Abstract
Purpose
To assess the relative radiosensitivities of a large collection of melanoma cell lines and to determine whether pharmacologic inhibition of mutant B-RAF with PLX-4032 can radiosensitize B-Raf+ melanoma cells.
Methods and Materials
A large collection of melanoma cell lines (n=37) were treated with 0 – 8 Gy IR and clonogenic survival assays used to generate survival curves to rank relative radiosensitivities among the cell lines. The ability of a B-RAF inhibitor, PLX-4032, to radiosensitize highly radioresistant, B-Raf+ cells was also assessed by clonogenic cell survival and spheroid invasion assays and the effects of treatment on the cell cycle assessed by FACS.
Results
Melanoma cell lines displayed a very large, heterogeneous range of SF2 values (1.002 – 0.053) with a mean of 0.51. Cell lines with surviving fractions of 0.29 or less at SF2 and SF4 were observed at a high frequency of 18.9% and 70.2%, respectively. Treatment of B-Raf+ cells with the B-RAF inhibitor PLX-4032 in combination with radiation provided enhanced inhibition of both colony formation and invasion, and radiosensitized cells through an increase in G1 arrest.
Conclusions
Our data suggest that melanomas are not uniformly radioresistant with a significant subset displaying inherent radiosensitivity. Pharmacologic inhibition of B-RAF with PLX-4032 effectively radiosensitized B-Raf+ melanoma cells suggesting this combination approach could provide improved radiotherapeutic response in B-Raf+ melanoma patients.
Keywords: melanoma, B-RAF, radiosensitization, PLX-4032
INTRODUCTION
Melanoma represents the sixth most common cancer diagnosed in the U.S. in both men and women and remains one of the mostly deadly forms of solid malignancies. Importantly, while overall cancer incidence rates continue to decrease, the incidence of melanoma continues to rise (1). Remarkably, current treatment options for advanced melanoma beyond surgical excision have changed little over the last few decades, and adjuvant chemotherapy provides only short-lived, partial responses in a small subset of patients (2, 3).
The role of adjuvant radiotherapy in malignant melanoma remains controversial (4, 5). Early in vitro radiation studies of melanoma cell lines showed wide initial shoulders on survival curves leading to a widespread belief that melanomas are radioresistant and thus require high dose per fraction regimens (5–7). These early assessments however have not been supported by clinical data. A study of 56 melanoma patients that examined locoregional control after adjuvant radiotherapy found hypofractionation and conventional fractionation equally efficacious with 5-yr in-field control rates of 87% (8). In the Radiotherapy and Oncology Group (RTOG) 83-05 prospective study, 137 patients were randomized to receive either hypofractionation vs conventional fractionation. This study was stopped early when an interim analysis show no difference between the two arms (complete response rates were 24% vs. 23%, and partial response rates 35% vs. 34%, respectively) (9). More recently, a randomized trial (TROG 02.01/ANZMTG 01.02) of melanoma patients at high risk of regional relapse was designed to assess the effect of radiotherapy after lymphadenectomy on the subsequent risk of regional relapse. Inclusion criteria included ≥1 parotid, ≥2 cervical or axillary, or ≥3 groin-positive nodes; or extra nodal spread of tumor; or minimum metastatic node diameter of 3 cm (neck or axilla) or 4 cm (groin). After a median follow-up of 27 months, the risk for local recurrence was significantly elevated in patients who received lymphadenectomy alone vs. those who received lymphadenectomy followed by adjuvant radiation (HR=1.77; CI [1.02 – 3.08], P=0.041) (10). While these studies collectively demonstrate that melanoma is not uniformly radioresistant and that higher dose per fraction regimens do not improve therapeutic success, the use of radiotherapy as a first-line treatment in the management of primary melanoma remains uncommon (11, 12) and when used, often incorporates riskier hypofractionation dosing regimens (4). Thus, there is great need not only to better identify those melanoma patients most likely to respond to radiotherapy and the optimal dosing regimens that provide the best therapeutic index, but also to discover the molecular underpinnings that enhance radiosensitivity by identifying novel targets as radiosensitizers.
Recent discoveries of the molecular aberrancies that promote melanomagenesis have provided potential new therapeutic targets (13). Mutational activation of the B-RAF serine/threonine kinase or its upstream activator, N-RAS (a small GTPase), occur at frequencies of approximately 50% and 15%, respectively, in a mutually exclusive manner and at an early stage of the disease (14, 15). The remaining 35% of melanomas are wild-type (WT) for both B-Raf and N-Ras (16, 17). RAS activation of RAF leads to phosphorylation and activation of MEK1/2 leading to phosphorylation and activation of ERK1/2 (mitogen-activated protein kinase; MAPK). Activated ERK1/2 then phosphorylates a number of cytoskeletal proteins, kinases, and transcription factors resulting in changes in cell proliferation, differentiation, apoptosis, invasion, angiogenesis and radioresistance (18–21).
The identification of mutant B-Raf in human cancers in 2002 (14) spawned the development of several B-RAF inhibitors currently being evaluated as monotherapies in clinical trials of melanoma (22). One such inhibitor, PLX-4032, recently completed a Phase I trial (23) and is currently entering into Phase II and III trials. Whether pharmacologic inhibition of B-RAF can radiosensitize melanoma cells harboring B-Raf mutations is currently unknown.
In this study, we characterized the relative radiosensitivities of a large panel of melanoma cell lines to begin to study the mechanisms that promote radiosensitivity vs. radioresistance in melanoma. We also examined whether the B-RAF inhibitor, PLX-4032, selectively radiosensitizes B-Raf+ melanoma cells.
MATERIALS AND METHODS
Cell lines, reagents and mutational analysis
Cell lines were obtained from the sources indicated (Supple. Table 1) and cultured as indicated. All cell lines were verified to be mycoplasma-free or, if contaminated, treated with Plasmocin (InvivoGen) and retested to verify mycoplasma-free status. Genomic DNA was isolated using Genomic Tips kits (Qiagen), and the mutational status of B-Raf (exons 11 and 15) and N-Ras (codons 12, 13, and 61) determined by direct sequencing of PCR amplification products as previously described (16). The B-RAF specific inhibitor, PLX-4032 (provided by Plexxikon Inc/F Hoffmann-La Roche Ltd), was dissolved with DMSO and stored frozen (< 1 month) at −20°C.
Western blot analyses
Cells were plated in complete media for 24 h and treated with drug or an equal amount of vehicle control (DMSO) at the times indicated and harvested with lysis buffer as previously described (18). Proteins (30 μg) were separated over 12% sodium dodecyl sulphate (SDS)/poly-acrylamide gels and electrophoretically transferred to polyvinyl difluoride (PVDF), blocked, probed with anti-phospho-ERK1/2 (T202/Y204, #9101) or anti-total ERK1/2 (#9102) (Cell Signaling Technology) followed by the appropriate secondary HRP-conjugated antibody and visualized by enhanced chemiluminescence (Amersham).
Colony-forming assays (CFA)
Cells were plated in triplicate at low density overnight in complete media, irradiated or sham-irradiated with the indicated graded, single doses using an RS2000 X-ray Biological Irradiator (RadSource) and the medium changed 2 h post-irradiation. Briefly, colonies >50 cells were counted approximately 2–3 weeks later, clonogenic surviving fractions were generated and survival curves fitted to the linear-quadratic model (SF=e−[α * D + β * D2]) using GraphPad Prism 5.0 according to a least squares fit, weighted to minimize the relative distances squared, and compared using the extra sum-of-squares F test as previously described (18). Graphs of survival curves for each individual cell line are shown in Supple. Fig. 1.
For drug treatments, cells were pretreated with DMSO or PLX-4032 at the doses and times indicated, irradiated at 6 Gy or sham-irradiated, trypsinized and plated at low density with fresh media without drug and the surviving fraction (SF) [number of colonies formed/number of cells plated × plating efficiency] calculated from the number of colonies (minimum of 50 cells/colony) formed in the treated dishes compared with the number formed in the non-treated control dishes and significance determined by t-test where *=P<0.05, **=P<0.01, ***=P<0.001.
Flow Cytometry
Cells were plated and treated as described for the CFA assays with PLX-4032 or DMSO, sham-irradiated or irradiated at 6 Gy, refed fresh media without drug, and cells harvested 24 h post-irradiation. Cells were collected by trypsinization, fixed in 70% ethanol, resuspended in 1X PBS containing RNase A (50 μg/ml) and propidium iodide (50 μg/ml) and DNA content determined by FACS using a Beckman-Coulter CyAn ADP.
Spheroid Invasion Assay
Melanoma spheroids were prepared by the liquid overlay method as described (24). Briefly, 5,000 cells/200 μl growth media/well were added to 96-well plates coated with 100 μl/well 1.5% Noble agar. After 72 h, cell spheroids were collected, treated with PLX-4032 at the indicated concentrations for 2 h, sham-irradiated or irradiated at 5 Gy, incubated 2 additional hours, centrifuged and resuspended in a gel (without drug) of bovine collagen I containing EMEM, Lglutamine, and 2% FBS and replated (4 spheroids/well/condition) in a 24-well plate placed at 37°C to allow the collagen to solidify. After 72 h of incubation, photomicrographs of the spheroids were used to calculate the area of invasion of cells that migrated out from the spheroids into the surrounding collagen using Adobe Acrobat Professional 9.0 and the invasion fraction determined by comparing the area of invasion of the drug-treated, irradiated spheroids with the area of invasion of the control non-drug-treated, unirradiated spheroids. Significance was determined by t-test.
RESULTS
While melanomas as a tumor class are relatively radioresistant, a significant subset do respond to radiotherapy (10). The molecular underpinnings that drive radioresistance in melanoma are poorly understood. To begin to uncover these underpinnings, we first characterized the relative radiosensitivities of a large collection of melanoma cell lines.
Melanoma cell lines show a large, heterogeneous range of radiosensitivities that does not correlate with mutational status of B-Raf or N-Ras
Our goal was to analyze the radiosensitivities of a collection of melanoma cell lines that harbored mutations in B-Raf and N-Ras at frequencies seen in patients. Melanoma cell lines were obtained from several sources, DNA extracted and mutational status of B-Raf and N-Ras determined (Supple. Table 1). In these melanoma cell lines (n=37), B-Raf and N-Ras mutations were mutually exclusive, and the frequency of B-Raf+, N-Ras+ and WT (B-Raf and N-Ras negative) subtypes were 54%, 24%, and 22%, respectively, and thus exhibit similar frequencies of these genes as seen in the clinic (14–17).
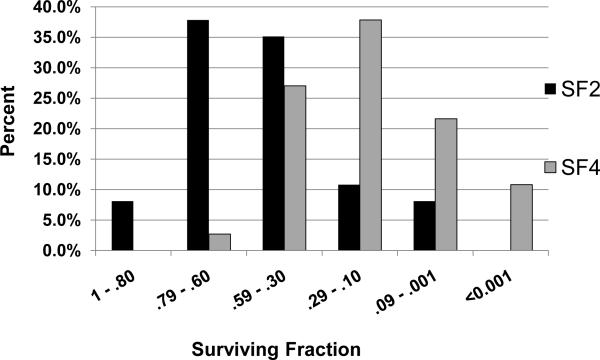
We next determined the relative radiosensitivities among the melanoma cell lines treated with ionizing radiation (IR) over 0–8 Gy. Supple. Table 1 shows the surviving fractions at 2 Gy (SF2) along with the genotype (B-Raf+, N-Ras+, or WT for B-Raf/N-Ras) for each cell line. As shown, the melanoma cell lines displayed a very large range of SF2 values ranging from highly radioresistant to highly radiosensitive (1.002 – 0.053). The mean SF2 value was 0.51 which is similar to those reported for other cancers commonly treated with radiotherapy such as colon, lung, and breast cancer (25). Cell lines with relatively low surviving fractions of ≤0.29 at SF2 and SF4 were observed at high frequencies of 18.9% and 70.2%, respectively (Fig. 1).
FIG. 1. A significant subset of melanoma cell lines are radiosensitive.
The percentage of cell lines with surviving fractions in the ranges indicated are shown for SF2 and SF4. A significant number of lines are radiosensitive with surviving fractions of 0.29 or less at SF2 and SF4 seen in 18.9 % and 70.2 %, respectively, of the cell lines.
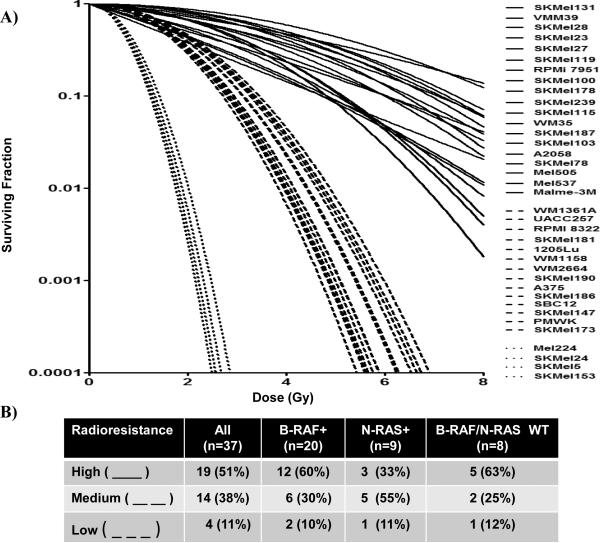
A graph of the survival curves (Fig. 2) showing clonogenic survival after 0–8 Gy revealed that the cell lines clustered into subgroups of high, medium or low radioresistance. All three radioresistance subgroups were found in all genotypes (B-Raf+, N-Ras+, or WT for B-Raf/N-Ras). Interestingly, the frequency of cell lines exhibiting high radioresistance for each genotype (B-Raf+, N-Ras+, or WT for B-Raf/N-Ras) were 60%, 33%, and 63%, respectively. While intriguing, these differences did reach statistical significance by Fishers' exact test (P=0.49).
FIG. 2. Melanoma survival curves.
(A) Survival curves over 0–8 Gy were generated for 37 melanoma all cell lines and relative radioresistance subgrouped as low ( ), medium (
), medium ( ), or high (
), or high ( ). (B) Statistical comparisons of relative radioresistance among B-Raf+, N-Ras+, or BRaf/N-Ras WT melanoma cell lines. Fisher's exact test showed no significant differences between genotypes (P=0.49).
). (B) Statistical comparisons of relative radioresistance among B-Raf+, N-Ras+, or BRaf/N-Ras WT melanoma cell lines. Fisher's exact test showed no significant differences between genotypes (P=0.49).
Inhibition of B-RAF with PLX-4032 radiosensitizes B-Raf+, but not WT or N-Ras+ melanoma cells in colony formation assays
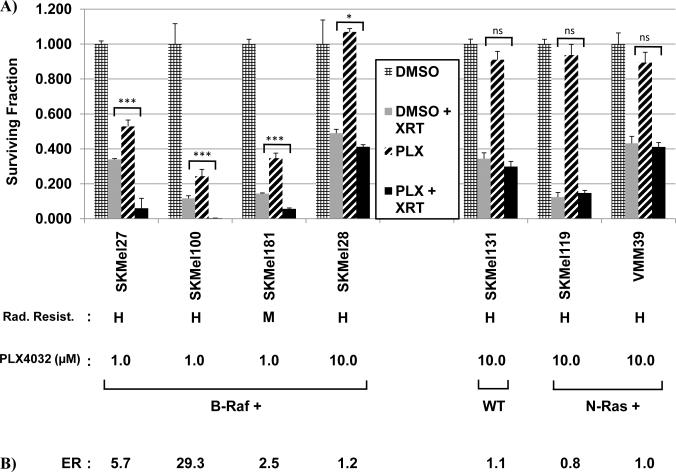
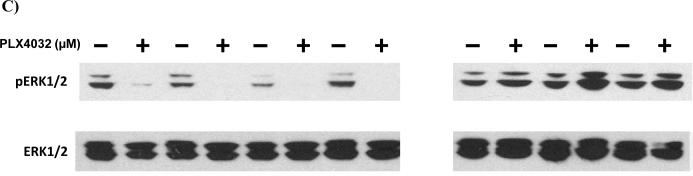
B-RAF is the most common oncogene found in malignant melanoma and many of the highly radioresistant cell lines were B-Raf+. Several B-RAF inhibitors are currently under clinical evaluation including PLX-4032 (22). We thus sought to determine whether pharmacologic inhibition of B-RAF could confer radiosensitization of melanoma cells using colony formation assays. Four highly or moderately radioresistant B-Raf+ cell lines (SKMel27, SKMel100, SKMel181, and SKMel28) were pretreated with PLX-4032 prior to irradiation and surviving fractions compared to cells incubated with DMSO alone. As controls, highly radioresistant cells genotyped as WT for B-Raf/N-Ras (SKMel131) or N-Ras+ (SKMel119 and VMM39) were also compared, as these should not be radiosensitized by PLX-4032. As shown in Fig. 3, all B-Raf+ cell lines showed statistically significant radiosensitization by PLX-4032 with a mean enhancement ratio of 9.7 (range 1.2 – 29.3) at doses that inhibited P-ERK1/2. In contrast, none of the N-Ras+ or WT cell lines were radiosensitized by PLX-4032 which exhibited a mean enhancement ratio of 0.97 (range 0.8 – 1.1). Survival curves over multiple doses of radiation are shown in Supple. Fig. 2 and likewise show radiosensitization B-Raf+, but not N-Ras+ or WT cell lines. Radiosensitization by PLX-4032 was also dose-dependent. Enhancement ratios for B-Raf+ SK-Mel-27 cells treated with either 1.0, 0.5 or 0.1 μM were 5.7, 2.5, and 1.9, respectively (data not shown). As reported by other groups for this class of drug, we likewise saw activation of P-ERK1/2 in the N-Ras+ and WT cell lines which reflects the ability of this drug class to activate c-RAF-1 in non-B-Raf + cells (26–29).
FIG. 3. PLX-4032 radiosensitizes B-Raf+ melanoma cells.
(A) B-Raf+ cells (SKMel27, SKMel100, SK-Mel-181, SK-Mel-28) were fed fresh media containing DMSO or PLX-4032 for 48 h at the indicated concentrations to achieve partial inhibition with drug alone, irradiated at 6 Gy, trypsinized 2 hr post-irradiation and replated as single cells and colonies stained and counted after 2–3 weeks incubation. Surviving fractions were calculated by comparison to DMSO control cells. Control B-Raf/N-Ras WT (SKMel131) and N-Ras+ (SKMel119 and VMM39) cells were pretreated similarly with the highest dose of PLX-4032 (10.0 μM). (B) Enhancement ratios (ER) = SF radiation alone/PLX-4032 + radiation. (C) Protein lysates were collected from cells treated with PLX-4032 for 48 h at the concentrations described in (A) and western blot analyses performed with anti-pERK1/2 or anti-ERK1/2 as a loading control to show the levels of pERK1/2 at the time of radiation.
Radiation in combination with PLX-4032 synergize to block invasion more effectively than either treatment alone
Activation of the RAF>MEK1/2>ERK1/2 pathway is known to be involved not only with proliferation and regulation of the cell cycle, but also with promoting motility and invasion (30, 31). To determine whether PLX-4032 could augment the ability of radiation treatment to block invasion, we utilized the 3D-spheroid collagen invasion assay. Here, the area of invasion by cells that migrate out from cell spheroids into the surrounding collagen was measured and the invasion fraction (IF) calculated by comparing the area of invasion of the treated spheroids relative to control, untreated spheroids. For this experiment we utilized a B-Raf+ cell line that shows robust growth characteristics in this assay. It should be noted that this cell line does not grow well at low density and could not be assessed in the colony formation assay. As shown in Supple. Fig. 3, spheroids of WM2664 cells treated with both PLX-4032 plus radiation showed a statistically significant greater inhibition (P=0.007) of cellular invasion in comparison to spheroids treated with PLX-4032 or radiation alone.
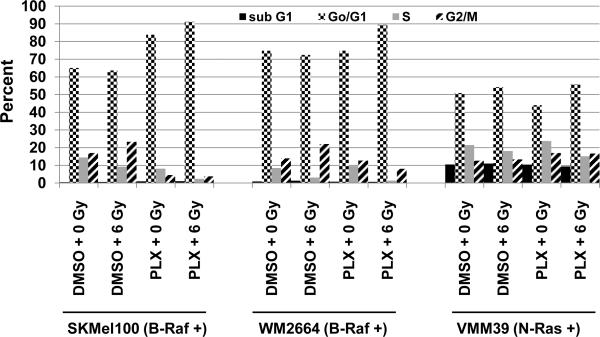
Radiosensitization by PLX-4032 is mediated by an increase in G1 arrest
To determine whether PLX-4032-mediated radiosensitization was due, in part, to alterations in the cell cycle distribution, two B-Raf+ cell lines (SKMel100 and WM2664) and one N-Ras+ line (VMM39) were pretreated with PLX-4032 with or without radiation as in Fig.3, and analyzed by flow cytometry 24 h post-irradiation. As shown in Fig. 4, both B-Raf+ cell lines showed an increase in G1 arrest when treated with PLX-4032 + radiation in comparison to radiation or drug alone and control, untreated cells. In contrast, the control N-Ras+ line showed no enhanced G1 arrest when treated with PLX-4032 + radiation relative to control, untreated cells.
FIG. 4. Radiosensitization by PLX-4032 correlates with an increase of cells in G1.
Melanoma cells were pretreated with PLX-4032 (as in Fig. 3) or DMSO for 48 h, cells irradiated with 6 Gy or sham-irradiated, and DNA content assessed by FACS analyses 24 h post-irradiation. Shown are percentages of cells in each stage of the cell cycle.
DISCUSSION
Our studies showed that melanoma cell lines display a very wide, heterogeneous range of radiosensitivities including not only radioresistant phenotypes, but also a radiosensitive phenotype which was observed in a significant number of cell lines. Comparisons of relative radiosensitivities among cells with genotypes of B-Raf+, N-Ras+ and WT for B-Raf/N-Ras suggested that while cells with genotypes of B-Raf+ and WT for B-Raf/N-Ras were more likely to be highly radioresistant than N-Ras+ cell lines, this difference did not reach statistical significance. Pharmacologic inhibition of B-RAF with PLX-4032 effectively radiosensitized B-Raf+ cells both by colony formation and invasion assays and was associated with enhancement of G1 arrest.
Previous in vitro studies with smaller numbers of melanoma cell lines also showed wide, heterogeneous ranges of radiosensitivities (32–34). Amundson et.al. recently analyzed the relative radiosensitivities of the NCI 60 panel of cancer cell lines and showed a similar heterogeneous range of radiosensitivities for several cancer subtypes often treated with adjuvant radiotherapy including those of the breast, colon and lung which exhibited similar average SF2 values (breast; 0.47, colon 0.52, lung; 0.54) (25). This study also included an analysis of 10 melanoma cell lines which showed an average SF2 of 0.58 and a range of radiosensitivity between 0.18 – 0.95 at SF2. Our analyses of 37 melanoma cell lines also showed similar values with an average of 0.51 and a larger range of radiosensitivities between 0.053 – 1.002 at SF2. In contrast, the percentage of melanoma cell lines exhibiting SF2 values ≤0.29 were found in only 1/10 (10%) of the cell lines examined in the Amundson study while in our study we found 7/37 (18.9%) of the lines to be sensitive (25). The differences between these studies are likely due to the sample size (n=10 vs. n=37). Thus, our study shows that while a majority (81%) of melanoma cells exhibit an intrinsic resistance to radiation, a significant percentage (19%) show an intrinsic sensitivity to radiation suggesting that this group of melanomas would be good candidates for radiotherapeutic control of local recurrence. In addition, melanoma cells exhibit radiosensitivity profiles similar to that of other carcinomas often treated with radiotherapy suggesting melanoma is not uniformly radioresistant. This is a concept supported not only by the early results of the TROG 02.01/ANZMTG 01.02 trial (10), but also by the use of radiotherapy in the management of melanoma brain metastases where local control rates range from about 50–85% with reported complete response rates of 14% (35–38). Critical to the treatment of the radioresistant melanomas will be the development of new and improved radiotherapeutic approaches, which will require a better understanding of the molecular pathways that drive radioresistance. The identification of these pathways could potentially be expose novel targets to radiosensitize melanoma. Future analyses of our collection of melanoma cell lines should provide the foundation to identify such molecular mechanisms of radioresistance leading to the discovery of novel radiosensitizers in melanoma.
Previous studies in both rodent and human cells have shown that activation of RAS and RAF promote radioresistance (21, 39, 40). In addition, in EGFR or HER2 positive breast cancer cells, blockade of MEK1/2>ERK1/2 signaling by pharmacologic inhibition of MEKI caused radiosensitization while expression of constitutively active RAF promoted radioresistance (18). These studies demonstrated that activation MEK1/2>ERK1/2 either by direct mutational activation of Ras or Raf or by indirect activation through upstream activators such as EGFR/HER2 results in promotion of radioresistance. While not statistically significant, our data suggest radioresistance might correlate more with B-Raf+ or B-Raf/N-Ras WT than Ras+ cells. Analyses with a larger number of WT and Ras+ cells would be required to statistically power these comparisons.
Biologic modulation of the radiation response involves the use of radiosensitizers that target signaling pathways resulting in enhanced loss of reproductive integrity or cell death. Preclinical studies using a variety of EGFR inhibitors in different model systems have shown their substantial promise as radiosensitizers (41). Cetuximab (Erbitux, ImClone Systems, Inc), a monoclonal antibody targeting EGFR, was the first biologic agent to show local control and survival advantage when used as a radiosensitizer in patients with head and neck cancer (42).
Metastatic melanoma has high propensity for both locoregional recurrence and distant metastasis and is a particularly difficult cancer to treat due to the limited systemic chemotherapeutic treatment options for this cancer. PLX-4032 is a novel, small-molecule B-RAF kinase inhibitor previously shown to inhibit P-ERK1/2 levels and growth of B-Raf+ melanoma cell lines (24). Importantly, results from a phase I trial of PLX-4032 showed tumor regression in 81% (26/32) of B-Raf+ melanoma patients treated at the MTD (23). Our studies showed that pretreatment of several B-Raf+ melanoma cell lines, including some of our most radioresistant lines, were effectively radiosensitized with PLX-4032 in both colony formation assays (average ER=8.1) and invasion assays through increased arrest in G1. The clinical relevance of our findings are important given that melanoma brain metastases are often treated with radiotherapy and that a similar B-Raf inhibitor developed by GlaxoSmithKline (GSK2118436) currently under clinical evaluation as a monotherapy has been reported in preliminary analyses to reduce the size of melanoma brain metastases in B-Raf+ patients (43). Interestingly, SK-Mel-28, despite being fairly resistant to PLX-4032 treatment alone, was slightly radiosensitized. The molecular mechanisms responsible for drug resistance to PLX-4032 in patients remains to be determined. SK-Mel-28 cells could provide an in vitro model to study this resistance.
In summary our data suggest that melanomas are not uniformly radioresistant, that a significant subset of melanomas are inherently radiosensitive and that inhibition of B-RAF with PLX-4032 effectively radiosensitizes B-Raf+ melanoma cells. This suggests that PLX-4032 or other B-RAF inhibitors in combination with radiation could provide improved radiotherapeutic response in B-Raf+ melanomas. Our study supports future clinical trials to evaluate the ability of PLX-4032 or similar pharmaceuticals that target BRAF to radiosensitize B-Raf+ melanomas.
Supplementary Material
Acknowledgements
Supported by CA115888 and ES014635, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors declare no conflicts of interest exist.
REFERENCES
- 1.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23:488–496. [PMC free article] [PubMed] [Google Scholar]
- 3.Mouawad R, Sebert M, Michels J, et al. Treatment for metastatic malignant melanoma: Old drugs and new strategies. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Guadagnolo BA, Zagars GK. Adjuvant radiation therapy for high-risk nodal metastases from cutaneous melanoma. Lancet Oncol. 2009;10:409–416. doi: 10.1016/S1470-2045(09)70043-9. [DOI] [PubMed] [Google Scholar]
- 5.Stevens G, McKay MJ. Dispelling the myths surrounding radiotherapy for treatment of cutaneous melanoma. Lancet Oncol. 2006;7:575–583. doi: 10.1016/S1470-2045(06)70758-6. [DOI] [PubMed] [Google Scholar]
- 6.Doss LL, Memula N. The radioresponsiveness of melanoma. Int J Radiat Oncol Biol Phys. 1982;8:1131–1134. doi: 10.1016/0360-3016(82)90060-8. [DOI] [PubMed] [Google Scholar]
- 7.Rofstad EK, Brustad T. Broad-shouldered survival curves of a human melanoma xenograft. Implications for radiation therapy in the absence and presence of misonidazole. Acta Radiol Oncol. 1981;20:261–265. doi: 10.3109/02841868109130204. [DOI] [PubMed] [Google Scholar]
- 8.Chang DT, Amdur RJ, Morris CG, et al. Adjuvant radiotherapy for cutaneous melanoma: comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys. 2006;66:1051–1055. doi: 10.1016/j.ijrobp.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 9.Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991;20:429–432. doi: 10.1016/0360-3016(91)90053-7. [DOI] [PubMed] [Google Scholar]
- 10.Burmeister B, Henderson M, Thompson J, et al. Adjuvant Radiotherapy Improves Regional (Lymph Node Field) Control in Melanoma Patients after Lymphadenectomy: Results of an Intergroup Randomized Trial (TROG 02.01/ANZMTG 01.02) Int J Radiat Oncol Biol Phys. 2009;75:S2. Abstract. [Google Scholar]
- 11.Cooper JS. Radiation therapy of malignant melanoma. Dermatol Clin. 2002;20:713–716. x. doi: 10.1016/s0733-8635(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 12.Testori A, Rutkowski P, Marsden J, et al. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi22–29. doi: 10.1093/annonc/mdp257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seetharamu N, Ott PA, Pavlick AC. Novel therapeutics for melanoma. Expert Rev Anticancer Ther. 2009;9:839–849. doi: 10.1586/era.09.40. [DOI] [PubMed] [Google Scholar]
- 14.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 15.Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483–6488. [PubMed] [Google Scholar]
- 16.Shields JM, Thomas NE, Cregger M, et al. Lack of extracellular signal-regulated kinase mitogen-activated protein kinase signaling shows a new type of melanoma. Cancer Res. 2007;67:1502–1512. doi: 10.1158/0008-5472.CAN-06-3311. [DOI] [PubMed] [Google Scholar]
- 17.Greshock J, Nathanson K, Medina A, et al. Distinct patterns of DNA copy number alterations associate with BRAF mutations in melanomas and melanoma-derived cell lines. Genes Chromosomes Cancer. 2009;48:419–428. doi: 10.1002/gcc.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambade MJ, Camp JT, Kimple RJ, et al. Mechanism of lapatinib-mediated radiosensitization of breast cancer cells is primarily by inhibition of the Raf>MEK>ERK mitogen-activated protein kinase cascade and radiosensitization of lapatinib-resistant cells restored by direct inhibition of MEK. Radiother Oncol. 2009;93:639–644. doi: 10.1016/j.radonc.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 20.Rajalingam K, Schreck R, Rapp UR, et al. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773:1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Grana TM, Rusyn EV, Zhou H, et al. Ras mediates radioresistance through both phosphatidylinositol 3-kinase-dependent and Raf-dependent but mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-independent signaling pathways. Cancer Res. 2002;62:4142–4150. [PubMed] [Google Scholar]
- 22.Brower V. BRAF Inhibitors: Research Accelerates in Wake of Positive Findings. J Natl Cancer Inst. 2010;102:214–215. doi: 10.1093/jnci/djq037. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amundson SA, Do KT, Vinikoor LC, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008;68:415–424. doi: 10.1158/0008-5472.CAN-07-2120. [DOI] [PubMed] [Google Scholar]
- 26.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010 doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010 doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 29.Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doehn U, Hauge C, Frank SR, et al. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol Cell. 2009;35:511–522. doi: 10.1016/j.molcel.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huntington JT, Shields JM, Der CJ, et al. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells: role of BRAF mutation and fibroblast growth factor signaling. J Biol Chem. 2004;279:33168–33176. doi: 10.1074/jbc.M405102200. [DOI] [PubMed] [Google Scholar]
- 32.Rofstad EK. Radiation biology of malignant melanoma. Acta Radiol Oncol. 1986;25:1–10. doi: 10.3109/02841868609136368. [DOI] [PubMed] [Google Scholar]
- 33.McKay MJ, Kefford RF. The spectrum of in vitro radiosensitivity in four human melanoma cell lines is not accounted for by differential induction or rejoining of DNA double strand breaks. Int J Radiat Oncol Biol Phys. 1995;31:345–352. doi: 10.1016/0360-3016(94)e0147-c. [DOI] [PubMed] [Google Scholar]
- 34.Barranco SC, Romsdahl MM, Humphrey RM. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971;31:830–833. [PubMed] [Google Scholar]
- 35.Manon R, O'Neill A, Knisely J, et al. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397) J Clin Oncol. 2005;23:8870–8876. doi: 10.1200/JCO.2005.01.8747. [DOI] [PubMed] [Google Scholar]
- 36.Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-Knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:809–816. doi: 10.1016/j.ijrobp.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Selek U, Chang EL, Hassenbusch SJ, 3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004;59:1097–1106. doi: 10.1016/j.ijrobp.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60:471–481. doi: 10.1227/01.NEU.0000255342.10780.52. discussion 481–472. [DOI] [PubMed] [Google Scholar]
- 39.Grana TM, Sartor CI, Cox AD. Epidermal growth factor receptor autocrine signaling in RIE-1 cells transformed by the Ras oncogene enhances radiation resistance. Cancer Res. 2003;63:7807–7814. [PubMed] [Google Scholar]
- 40.Pirollo KF, Hao Z, Rait A, et al. Evidence supporting a signal transduction pathway leading to the radiation-resistant phenotype in human tumor cells. Biochem Biophys Res Commun. 1997;230:196–201. doi: 10.1006/bbrc.1996.5922. [DOI] [PubMed] [Google Scholar]
- 41.Sartor CI. Mechanisms of disease: Radiosensitization by epidermal growth factor receptor inhibitors. Nat Clin Pract Oncol. 2004;1:80–87. doi: 10.1038/ncponc0048. [DOI] [PubMed] [Google Scholar]
- 42.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 43.G.V. Long GV, Kefford RF, Carr PJA, et al. GSK2118436 Phase 1/2 study of a selective inhibitor of V600 mutant Braf kinase: evidence of activity in melanoma brain metastases. Annals of Oncology. 2010:LBA27. Abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.