F1,45 = 1.09, p = 0.30), but a significant main effect of valence (F1,45 = 148.80, p < 0.001) and a significant group by valence interaction (F1,45 = 12.13, p = 0.001). Decomposing the interaction revealed that controls attributed a greater proportion of positive adjectives to themselves (90%) than did patients (72%) (pairwise comparison, Bonferroni corrected: p < 0.001). Additionally, there was a trend towards greater self attribution of negative adjectives in patients (35%) compared to controls (23%) (pairwise comparison, Bonferroni corrected: p = 0.07).
Recognition phase
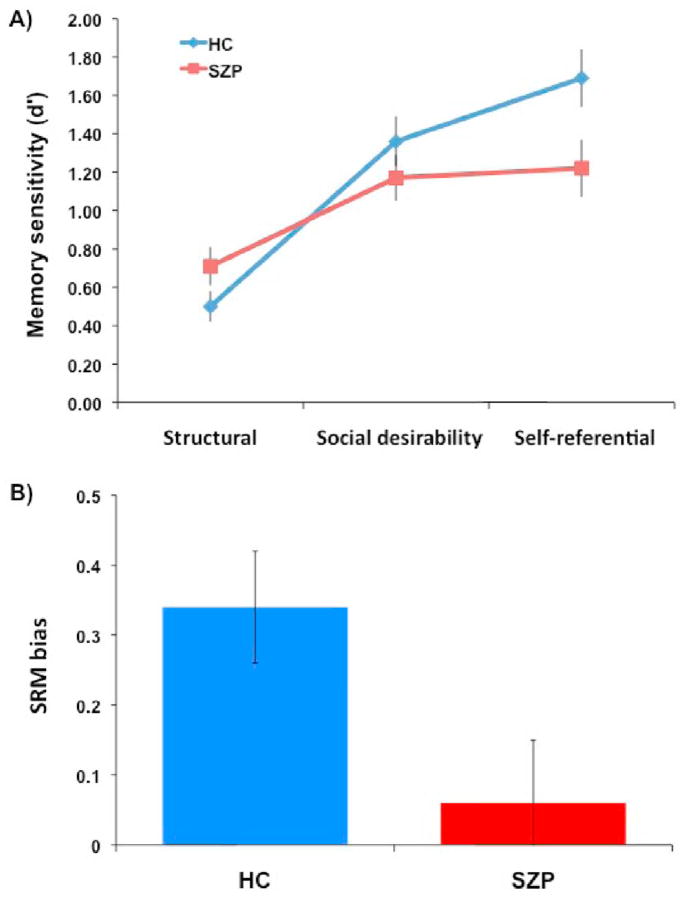
For sensitivity (d′), we found a significant main effect of condition (F2,90 = 51.25, p < 0.001), a significant group by condition interaction (F2,90 = 8.61, p < 0.001), and a significant main effect of valence (F1,45 = 9.16, p = 0.004). Other effects were not significant (ps varying from 0.27 to 0.79). As we hypothesized, patients had reduced recognition sensitivity compared to controls for the self-referential condition only (p = 0.03). Indeed, patients and controls were not significantly different in their responses for the structural (p = 0.12) and social desirability (p = 0.30) conditions. The main effect of valence indicates that participants had better recognition for negative adjectives compared to positive ones (d′ negative = 1.26, SD = 0.59; d′ positive = 1.06, SD = 0.55). Figure 1A shows the recognition sensitivity by group and condition, collapsed across valence.
Figure 1.
Part A depicts the memory sensitivity (d′) for each group (HS = healthy controls and SZP = schizophrenia patients) and each encoding condition (Structural, Social desirability, Self-referential). Part B shows the mean magnitude of SRM bias (d′ self-referential - d′ social desirability) for each group. The bars in both panels represent standard errors.
Recognition was quite poor for both groups for the structural condition, and patients showed non-significantly greater recognition than controls. To determine whether the results for the interaction were driven by the structural condition, we conducted an additional ANOVA (2 × 2 × 2) on d′ using only the social desirability and self-referential conditions. The effects were essentially the same with a significant main effect of condition (F1,45 = 10.94, p = 0.002), a significant group by condition interaction (F1,45 = 6.30, p =0.02), and a significant main effect of valence (F1,45 = 2.37, p = 0.01). Other effects were not significant (ps varying from 0.23 to 0.76). Finally, the analysis for SRM bias showed that controls (mean = 0.33) had a significantly greater memory boost for self-referenced information compared to patients (mean = 0.05) (t = 2.19, df = 45, p = 0.03). Figure 1B illustrates the mean magnitude of SRM bias for each group.
DISCUSSION
In this study we employed a version of the self-referential recognition memory paradigm to determine whether patients with schizophrenia benefit from a memory boost for personality adjectives encoded in a self-referential manner. Healthy controls demonstrated a typical memory boost for self-referential information. In contrast, patients failed to show enhanced recognition sensitivity for adjectives encoded with reference to the self as compared to those encoded with referenced to their general social desirability. Both groups, however, showed significantly greater recognition sensitivity for both self-referential and social desirability conditions compared to the structural condition. The latter observation is in accordance with enhanced depth of processing for semantic information and suggests that schizophrenia patients did benefit from elaborative encoding, but only up to a point. They showed the expected benefits of enhanced processing in the social desirability condition, but this benefit did not extend to self-processing.
A recent study also explored self-related memory processes in schizophrenia, but in a different way . Using a source monitoring paradigm, Fisher et al. observed that schizophrenia patients had more difficulty than controls in recognizing that they were the source of words generated during an earlier sentence completion task. Our results extend those of Fisher et al. by demonstrating that self-related memory deficits in schizophrenia are not limited to source monitoring, but also apply to retrieval of information processed in reference to the self.
Why might patients with schizophrenia not show a memory boost for self-referenced adjectives? Early theories argued that the SRM effect was mainly explained by increased depth of processing for self-relevant information, within the same systems that underlie memory for other types of information . It was suggested that the extensive knowledge that we have about ourselves simply favors more elaborative encoding of self-referenced information . However, there may be a distinct type of encoding that is conducted when we process information about ourselves. Recent behavioral and neuroimaging studies suggest that the concept of self relies on brain structures beyond classical memory systems, including the mPFC . In healthy subjects, activity of the mPFC during encoding of stimuli attributed to the self predicts subsequent memory performance . Thus, impaired SRM in schizophrenia may be explained by an impairment in self processing that is subserved by abnormal neural functioning within the mPFC . Our main finding is also consistent with the emotional memory literature in schizophrenia. Past studies have demonstrated that schizophrenia patients do not show the same memory boost as controls for emotional stimuli compared to neutral stimuli . Because stimuli processed in reference to the self are likely more emotionally colored than those processed in other ways, one might wonder whether SRM is simply one aspect of emotional memory. Indeed, memory for emotional stimuli and self-referenced information both involve enhanced types of memory processing, and are both thought to involve enhanced long-term potentiation in hippocampus. However, while self-referential processing is mainly associated with cortical midline structures, emotion processing might predominantly take place in subcortical and other cortical regions including the insula and amygdala.
One question is whether our main result can be explained by group differences in “event congruency” during encoding . Event congruency refers to encoding events (or items) eliciting “yes” answers. Event congruency has been shown to promote more successful memory formation (i.e. than items eliciting a “no” response) through enhanced semantic elaboration and relational binding operations. As mentioned above, patients and controls did not differ significantly in their overall proportion of “yes” answers during the self-referential condition (53% versus 57% for patients and controls, respectively). We examined whether the groups differed in the effect of event congruency on word recognition with a post hoc 2 (group) x 2 (yes versus no answers) x 2 (valence) ANOVA on d′ data for the self-referential condition. None of the interactions were significant. Hence even though patients endorsed fewer positive adjectives and somewhat more negative adjectives than controls during the self-referential condition, we did not find a memory benefit for event congruency (yes responses) across valence and across group.
The social desirability condition used in the current study required participants to evaluate the desirability of personality adjectives for other people in general. This type of semantic condition has also been used in some studies investigating the SRM effect in healthy controls and depressed patients . Some other studies have employed a more specific other condition that required participants to indicate whether a personality adjectives described a particular person, such as a U.S. President, Angelina Jolie, or Harry Potter .
One limitation of the social desirability approach used in this study is that the impaired SRM observed in schizophrenia could reflect a more general difficulty in thinking about a particular person (in this case, themselves) as opposed to a specific self-referential processing impairment. It is possible that healthy controls may have benefitted more than patients from deeper elaboration during the self condition, for reasons that are not necessarily linked to self-processing. One argument against this suggestion is that a desirability task is different from other semantic-encoding tasks (e.g. synonym judgment) in that it involves deeper processing, because it promotes both relational and item-specific processing . It should be noted that there are potential confounds with the use of a specific other condition with a schizophrenia population.
For example, reference to a specific well-known person raises questions about potential group differences in level of familiarity with the named other person. In addition, the use of an intimate other (e.g. mother/father, wife/husband, best friend) may partly eliminate the familiarity confound, but is also likely to engage self-referential processes, thereby complicating interpretation. Nevertheless, future SRM effect studies on schizophrenia should confirm the impaired SRM in patients by using a specific other condition that has been controlled for group differences in other person familiarity.
A potential confound is the patients’ medication. All patients were taking antipsychotic medication at the time of testing, which may have affected their performances. However, the fact that we did not find an overall memory problem in patients, but rather a specific impairment for the self condition strongly argues against medication as an explanation for our main finding.
Social cognitive deficits are key features of schizophrenia and important determinants of functional outcome . These social cognitive deficits are distinct from, yet related to, non-social neurocognitive impairments (i.e. deficits in attention, memory, and executives functions) . Our study examined one aspect of social cognition and revealed a rather specific failure to show a memory boost for self-referenced information in schizophrenia. A reduced memory for personally relevant information may adversely impact social functioning in schizophrenia. This has been supported by recent findings of significant associations between SRM and social functioning in autistic patients . Although such an impact may be present in schizophrenia, community functioning was not assessed in this study. Other studies with longitudinal designs and larger samples can determine whether the reduced SRM is related to social functioning.
Acknowledgments
We thank the people at the Green lab who were responsible for the clinical evaluation of the participants and the assessment of the main task.
Role of the funding source
Funding for this study was provided by NIMH Grant MH043292 to Dr. Green. Dr Harvey has received a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR). The NIMH and CIHR had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
Contributors
Dr Harvey and Dr Green designed the study and wrote the protocol. Dr Harvey managed the literature searches, statistical analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Allen PP, Johns LC, Fu CH, Broome MR, Vythelingum GN, McGuire PK. Misattribution of external speech in patients with hallucinations and delusions. Schizophr Res. 2004;69:277–287. doi: 10.1016/j.schres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. The self. In: Gilbert DT, Fiske ST, editors. The handbook of social psychology. McGraw-Hill; New York: 1998. pp. 680–740. [Google Scholar]
- Bell M, Tsang HW, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophr Bull. 2009;35:738–747. doi: 10.1093/schbul/sbm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30:1131–1139. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Brophy AL. Alternatives to a table of criterion values in signal detection theory. Behavior Research Methods, Instruments, & Computers. 1986;18:285–286. [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Carruthers P. How we know our own minds: the relationship between mindreading and metacognition. Behav Brain Sci. 2009;32:121–138. doi: 10.1017/S0140525X09000545. discussion 138–182. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Craik FIM, Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology. 1975;11:268–294. [Google Scholar]
- Damasio A. Feelings of emotion and the self. Ann N Y Acad Sci. 2003;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Dimaggio G, Lysaker PH, Carcione A, Nicolo G, Semerari A. Know yourself and you shall know the other... to a certain extent: multiple paths of influence of self-reflection on mindreading. Conscious Cogn. 2008;17:778–789. doi: 10.1016/j.concog.2008.02.005. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Fisher M, McCoy K, Poole JH, Vinogradov S. Self and other in schizophrenia: a cognitive neuroscience perspective. Am J Psychiatry. 2008;165:1465–1472. doi: 10.1176/appi.ajp.2008.07111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophr Res. 2009;115:74–81. doi: 10.1016/j.schres.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, Kring AM, Park S, Silverstein SM, Heinssen R. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U, Chechko N, Pauly K, Koch K, Backes V, Seiferth N, Shah NJ, Stocker T, Schneider F, Kellermann T. Neural correlates of emotion recognition in schizophrenia. Schizophr Res. 2010 doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Hall J, Harris JM, McKirdy JW, Johnstone EC, Lawrie SM. Emotional memory in schizophrenia. Neuropsychologia. 2007;45:1152–1159. doi: 10.1016/j.neuropsychologia.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Zahka NE, Kojkowski NM, Inge AP, Schwartz CB, Hileman CM, Coman DC, Mundy PC. Self-referenced memory, social cognition, and symptom presentation in autism. J Child Psychol Psychiatry. 2009;50:853–861. doi: 10.1111/j.1469-7610.2008.02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbener ES. Emotional memory in schizophrenia. Schizophr Bull. 2008;34:875–887. doi: 10.1093/schbul/sbn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson PR, Chidambi G, Lee A, Meyer J. Foundations for self-awareness: An exploration through autism. Monogr Soc Res Child Dev. 2006;71:vii–166. doi: 10.1111/j.1540-5834.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21:1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J. The nature of self-referent encoding - The contributions of elaborative and organizational processes. Journal of personality and social psychology. 1988;55 [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial Emotion Perception in Schizophrenia: A Meta-analytic Review. Schizophr Bull. 2009 doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychol Rev. 2009;19:312–323. doi: 10.1007/s11065-009-9107-0. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- Lee J, Kwon JS, Shin YW, Lee KJ, Park S. Visual self-recognition in patients with schizophrenia. Schizophr Res. 2007;94:215–220. doi: 10.1016/j.schres.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. Br Med Bull. 2008;88:43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond B Biol Sci. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Newell L. Attention, Joint Attention, and Social Cognition. Curr Dir Psychol Sci. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. At the interface of the affective, behavioral, and cognitive neurosciences: decoding the emotional feelings of the brain. Brain Cogn. 2003;52:4–14. doi: 10.1016/s0278-2626(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Park IH, Park HJ, Chun JW, Kim EY, Kim JJ. Dysfunctional modulation of emotional interference in the medial prefrontal cortex in patients with schizophrenia. Neurosci Lett. 2008;440:119–124. doi: 10.1016/j.neulet.2008.05.094. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Achim AM, Montoya A, Lal S, Lepage M. Cognitive and clinical moderators of recognition memory in schizophrenia: a meta-analysis. Schizophr Res. 2005;74:233–252. doi: 10.1016/j.schres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Canales-Rodriguez EJ, Salvador R, Sarro S, Gomar JJ, Vila F, Ortiz-Gil J, Iturria-Medina Y, Capdevila A, McKenna PJ. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry. 2010;15:823–830. doi: 10.1038/mp.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr Res. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Gray JC, Davachi L. Event congruency enhances episodic memory encoding through semantic elaboration and relational binding. Cereb Cortex. 2009;19:1198–1207. doi: 10.1093/cercor/bhn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychol Bull. 1997;121:371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Cunningham SJ, Macrae CN. Self-memory biases in explicit and incidental encoding of trait adjectives. Conscious Cogn. 2008;17:1040–1045. doi: 10.1016/j.concog.2008.02.004. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993;3:227–243. [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97:129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc Natl Acad Sci U S A. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]