Abstract
Cell death induction by TNF has been an intensively studied area for the last two decades. Although it may appear that the skeleton should have been picked clean by now, new secrets about TNF death signaling are still being uncovered. In particular, the recent evidence that ubiquitination of the death kinase RIP1 regulates its participation in apoptotic and necrotic cell death is opening up unexplored avenues in the catacombs of TNF death signaling. In this minireview, we focus on two major cell death checkpoints that determine whether RIP1 functions as a pro-survival or pro-death molecule.
The cytokine TNF can communicate a diverse array of inflammatory and immune gene expression programs by activating different transcription factors. TNF signaling can be translated into two opposing cell fate outcomes: cell survival or cell demise. The signaling complexes that prolong cell life or instigate cell death often form simultaneously within a single cell type. A central problem in TNF signaling has been to find out what bestows TNF with this antagonymic quality: what determines life versus death?
One of the earliest observations in the TNF signaling field was that most cell types do not die when treated with TNF. However, TNF treatment could provoke apoptosis if protein synthesis inhibitors are present, suggesting that (1) TNF must trigger expression of pro-survival genes for cells to live but (2) paradoxically, the apoptotic machinery is pre-existing and new protein synthesis is not required for cell death. Most subsequent studies of TNFR death signaling focused on the ability of inducible anti-apoptotic factors to prevent cell death. In particular, translocation of NFκB transcription factors to the nucleus to drive expression of anti-apoptotic proteins such as cFLIP, Bcl2 family members, TRAFs and cIAPs was identified as a key checkpoint in TNFR1 death signaling [1]. Blockade of NFκB activity by expression of the IκBα super-repressor [2,3] or knockout of the p65/RelA member of the NFκB family [4], sensitized cells to apoptosis when stimulated with TNF, which correlated nicely with the ability of protein synthesis inhibitors to switch the TNFR1 response from life to death. However, as originally proposed by Natoli et al [5], this presents a problem: since the apoptotic machinery is already present, why is cell death not the default pathway? Why do cells not die before protective protein synthesis has occurred? We have termed this the ‘NFκB paradox’ because NFκB-dependent synthesis of anti-death genes is insufficient to account for the dominant effect of survival in most cell types since the death machinery is pre-existing whereas the NFκB survival response is dependent on new protein synthesis. Presciently, Natoli et al predicted that there are cytoprotective mechanisms that do not require waiting for the intracellular signaling events, NFκB-dependent gene transcription program and protein synthesis processes to block cell death. A series of recent studies have exhumed the molecular details of this early NFκB-independent cytoprotective mechanism. They indicate that ubiquitination of the signaling adaptor RIP1 functions as a major cytoprotective event. Conversely, disruption of RIP1 ubiquitination converts RIP1 into a death-inducing molecule.
The kinase RIP1 was originally discovered via its interaction with the death domain of TNFR1 and Fas [6]. The acronym RIP1 reflects the striking ability of RIP1 to trigger apoptotic cell death upon overexpression in BHK cells. However, RIP1 was quickly implicated in the activation of NFκB downstream of TNFR1, appeared dispensable for apoptosis, and thus most subsequent studies focused on RIP1’s pro-survival activity. The potential role for RIP1 in triggering apoptosis downstream of TNFR1 remained buried for more than a decade.
RIP1 is recruited to the TNFR1 signaling complex within seconds of TNF stimulation and overexpression of RIP1 activates NFκB [7], which pointed to a role for RIP1 in NFκB activation by TNFR1. Conclusive evidence that RIP1 transmits the NFκB-activating signal from TNFR1 was provided by two separate studies. Ting et al [8] isolated a human Jurkat T cell clone that became non-responsive to TNF after chemical mutagenesis. This T cell mutant was found to lack RIP1 protein; reconstitution of the mutant cell line with RIP1 restored NFκB responses to TNF stimulation, proving that RIP1 is a critical requirement for optimal NFκB-mediated gene transcription by TNFR1. Likewise, various cell types isolated from RIP1 knockout mice poorly activate NFκB when treated with TNF; activation of the IKK complex can be restored in these cells by expression of RIP1 [9]. In human T cells, the absence of RIP1 had a minimal effect on apoptosis triggered by either Fas or TNFR1 [8]. In fact, fibroblasts and thymocytes from RIP1 knockout mice are more sensitive to apoptosis when treated with TNF [9,10]. These early studies revealed that RIP1 could function as a pro-survival signaling molecule, probably by activating NFκB. Much research attention was concentrated on elucidating the molecular mechanisms that permit RIP1 to activate NFκB. However, this leaves us with several enigmatic questions. In order to activate NFκB, TNFR1 rapidly recruits an adaptor molecule that is also a potent trigger of apoptosis, yet most cells do not die when stimulated with TNF. Even more surprisingly, despite being a death domain protein, RIP1 appears dispensable for the induction of apoptosis by either Fas or TNFR1. So what keeps the death promoting potential of RIP1 in check when it is bound to TNFR1 and in what circumstances is the pro-apoptotic activity of RIP1 utilized by death receptors? Closer inspection of the receptor proximal events that regulate activation of NFκB by RIP1 has unraveled part of this enigma.
RIP1 recruited to TNFR1 is rapidly and substantially modified with non-degradative polyubiquitin chains [11,12]. Modification of RIP1 with ubiquitin is coincident with recruitment of the IKK complex to TNFR1 and phosphorylation of IκBα This implied that polyubiquitination of RIP1 may regulate the activation of NFκB, particularly since emerging studies suggested that activation of kinase complexes requires non-degradative ubiquitin chains [13]. Wertz et al reported that A20, an inhibitor of NFκB activation by TNF, functions as a dual ubiquitin editing enzyme towards RIP1 [14]. The deubiquitinase domain of A20 first removes non-degradative ubiquitin chains from RIP1 and then the E3 ligase domain of A20 attaches degradative ubiquitin chains to RIP1, which targets RIP1 to the proteasome. The deubiquitinase and the E3 ligase activity of A20 are required for A20 to block activation of NFκB, which leads to the obvious hypothesis that non-degradative ubiquitination of RIP1 may mediate activation of the IKK complex. Ea et al [15] and Li et al [16] identified lysine 377 as the site of non-degradative ubiquitination on RIP1 and reported that mutation of lysine 377 abrogated the ability of RIP1 to activate NFκB. The non-degradative ubiquitin chains attached to RIP1 form a scaffold for proteins that contain ubiquitin-binding domains. RIP1-deficient T cells reconstituted with the point mutant RIP1-K377R are unable to recruit TAB2 or NEMO to the TNFR1 complex [15,16]. The NFκB essential modulator (NEMO), as its name suggests, is crucial for formation of an active IKK complex. The Chen and Ashwell laboratories characterized a ubiquitin-binding domain in NEMO (NOA/UBAN) that specifically recognizes non-degradative polyubiquitin [15,17]. The ability of NEMO to bind ubiquitin chains is required for NEMO to interact with RIP1 at TNFR1 in a stimulus-dependent manner and to activate NFκB. Competent activation of NFκB by TNF thus requires ubiquitination of lysine 377 of RIP1 and subsequent binding to the ubiquitin recognition domain of NEMO. NEMO itself can be conjugated to linear polyubiquitin chains joined head to tail by the LUBAC complex and this is required for efficient activation of NFκB [18,19]. The NOA/UBAN ubiquitin recognition domain is able to recognize linear ubiquitin linkages[20], but in the context of full-length NEMO a C-terminal ubiquitin binding zinc finger in conjunction with the NOA/UBAN confers much higher affinity for binding K63-linked polyubiquitin chains than head to tail conjugated linear polyubiquitin [21]. Since RIP1 is modified with predominantly K63-linked polyubiquitin chains [22], specific binding of NEMO to this form of ubiquitinated RIP1 is a major factor in the activation of NFκB. Not surprisingly, cells that express RIP1-K377R are more sensitive to TNF-triggered apoptosis and this was assumed to be because they are unable to instigate pro-survival NFκB-responses [15,16] though as will be discussed below, this assumption was not entirely correct.
So what enzymes carry out the non-degradative ubiquitination of RIP1? Lee et al showed that RIP1 recruited to TNFR1 is not ubiquitinated in TRAF2 knockout fibroblasts; expression of wild-type TRAF2 but not a TRAF2 mutant lacking the E3 RING domain restored polyubiquitination of RIP1 [19,23]. Similarly, overexpression of TRAF2 can trigger RIP1 ubiquitination in HEK 293 cells [14]. However, the defect in NFκB activation by TRAF2 knockout cells is modest [24] and TRAF2 is unable to directly conjugate polyubiquitin chains to RIP1 during in vitro reactions [25], which called into question whether or not TRAF2 functions as an E3 ligase for RIP1. In contrast, the IAPs readily ubiquitinate RIP1 during in vitro reactions and the loss of cIAP1 and cIAP2 abrogates ubiquitination of RIP1 in response to TNF [17,25,26]. Loss of cIAP activity leads to diminished NFκB activity [26], which corroborates the requirement for polyubiquitin chains on lysine 377 of RIP1 in NFκB responses. These groups proposed that cIAP1 and cIAP2 were most likely to be the E3 ligases responsible for non-degradative ubiquitination of RIP1 in the TNFR1 complex. However, a recent study has shed new light on this issue by revealing that the ability of TRAF2 to function as an E3 ligase and attach ubiquitin chains to RIP1 requires the lipid mediator sphingosine-1-phosphate (S1P): production of S1P is essential for TNF to activate NFκB [27]. TRAF2 and the cIAPs interact [28] and cIAP1 protein levels are kept stable by TRAF2 [29], indicating that the function of TRAF2 and the cIAPs is intimately associated. Significantly, a side-by-side comparison of the active TNFR1 complex from TRAF2 knockout and cIAP1/2 knockout fibroblasts reveals that the ubiquitination of RIP1 is impaired to the same degree in the absence of either TRAF2 or cIAP1/2 [19]. Together, these reports suggest that the concerted action of TRAF2 and the cIAPs is required for ubiquitination of RIP1 and proficient activation of the pro-survival NFκB pathway. These studies highlight the importance of non-degradative ubiquitination of RIP1 in NFκB signaling, but what effect do these ubiquitination events have on the pro-apoptotic activity of RIP1?
The initial report by Yeh et al describing the phenotype of TRAF2 knockout mice included the interesting observation that activation of NFκB was relatively normal in TRAF2 knockout fibroblasts, but they were sensitive to apoptosis when stimulated with TNF [24]. TRAF2 knockout fibroblasts undergo more apoptosis than their TRAF2 wild-type counterparts when treated with TNF, both in the absence or presence of protein synthesis inhibitors. Similarly, the TRAF2 mutant lacking the E3 RING domain works as a dominant negative protein and can trigger apoptosis in cells that lack any NFκB activity due to overexpression of the IκBαSR [30,31]. TRAF2’s E3 ligase activity, therefore, can prevent TNF from causing apoptotic cell death by a mechanism that does not depend on either new protein synthesis or the activation of NFκB. Since the ubiquitination of RIP1 is absent in TRAF2 knockout fibroblasts, we hypothesized that the non-degradative ubiquitin chains prevent RIP1 from triggering apoptosis when it is associated with TNFR1. This hypothesis leads to two predictions: (1) the cytoprotective effect of the ubiquitination of RIP1 does not require activation of NFκB and (2) apoptosis in cells that lack E3 ligase activity for RIP1 should be RIP1-dependent. To test this hypothesis, RIP1-deficient Jurkat T cells were reconstituted with either a control protein, RIP1 wild-type (RIP1-WT) or RIP1-K377R [32]. Those experiments indicated that there is no overall correlation between protection from TNF-induced apoptosis and NFκB activation [32]. What was striking in those experiments was that T cells that express RIP1-K377R, which cannot undergo ubiquitination, were very susceptible to TNF-induced apoptosis, more so than cells without RIP1. This gain-of-function in cell death by RIP1-K377R was evident even at early time points. On the other hand, T cells that express RIP1-WT, which undergoes ubiquitination, were resistant to apoptosis, more so than cells without RIP1. Those observations suggested that when RIP1 is ubiquitinated, it generates survival signals whereas when RIP1 is not ubiquitinated, RIP1 generates a death signal. Furthermore, the survival signal generated by ubiquitinated RIP1 is NFκB-independent at early time points but shifts to a NFκB-dependent manner at later time points. The molecular basis for this dichotomous behavior of RIP1 was explained by the fact that when RIP1 is ubiquitinated, it does not associate with caspase-8 whereas when it is not ubiquitinated, it rapidly associates with caspase-8 to trigger apoptosis. Moreover, blockade of the E3 ligase activity of TRAF2 in NFκB-deficient T cells, results in apoptosis that is dependent on RIP1 [32]. These observations fit the predictions of the hypothesis that non-degradative ubiquitin chains prevent RIP1 from triggering apoptosis.
So what signals might lead to a situation whereby RIP1 is not ubiquitinated and free to engage the apoptotic machinery? Such a situation can occur in cells that express both TNFR1 and TNFR2. Ligation of TNFR2 leads to degradation of TRAF2, cIAP1 and cIAP2 [33] and this correlates with the ability of TNFR2 to enhance apoptosis through TNFR1, despite elevated levels of NFκB activity [34,35]. Therefore, we postulate that physiologically the apoptosis-enhancing activity of RIP1 can be resurrected by ligation of receptors that trigger degradation of these E3 ligases. In addition to TNFR2, other members of the TNFRSF such as CD30, CD40 and TWEAK can degrade these E3 ligases [35,36]. The ability of non-ubiquitinated RIP1 to trigger apoptosis can also be unveiled by pharmacological agents. SMAC mimetics are tetrapeptides based on the amino-acid sequence from the SMAC protein that binds to IAP family members [37] and were originally designed to repress IAP inhibition of the caspases. Surprisingly, treatment of cells with SMAC mimetics prompts cIAP1 and cIAP2 to autoubiquitinate, leading to their degradation via the proteasome [25,38]. The Wang and Barker groups report that treatment of cancer cell lines with SMAC mimetics induces RIP1 to bind caspase-8 and trigger apoptosis [25,39]. The SMAC-mimetic induced complex of RIP1 and caspase-8 forms rapidly after TNFR1 ligation and triggers apoptosis, despite efficient expression of cFLIP, the main target of NFκB-dependent pro-survival activity [39]. These two studies provide additional evidence that the loss of the E3 ligases for RIP1 permits RIP1 to function as a pro-apoptotic molecule and supports our earlier work indicating that the ubiquitination of RIP1 on lysine 377 prevents RIP1 from engaging caspase-8.
So what apoptosis-inducing complex is subject to regulation by NFκB-induced pro-survival molecules such as cFLIP? Micheau and Tschopp demonstrated that ligation of TNFR1 results in the formation of two signaling complexes separated temporally and spatially [11]. The signaling molecules RIP1, TRADD, TRAF2 are recruited to the TNFR1 trimers in the plasma membrane early after receptor ligation whereas the cell death regulators FADD and caspase 8 are recruited to a pro-apoptotic complex that forms slowly in the cytoplasm. This pro-apoptotic complex II comprises several molecules that clearly have the potential to trigger apoptosis: TRADD, RIP1, FADD, caspase 8, and caspase 10. In the presence of ongoing NFκB activity, cFLIP protein is produced and translocates to complex II to prevent activation of caspase 8. If NFκB activity is blocked, cFLIP is absent and cells die by apoptosis. Surprisingly, RIP1 appears to be dispensable for complex II to trigger apoptosis whereas TRADD [40,41], FADD [42,43] and caspase 8 [44,45] are essential. Within complex II, RIP1 protein is heavily modified with what may be polyubiquitin chains; the nature of this modification, the enzymes responsible and the effect of this modification on the activity of complex II are unclear. E3 ligases that can target RIP1 for ubiquitination such as A20, cIAP1 and TRAF2 are present in complex II, therefore, it is possible that non-degradative or degradative ubiquitination of RIP1 occurs to prevent RIP1 from actively participating in apoptosis initiated by complex II. So how does this complex II, which triggers apoptosis in a RIP1-independent fashion and is subject to regulation by NFκB pro-survival factors such as cFLIP, relate to the RIP1 and caspase 8 complexes that form when RIP1 ubiquitination is blocked? Wang et al have shown that the Caspase 8 and RIP1 complex that forms upon treatment of cells with SMAC mimetic also contains FADD, but unlike complex II, apoptosis initiated by this complex is RIP1-dependent and not sensitive to inhibition by cFLIP [39]. It seems likely that the exact components of the apoptosis-inducing complexes are very different in the presence of ubiquitinated and non-ubiquitinated RIP1, for example pro-survival factors that contain ubiquitin binding domains such as the IAPs [46,47] and ABIN1 [40,48] could be recruited to complex II in the presence of ubiquitinated RIP1. Moreover, the stochiometry of the caspase 8 complexes may be altered by the presence of ubiquitinated RIP1 and thus caspase 8 and FADD may be activated in a very different manner in the context of RIP1-independent and dependent apoptosis. In summary, RIP1 does not normally participate in apoptosis initiated by complex II but RIP1 may enhance apoptosis triggered by complex II when the ubiquitination status of RIP1 is blocked. In the absence of ubiquitination, RIP1 interacts with caspase-8 and enhances apoptosis, but how does non-degradative ubiquitination restrain RIP1 from binding caspase-8?
A key downstream molecule that contributes to the NFκB-independent protective effect of RIP1 ubiquitination is, ironically, recruitment of the NFκB essential modulator NEMO. Enhanced sensitivity to apoptosis in NEMO-deficient cells has been attributed to loss of pro-survival NFκB-mediated gene transcription [49]. However, a more thorough post-mortem of apoptosis in NEMO-deficient T cells reveals that they are more susceptible to TNF-mediated cell death than T cells rendered sensitive by NFκB-blockade [50]. The apoptosis of NEMO-deficient T cells is abrogated by knockdown of RIP1. Therefore, we proposed the model that binding of NEMO to ubiquitinated RIP1 prevents RIP1 from interacting with caspase-8. Consistent with the model, reconstitution of NEMO-deficient T cells with NEMO mutants that are unable to bind polyubiquitin chains did not prevent apoptosis whereas reconstitution with the wildtype NEMO prevented apoptosis. Therefore, NEMO must bind to ubiquitinated RIP1 in order to restrain RIP1 from binding caspase-8, and this pro-survival activity of NEMO does not require activation of NFκB.
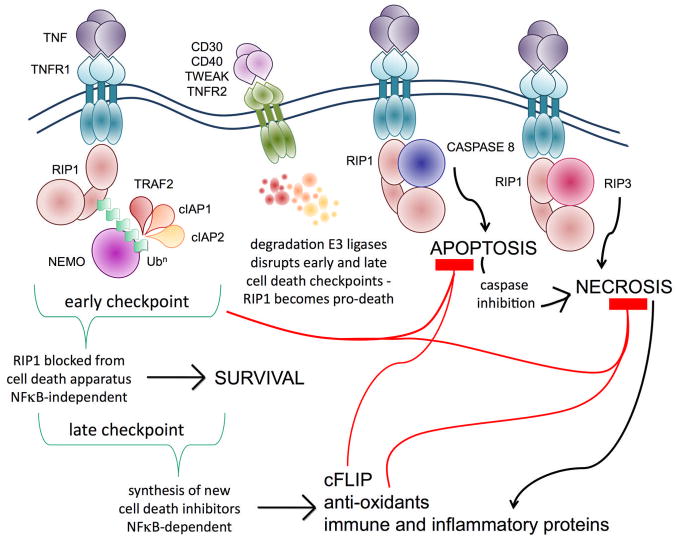
The combination of these studies suggests a model whereby there are two major cell death checkpoints in TNFR death signaling controlled by RIP1. The ubiquitination of RIP1 and recruitment of NEMO functions as the first pro-survival checkpoint at early time-points after TNFR1 ligation because this restrains the apoptosis-inducing property of RIP1 by sequestering it from caspase-8. This early cytoprotective effect does not require NFκB-driven gene transcription or the synthesis of new anti-apoptotic factors. However, this same interaction between NEMO and ubiquitinated RIP1 subsequently leads to the activation of NFκB. NFκB-dependent gene transcription acts as the second cell death checkpoint to inhibit apoptosis at later time-points and this delayed protection from apoptosis does depend on the synthesis of new proteins such as cFLIP. Disruption of the first checkpoint results in rapid entry of cells into apoptosis mediated by RIP1 binding caspase-8. This model reconciles the pro-survival and pro-apoptotic activities of RIP1 and provides a framework for understanding why physiological triggers such as TNFR2 or TWEAK ligation predispose cells to undergo apoptosis when stimulated through TNFR1 (Figure 1). These studies exhumed interest in the pro-apoptotic function of RIP1, but leaves open the question if the early cell death checkpoint also regulates a cell death process in which RIP1 is a notorious executioner: programmed necrosis.
Figure 1. Control of RIP1’s pro-life and pro-death activity by ubiquitination resolves the NFκB paradox and the co-operation of TNFR signaling in immunity.
There are two cell death checkpoints in the TNFR1 pathway. In the first checkpoint, ubiquitination of RIP1 by the E3 ligases TRAF2, cIAP1 and cIAP2 hinders RIP1 from binding cell death molecules early after TNF stimulation. This checkpoint does not require new protein synthesis. In the second checkpoint, NFκB activation by ubiquitinated RIP1 leads to the production of new proteins that can block cell death, keeping cells alive in the long-term, which explains why cell survival is the default outcome of TNFR1 signaling. However, when cell death is desirable, the early checkpoint can be rapidly altered by ligation of the other TNFR family members, which trigger degradation of the E3 ligases. Non-ubiquitinated RIP1 becomes a pro-death signaling molecule and quickly binds caspase-8 to initiate apoptosis. If caspase activity is blocked, RIP1 binds RIP3 and triggers the back-up cell death pathway by programmed necrosis. Ubiquitination controls the switch between RIP1’s pro-life and pro-death activity: this resolves the NFκB paradox – ubiquitination of RIP1 prevents TNFR1 from triggering cell death until an effective pro-survival gene expression program has been implemented.
NFκB activated by TNFR1 drives expression of immune and inflammatory genes during responses to pathogens. Some pathogens attempt to block NFκB and dampen down inflammation, in order to prolong the survival of infected cells. The other TNFRs like TNFR2 can unmask the pro-death activity of RIP1 and trigger pro-inflammatory necrotic death. This co-operation between the TNFRs allows TNFR1 to circumvent the immune evasion strategies used by pathogens to block NFκB activity or apoptosis.
TNFR2 colludes with TNFR1 to trigger apoptosis, but caspase inhibitors are unable to prevent TNF-mediated cell death in this situation. Instead, the cells switch from caspase-dependent apoptosis to a cell death program with necrotic morphology [51]. The kinase activity of RIP1 has been known for a long time to be essential for the ability of TNF to trigger necrotic cell death when caspases are inhibited [52–54]. The degradation of the E3 enzymes TRAF2, cIAP1 and cIAP2 triggered by TNFR2 correlates with the induction of RIP1-dependent programmed necrosis. Therefore, it is possible that the non-degradative ubiquitination of RIP1 and subsequent binding of NEMO may also hinder RIP1 from functioning as a pro-necrotic signaling molecule. Indeed TRAF2/TRAF5 knockout cells undergo necrotic cell death when treated with TNF in the presence of caspase inhibitors [55]. If we disrupt the early cell death checkpoint, for example by mutating lysine 377 of RIP1 to arginine, or blocking the activity of TRAF2 and the cIAPs, T cells become sensitized to cell death by programmed necrosis when stimulated with TNF in the presence of caspase inhibitors. Similarly, binding of NEMO to ubiquitinated RIP1 inhibits programmed necrosis and, like the anti-apoptotic effect of this early checkpoint, this does not require NFκB activity [56]. TRADD is required for recruitment of TRAF2 to TNFR1 and subsequent ubiquitination of RIP1 [40]. Knockdown of TRADD enhances necrosis in Caspase 8 deficient T cells [57], which suggests that TRADD may inhibit necrosis by orchestrating the ubiquitination of RIP1. Similarly, expression of a FADD dominant negative [58] or FADD deficiency [51] can greatly sensitise cells to necrosis and this correlates with loss of the ubiquitin-like modification of RIP1 in complex II when FADD is deficient or inhibited [11]. The mechanism by which FADD may contribute to RIP1 ubiquitination in complex II remains to be investigated. These studies of TRADD and FADD suggest that some of the molecules that inhibit necrosis by activating caspase 8 may have additional necrosis-blocking activity by contributing to RIP1 ubiquitination. RIP1 functions as a pro-apoptotic molecule by binding caspase-8, but how does it function as a pro-necrotic molecule? Three recent studies have illuminated the molecular mechanism utilized by TNFRs to trigger cell death by necrosis [59–61]. The Wang and Chan groups identified by siRNA screens the kinase RIP3 as a crucial downstream mediator of programmed necrosis. Interestingly, in order to trigger necrotic cell death, both groups utilize cell death stimuli that we predict would disrupt the early cell death checkpoint. He et al [59] found that SMAC mimetics can trigger programmed necrosis in cell lines that express RIP3 when stimulated with TNF during caspase blockade. Likewise, Cho et al [61] describe how ligation of TNFR2 during infection with vaccinia virus, which encodes the Spi2 caspase inhibitor, requires RIP3 to induce necrotic cell death. In both scenarios, the ubiquitination of RIP1 should be defective. SMAC mimetics and ligation of TNFR2 can both activate NFκB, so this opens up the question of whether or not the late checkpoint mediated by NFκB offers any reprieve from a death sentence by programmed necrosis.
A few studies have pointed to a protective gene expression program mediated by NFκB and other transcription factors that can reduce cell death by necrosis. In cell types such as fibroblasts, the cell death process during programmed necrosis requires the production of reactive oxygen species [54,62]. Fibroblasts from p65/RelA knockout mice produce reactive oxygen species when stimulated with TNF in the presence of caspase blockade and undergo cell death by necrosis [55]. Soaking up the reactive oxygen species with powerful pharmacological antioxidants can prevent this necrotic cell death. NFκB can drive expression of many proteins with antioxidant activity such as ferritin, manganese superoxide dismutase and glutathione-s-transferase [63–65], which suggests that the second cell death checkpoint may block programmed necrosis. The main cellular scavenger for free radicals is catalase and degradation of this enzyme during autophagy is associated with the necrotic cell death of mouse fibrosarcoma cells [66]. In addition, it should be remembered that the pro-survival function of NFκB was originally attributed to the inducible expression of genes such as TRAF2, cIAP1 and cIAP2 [1], which suggests that NFκB activity might be important for maintaining expression of the proteins that control the early cell death checkpoint. Whether or not cFLIP, the main target responsible for NFκB anti-apoptotic activity [67], can prevent programmed necrosis has not been fully addressed. Viral FLIPs such as MCF159 from molluscum contagiosum virus or K13 from Kaposi’s sarcoma herpesvirus block necrotic cell death [51] and knockdown of cFLIP can sensitize HeLa cells to both TNF-induced apoptosis and necrosis [68]. Therefore, the contribution of NFκB-dependent gene transcription programs to protection from necrotic cell death remains to be fully clarified.
It is clear from in vitro studies that the second cell death checkpoint, i.e., NFκB activity, can prevent both apoptosis and necrosis, which implies that there must be a physiological situation in which loss of NFκB activity induces cell death as a favorable outcome. NFκB activation by TNF is required for expression of immune and inflammatory genes that are important for host responses to pathogens. Not surprisingly, there are numerous examples of viral and bacterial pathogens that trigger cell death when NFκB is inhibited, for example by YopJ during Yersinia infection [69] and herpes simplex virus triggered apoptosis requires loss of NFκB signaling [70]. However, many viruses activate NFκB in order to control their own replication and promote cell survival. In such an event, it may be advantageous to the host to possess the capability to disrupt the first cell death checkpoint in order to trigger apoptosis or necrosis of the infected cells. Induction of programmed necrosis in virally-infected cells by disruption of the first death checkpoint may be particularly advantageous to the host as this type of death may be more immunogenic. Chan and colleagues have shown that TNFR2 is required for necrotic cell death to occur during infection with vaccinia virus, which encodes a potent inhibitor of apoptotic and inflammatory caspases [51]. In the context of vaccinia virus infection, the function of virus-triggered programmed necrosis is clearly to enable removal of infected cells and a pro-inflammatory response to be initiated that circumvents the virus immune evasion strategy [51,61]. The increasing number of pathogen components that have been reported to block necrotic cell death [71] underscores the importance of programmed necrosis to the development of protective immunity. Cell survival at both checkpoints requires NEMO-binding to ubiquitinated RIP1. NEMO is a critical requirement for the activation of several kinase complexes that mediate immune responses to pathogens, from activation of NFκB to IRF3 [72,73] and thus required for cytokine and interferon production. It is likely that pathogens may try to downregulate NEMO protein levels in an attempt to evade this response. However, pathogen-mediated loss of NEMO should result in disruption of the first cell death checkpoint rendering infected cells sensitive to programmed necrosis and the ensuing immunogenic consequences of necrotic death may serve as a backup host defense mechanism. Support for this idea has come from a recent report that an E3 ligase encoded by Shigella induces degradation of NEMO and blocks NFκB-mediated immune gene expression programs [74]. Other groups have shown that Shigella infection of certain cell types leads to necrotic cell death [75,76]. Therefore, mammalian cells may have evolved the two cell death checkpoints in the TNFR1 pathway in order to rapidly respond to interference with NEMO’s pro-survival and immune functions (Figure 1).
So what is the broader mechanism that enables NEMO-binding to ubiquitinated RIP1 to prevent both apoptosis and programmed necrosis so effectively? The fact that TNFR2 predisposes cells to undergo apoptosis or necrosis when stimulated with TNF despite the requirement for two very different sets of executioner proteins suggests that a common overall mechanism underlies the inhibition of RIP1-dependent cell death processes by NEMO. There are several possible effects of NEMO binding to ubiquitinated RIP1 that we envisage may restrain RIP1 from engaging different cell death apparatus. The simplest explanation is that ubiquitination of RIP1 at lysine 377 and the recruitment of ubiquitin binding proteins such as NEMO may sterically hinder RIP1 from interacting with downstream death mediators such as caspase-8 or RIP3, which do not have ubiquitin binding domains. Ubiquitination of RIP1 may also prevent RIP1 oligomerisation, which has been suggested to play a role in its ability to trigger cell death [77]. Oligomerisation of the death domain of RIP1 requires the presence of an alpha-helix that is immediately adjacent to the lysine 377 acceptor site of RIP1. This alpha-helix region also contains the RIP homologous interaction domain (RHIM) required for interaction with RIP3. Therefore, it is conceivable that oligomerisation of RIP1 might be required for RIP1 to bind or activate either caspase-8 or RIP3 and that extensive modification of the intermediate domain by ubiquitin chains would prevent these structures from forming. Ubiquitination of plasma membrane receptors is a well-known signal that leads to receptor internalization. Endocytosis of the interferon alpha receptor requires phosphorylation and ubiquitination on a motif that is very similar to the degradation motif of IκBα targeted by the IKK complex [78]. As reported by Schutze et al, internalization of TNFR1 is important for the activation of downstream signaling pathways, particularly cell death [79]. Therefore, ubiquitination of RIP1 when it is recruited to TNFR1 might modulate the internalization or subcellular trafficking of the TNFR1 complex, which is another mechanism by which access of RIP1 to different cell death machinery might be regulated. Alternatively, NEMO binding to RIP1 may regulate the activity of kinase complexes such as IKKε or IKKα/β that specifically phosphorylate and inhibit the activity of cell death mediators, prior to the activation of gene expression programs by these kinase complexes. Reiley et al have shown that NEMO is required for the IKK complex to phosphorylate and inhibit the deubiquitinase CYLD [80], a critical component of the necrotic machinery [81], as can the TNF-inducible IKKε [82]. As alluded to earlier, activation of these kinases may also influence internalization of the TNFR1 complex itself.
In conclusion, there are two cell death checkpoints downstream of TNFR1 that determine whether cells live or die. Pro-survival effects from the first checkpoint that do not require gene expression can be as potent as the pro-survival impact of new proteins synthesized after the second checkpoint. A thorough examination of the molecular mechanisms that regulate the pro-survival activity of NEMO binding to ubiquitinated RIP1 should reveal new strategies to instigate or prevent cell death when appropriate in a clinical setting.
Acknowledgments
ATT is supported by NIH grant AI052417. MAO’D is a recipient of a Research Fellowship Award from the Crohn's and Colitis Foundation of America.
Abbreviations
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- RIP1
receptor-interacting protein 1
- cFLIP
cellular FLICE-like inhibitory protein
- Bcl2
B-cell CLL/lymphoma 2
- TRAF
TNF receptor-associated factor
- cIAP
cellular inhibitor of apoptosis protein
- IκBα
inhibitor of kappaB alpha
- NFκB
nuclear factor kappaB
- NEMO
NFκB essential modulator
- IKK
IκB kinase
- TAB2
TAK1-binding protein 2
- SMAC
second mitochondria-derived activator of caspase
- TRADD
TNFR1-associated via death domain
- FADD
FAS-associated via death domain
- ABIN1
A20-binding inhibitor of NFκB
- RIP3
receptor-interacting protein 3
References
- 1.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 2.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–76. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 3.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 4.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–4. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 5.Natoli G, Costanzo A, Guido F, Moretti F, Levrero M. Apoptotic, non-apoptotic, and anti-apoptotic pathways of tumor necrosis factor signalling. Biochem Pharmacol. 1998;56:915–20. doi: 10.1016/s0006-2952(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 6.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–23. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 7.Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–96. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 8.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–96. [PMC free article] [PubMed] [Google Scholar]
- 9.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 10.Cusson N, Oikemus S, Kilpatrick ED, Cunningham L, Kelliher M. The death domain kinase RIP protects thymocytes from tumor necrosis factor receptor type 2-induced cell death. J Exp Med. 2002;196:15–26. doi: 10.1084/jem.20011470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 12.Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18:655–64. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 13.Deng L, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–61. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 14.Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–9. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 15.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–57. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J Biol Chem. 2006;281:13636–43. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- 17.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 18.Tokunaga F, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–32. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 19.Haas TL, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–44. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Rahighi S, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136:1098–109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Laplantine E, Fontan E, Chiaravalli J, Lopez T, Lakisic G, Veron M, Agou F, Israel A. NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain. EMBO J. 2009;28:2885–95. doi: 10.1038/emboj.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–78. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279:33185–91. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 24.Yeh WC, et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–25. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand MJ, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–9. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 465:1084–8. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Kabaleeswaran V, Wang Y, Cheng G, Wu H. Crystal structures of the TRAF2: cIAP2 and the TRAF1: TRAF2: cIAP2 complexes: affinity, specificity, and regulation. Mol Cell. 38:101–13. doi: 10.1016/j.molcel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csomos RA, Brady GF, Duckett CS. Enhanced cytoprotective effects of the inhibitor of apoptosis protein cellular IAP1 through stabilization with TRAF2. J Biol Chem. 2009;284:20531–9. doi: 10.1074/jbc.M109.029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natoli G, Costanzo A, Guido F, Moretti F, Bernardo A, Burgio VL, Agresti C, Levrero M. Nuclear factor kB-independent cytoprotective pathways originating at tumor necrosis factor receptor-associated factor 2. J Biol Chem. 1998;273:31262–72. doi: 10.1074/jbc.273.47.31262. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Kaufman DR, Mora AL, Santana A, Boothby M, Choi Y. Stimulus-dependent synergism of the antiapoptotic tumor necrosis factor receptor-associated factor 2 (TRAF2) and nuclear factor kappaB pathways. J Exp Med. 1998;188:1381–4. doi: 10.1084/jem.188.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–24. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–7. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 34.Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–60. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Fotin-Mleczek M, et al. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757–70. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- 36.Vince JE, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol. 2008;182:171–84. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 38.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 40.Ermolaeva MA, Michallet MC, Papadopoulou N, Utermohlen O, Kranidioti K, Kollias G, Tschopp J, Pasparakis M. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol. 2008;9:1037–46. doi: 10.1038/ni.1638. [DOI] [PubMed] [Google Scholar]
- 41.Pobezinskaya YL, Kim YS, Choksi S, Morgan MJ, Li T, Liu C, Liu Z. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008;9:1047–54. doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 43.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 44.Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol. 1998;8:1001–8. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- 45.Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 46.Blankenship JW, et al. Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2(1) Biochem J. 2009;417:149–60. doi: 10.1042/BJ20081885. [DOI] [PubMed] [Google Scholar]
- 47.Gyrd-Hansen M, et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat Cell Biol. 2008;10:1309–17. doi: 10.1038/ncb1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oshima S, et al. ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature. 2009;457:906–9. doi: 10.1038/nature07575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–62. [PMC free article] [PubMed] [Google Scholar]
- 50.Legarda-Addison D, Hase H, O'Donnell MA, Ting AT. NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 2009;16:1279–88. doi: 10.1038/cdd.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan FK, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–21. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 52.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 53.Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol. 2000;74:7470–7. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Y, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–8. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 55.Sakon S, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hase H, O'Donnell Marie Anne, Legarda-Addison Diana, Skountzos Penelopi, Ting Adrian T. NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1. 2010 doi: 10.1371/journal.pone.0041238. manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng L, Bidere N, Staudt D, Cubre A, Orenstein J, Chan FK, Lenardo M. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol. 2006;26:3505–13. doi: 10.1128/MCB.26.9.3505-3513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanden Berghe T, van Loo G, Saelens X, Van Gurp M, Brouckaert G, Kalai M, Declercq W, Vandenabeele P. Differential signaling to apoptotic and necrotic cell death by Fas-associated death domain protein FADD. J Biol Chem. 2004;279:7925–33. doi: 10.1074/jbc.M307807200. [DOI] [PubMed] [Google Scholar]
- 59.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 61.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–85. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasazuki T, et al. Genome wide analysis of TNF-inducible genes reveals that antioxidant enzymes are induced by TNF and responsible for elimination of ROS. Mol Immunol. 2004;41:547–51. doi: 10.1016/j.molimm.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 64.Pham CG, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–42. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Wong GH, Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–4. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 66.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci U S A. 2006;103:4952–7. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeh WC, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–42. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima A, Kojima Y, Nakayama M, Yagita H, Okumura K, Nakano H. Downregulation of c-FLIP promotes caspase-dependent JNK activation and reactive oxygen species accumulation in tumor cells. Oncogene. 2008;27:76–84. doi: 10.1038/sj.onc.1210624. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Ting AT, Marcu KB, Bliska JB. Inhibition of MAPK and NF-kappa B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J Immunol. 2005;174:7939–49. doi: 10.4049/jimmunol.174.12.7939. [DOI] [PubMed] [Google Scholar]
- 70.Goodkin ML, Ting AT, Blaho JA. NF-kappaB is required for apoptosis prevention during herpes simplex virus type 1 infection. J Virol. 2003;77:7261–80. doi: 10.1128/JVI.77.13.7261-7280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 7:302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 73.Zhao T, Yang L, Sun Q, Arguello M, Ballard DW, Hiscott J, Lin R. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
- 74.Ashida H, Kim M, Schmidt-Supprian M, Ma A, Ogawa M, Sasakawa C. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat Cell Biol. 12:66–73. doi: 10.1038/ncb2006. sup pp 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galluzzi L, Kroemer G. Shigella targets the mitochondrial checkpoint of programmed necrosis. Cell Host Microbe. 2009;5:107–9. doi: 10.1016/j.chom.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Carneiro LA, et al. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe. 2009;5:123–36. doi: 10.1016/j.chom.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Grimm S, Stanger BZ, Leder P. RIP and FADD: two “death domain”-containing proteins can induce apoptosis by convergent, but dissociable, pathways. Proc Natl Acad Sci U S A. 1996;93:10923–7. doi: 10.1073/pnas.93.20.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J Biol Chem. 2004;279:46614–20. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- 79.Schneider-Brachert W, et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–28. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 80.Reiley W, Zhang M, Wu X, Granger E, Sun SC. Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma-dependent phosphorylation. Mol Cell Biol. 2005;25:3886–95. doi: 10.1128/MCB.25.10.3886-3895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–23. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hutti JE, Shen RR, Abbott DW, Zhou AY, Sprott KM, Asara JM, Hahn WC, Cantley LC. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol Cell. 2009;34:461–72. doi: 10.1016/j.molcel.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]