Abstract
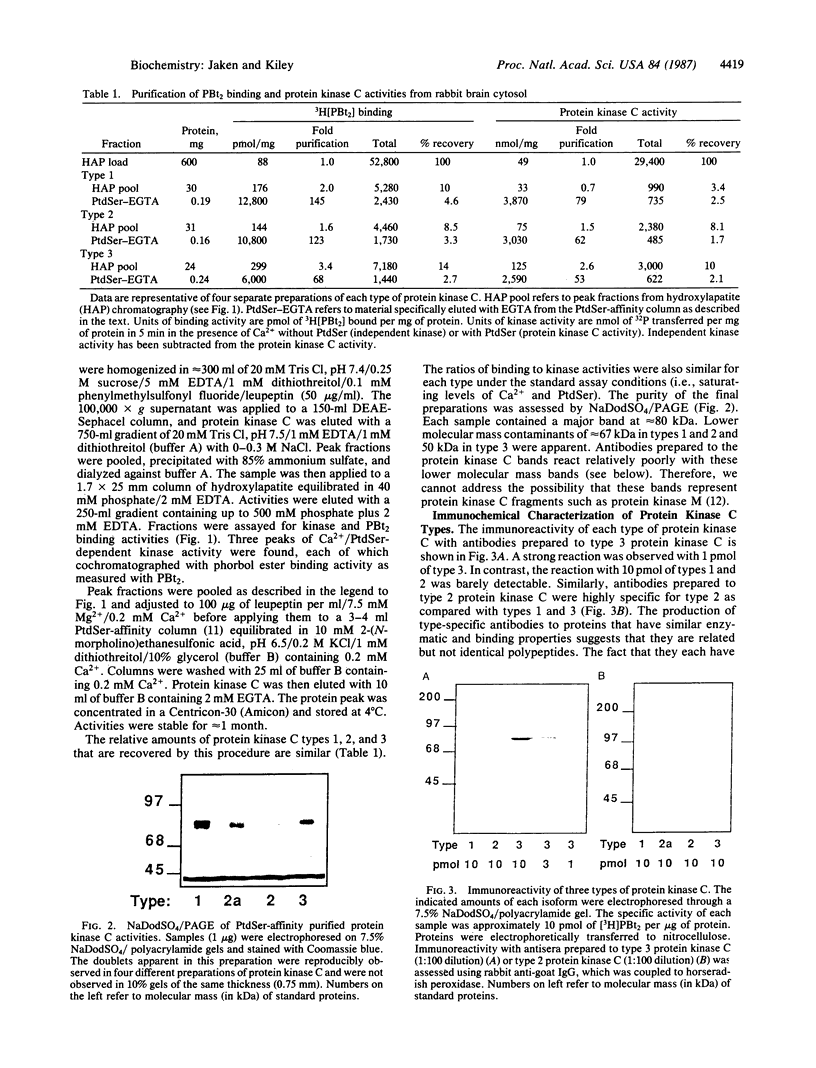
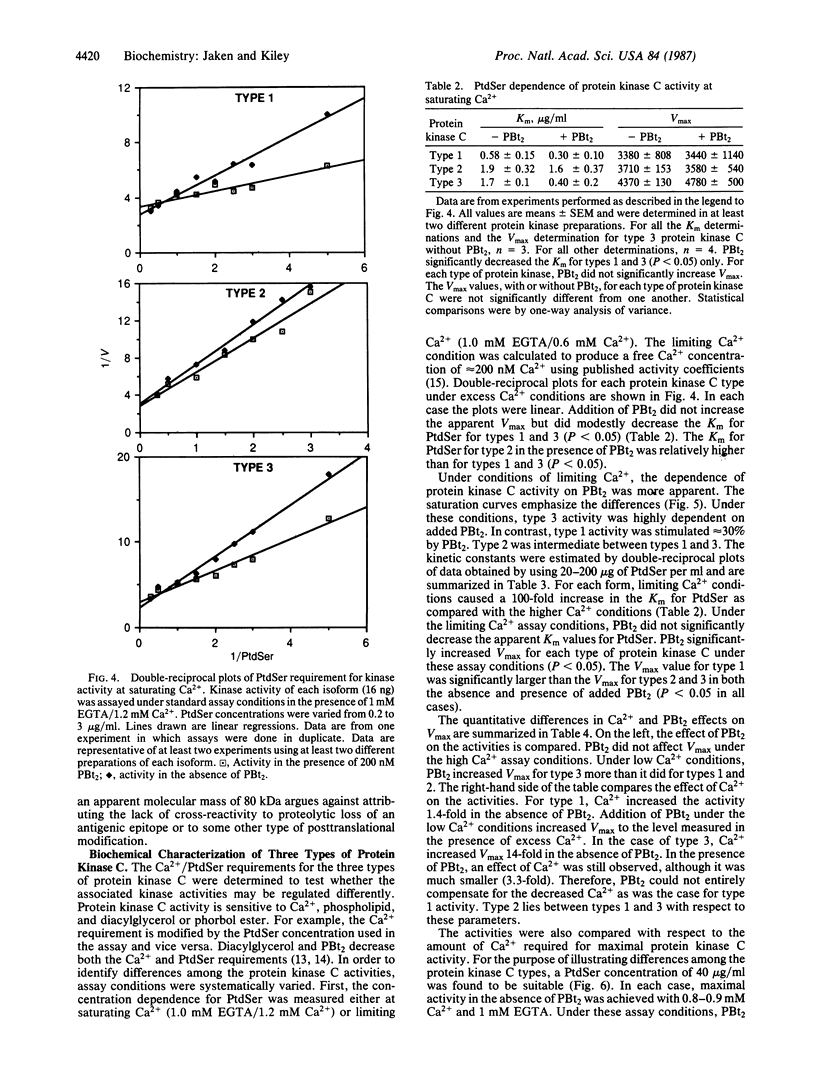
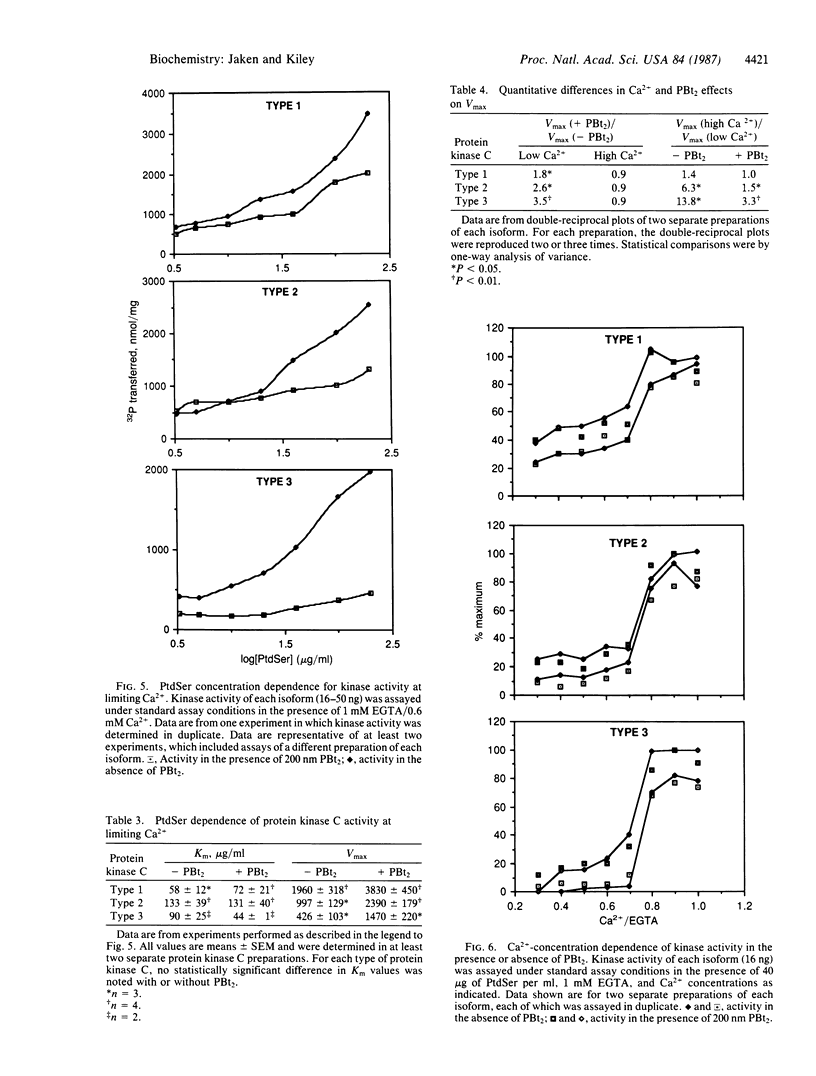
Three types of protein kinase C were purified from rabbit brain cytosol. Each type has a molecular mass of approximately 80 kDa and serves as a receptor for phorbol esters. Polyclonal antibodies produced to two protein kinase C types were relatively type-specific, indicating that these proteins have unique antigenic determinants. We, therefore, characterized the enzymatic activities to determine if these proteins also had distinct biochemical properties. Type 1 protein kinase C was relatively less Ca2+-dependent than types 2 and 3. The addition of Ca2+ increased Vmax approximately 40% for type 1,600% for type 2, and 1400% for type 3 as compared to the Vmax measured at lower Ca2+ conditions. These results suggest that differences in primary structure can confer type-specific biochemical properties, and this in turn may provide the basis for protein kinase C type-specific stimulus-response coupling.
Full text
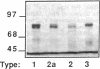
PDF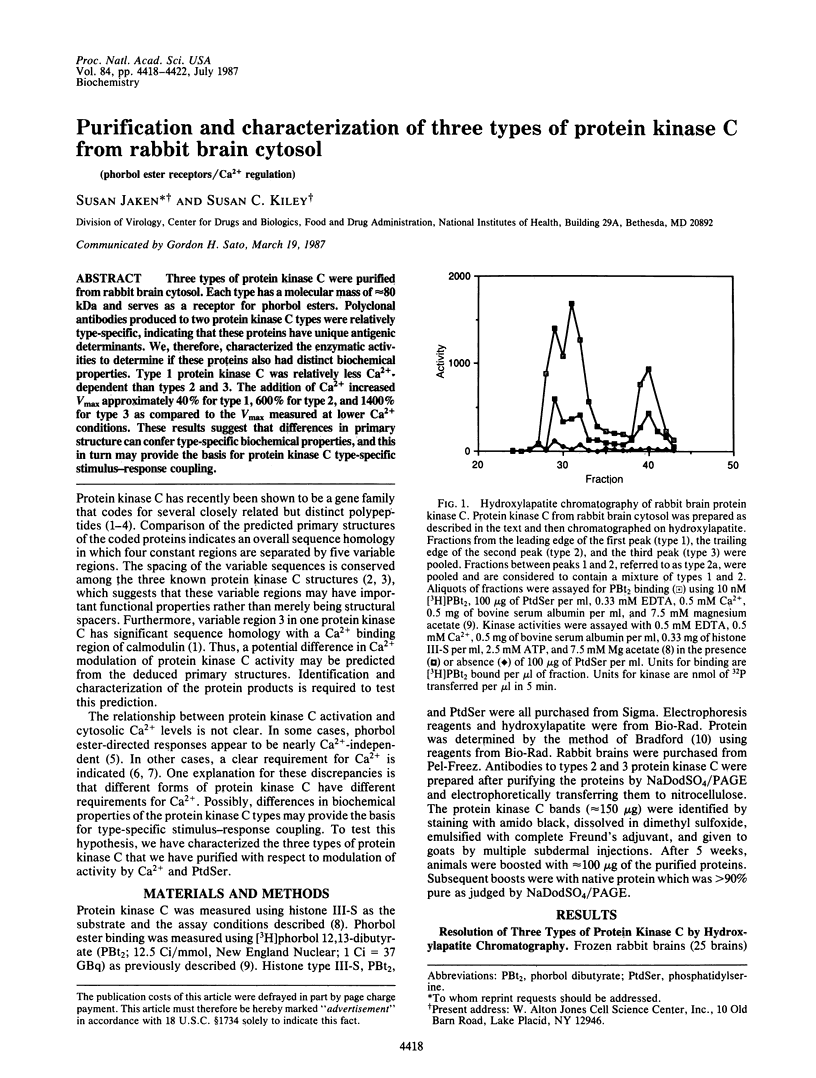

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Protein kinase C translocates from cytosol to membrane upon hormone activation: effects of thyrotropin-releasing hormone in GH3 cells. Biochem Biophys Res Commun. 1985 Apr 30;128(2):531–537. doi: 10.1016/0006-291x(85)90079-8. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Nakabayashi H., Huang F. L. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8535–8539. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Jaken S. Initial events in phorbol ester binding to GH4C1 rat pituitary cells. Endocrinology. 1985 Dec;117(6):2293–2300. doi: 10.1210/endo-117-6-2293. [DOI] [PubMed] [Google Scholar]
- Jaken S. Measurement of phorbol ester receptors in intact cells and subcellular fractions. Methods Enzymol. 1987;141:275–287. doi: 10.1016/0076-6879(87)41075-6. [DOI] [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Ono Y., Kurokawa T., Kawahara K., Nishimura O., Marumoto R., Igarashi K., Sugino Y., Kikkawa U., Ogita K., Nishizuka Y. Cloning of rat brain protein kinase C complementary DNA. FEBS Lett. 1986 Jul 28;203(2):111–115. doi: 10.1016/0014-5793(86)80724-4. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Uchida T., Filburn C. R. Affinity chromatography of protein kinase C-phorbol ester receptor on polyacrylamide-immobilized phosphatidylserine. J Biol Chem. 1984 Oct 25;259(20):12311–12314. [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]