Abstract
Feline immunodeficiency virus (FIV), like human immunodeficiency virus (HIV)-1, is a neurotropic lentivirus, and both natural and experimental infections are associated with neuropathology. FIV enters the brain early following experimental infection, most likely via the blood-brain and blood-cerebrospinal fluid barriers. The exact mechanism of entry, and the factors that influence this entry, are not fully understood. As FIV is a recognised model of HIV-1 infection, understanding such mechanisms is important, particularly as HIV enters the brain early in infection. Furthermore, the development of strategies to combat this central nervous system (CNS) infection requires an understanding of the interactions between the virus and the CNS. In this review the results of both in vitro and in vivo FIV studies are assessed in an attempt to elucidate the mechanisms of viral entry into the brain.
Keywords: Feline immunodeficiency virus (FIV), Human immunodeficiency virus (HIV), Neuropathology, Blood-brain barrier, CNS
Introduction
Feline immunodeficiency virus (FIV) is a member of the retroviridae, as are the other immunodeficiency-inducing lentiviruses, human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV). FIV was first isolated in 1987 from a group of domestic cats suffering from an acquired immune deficiency syndrome (AIDS)-like condition (Pedersen et al., 1987). However, based on phylogenetic analysis, it is likely that FIV may represent a more primitive lentivirus which has been prevalent in cat populations even longer than HIV has been present in humans (Sodora et al., 1994). In addition, FIV appears to have evolved independently of both HIV and SIV (Olmsted et al., 1989; Elder and Phillips, 1993).
FIV resembles HIV and SIV in its structure and biochemical properties, but is not antigenically related to either virus. FIV does not infect humans or human cells (Poeschla and Looney, 1998), although a chimeric clone was shown to establish pro-virus in human MOLT4 cells, without infectious virions being produced (Ikeda et al., 1996). FIV has been sub-divided into five clades (A–E) based on sequence similarities in the V3-V5 regions of envelope proteins (Sodora et al., 1995). The virus is mainly transmitted by biting during fighting and sexual activity, and therefore its prevalence worldwide is at least twice as high in males as in females (Bendinelli et al., 1995). Vertical transmission has also been described during acute maternal infection, leading to an overall infection rate of 70% (O’Neil et al., 1995).
Experimental infection with FIV results in early, acute infection characterised by pyrexia, dullness, leucopenia and is associated with an inversion of the CD4+/CD8+ T cell ratio, followed by a progressive decline in circulating CD4+ T cells (Ackley et al., 1990; Tompkins et al., 1991; Callanan et al., 1992) and a sustained increase in CD8+ T cells (Willett et al., 1993; Bucci et al., 1998; Gebhard et al., 1999). A prolonged subclinical phase then occurs (Diehl et al., 1995) and in many specific-pathogen free environments, this phase of infection continues beyond the time-span of many studies, although one long-term experiment reported 6/24 cats progressing to the AIDS stage of disease between 3.8 and 5.8 years post-infection (Mathiason-DuBard et al., 1998). However, in many naturally-infected animals, severe immunodeficiency develops with accompanying opportunist infections (Brown et al., 1991; English et al., 1994).
Although most primary isolates of FIV will infect T lymphocytes, B lymphocytes and monocytes, productive infection in vivo is largely restricted to T lymphocytes (Dow et al., 1992; Beebe et al., 1994; Dow et al., 1999). FIV is considered a more lymphotropic lentivirus than HIV which can either be lymphotropic or, more commonly, macrophage-tropic, with both strain types existing simultaneously in the HIV-1 infected patient (Moses and Nelson, 1994; Kramer Hammerle et al., 2005; Willett et al., 2008). The primary receptor for FIV has been identified as CD134 (Shimojima et al., 2004) in contrast to CD4 which is the primary receptor used by HIV (Gonzalez-Scarano and Martin-Garcia, 2005). However, CD134 is thought to support infectious interactions in much the same manner as CD4. Both HIV and FIV lymphotropic-strains use CXCR4 as a co-receptor, and there is close homology between feline and human CXCR4. Following ectopic expression of human CXCR4 on non-permissive human cells, these cells can fuse with FIV-infected feline cells. Moreover, fusion between FIV-infected feline cells and CXCR4-transfected human cells is inhibited by both anti-CXCR4 and anti-FIV antibodies (Willett et al., 1997). In contrast, SIV exclusively uses CCR5 as a co-receptor. However, all three viruses have tropisms for similar cell populations including cells of monocyte lineage (Willett et al., 1997, 1998; Egberink et al., 1999).
FIV is neurotropic and neurovirulent
Like all lentiviruses, FIV invades the central nervous system (CNS) (Power et al., 2004) and has been detected in the brain and cerebrospinal fluid (CSF) at the time of acute infection. In one study using FIV Glasgow-8 strain (FIVGL8), virus was observed in animals as early as 1 week after infection and in 9/12 animals by 23 weeks post-infection (Ryan et al., 2003). In HIV infection, the rapidity of viral entry to the brain was first demonstrated in a patient 15 days after accidental inoculation with a HIV-1-contaminated product (Davis et al., 1992). This early penetration of virus into the brain and CSF suggests the existence of efficient transmission mechanisms across the blood-brain barrier (BBB) and blood-CSF barrier.
The first reports of the neurological effects of natural FIV infection in cats highlighted the altered behaviour of the animals (Pedersen et al. 1987). Subsequently, it was shown that experimental infection with FIV Petaluma could induce CSF pleocytosis with intra-thecal anti-FIV antibody production (Dow et al., 1990). At the termination of this experiment, 12 months after infection, virus was isolated from sub-cortical brain structures, and encephalitis was reported. Antibodies to FIV were detected in the CSF of 9/10 naturally-infected cats, and the virus was cultured from the CSF of 5/9 of these animals (Dow et al., 1990).
Since these initial observations with the original Petaluma isolate, several other FIV strains have been reported to cause neuropathology in domestic cats including: FIVMD (Phillips et al., 1994; Podell et al., 1999); FIVPPR (Phillips et al., 1996, 2000); FIVNCSU1 (Meeker et al., 1997; Bragg et al., 2002); FIVGL8 (Ryan et al., 2003); and the CSF-derived isolate, FIVV1CSF (Power et al., 1998). Furthermore, many of these viruses have induced neurological signs including abnormal stereotypic motor behaviours, increased aggression, anisocoria, delayed righting and papillary light reflexes, marked changes in sleep architecture and deficits in cognitive motor function (Podell et al., 1993, 1997; Phillips et al., 1994, 1996; Prospero-Garcia et al., 1994; Steigerwald et al., 1999; Meeker, 2007).
The fundamental pathology induced by FIV is an encephalitis similar to that reported in HIV-1-infected patients, although the lesions are typically less severe (Meeker, 2007), and, in contrast to both HIV and SIV infection, multi-nucleated giant cells are rarely observed (Fox and Phillips, 2002). Proliferation and activation of microglial and astrocytic cells (gliosis), often with nodular formations, are typical features. This is sometimes accompanied by degenerative processes such as myelin damage manifested as myelin pallor (Hurtrel et al., 1992; Abramo et al., 1995; Boche et al., 1996; Poli et al., 1997; Silvotti et al., 1997). Neuronal loss has also been reported using FIV-NCSU1, beginning in the sub-clinical phase of infection (Meeker et al., 1997; Power et al., 1998). Viral cell tropism in the context of CNS infection remains controversial in HIV-1 infection and studies to date with FIV have been limited, involving only the Petaluma isolate, suggesting that infection of macrophages, microglia and possibly astrocytes, but not neurons, occurs (Dow et al., 1990, 1992; Poli et al., 1999).
While it is now established that most isolates of FIV are neurotropic, there is mounting evidence of variations in neurovirulence, similar to HIV (Johnston et al., 2000, 2002). It has been proposed that this variation in virulence is potentially due to the emergence of distinct neuropathogenic strains resulting from adaptation following passages through host populations and the independent evolution of HIV and FIV in humans and cats, respectively (Lackner et al., 1991; Albright et al., 2003; van Marle and Power, 2005). However it is also proposed that there are adaptations of virus within brain compartments in individual hosts (van Marle and Power, 2005; Liu et al., 2006a). When cats were inoculated with cell-free FIVNCSU1, by the intra-cerebroventricular or intra-peritoneal routes, rapid compartmentalisation of envelope variants between the CNS and peripheral tissues was observed suggesting that regional influences quickly influence the viral genome (Liu et al., 2006a).
Although cats intra-cranially inoculated with virus were more likely to develop variations in the V3-V4 envelope sequence, no clear relationship between specific variants and CNS disease could be established. Highly efficient movement of virus from the CSF to the systemic tissues in this model indicated that any adaptations that develop in the CNS could be rapidly transferred systemically (Liu et al. 2006b). As with studies of HIV, the FIV recovered from CSF at all time points contained a mix of envelope from both plasma and local sources. This suggests continuous exchange of virus between the systemic circulation into the CSF. Studies of HIV clearance from the CSF following anti-retroviral therapy support this view with a rapid early decline in CSF HIV load that paralleled decreases in the amount of virus in plasma (Haas et al., 2003). This rapid decline in CSF HIV was consistent with virus replication in CD4+ T cells suggesting a prominent contribution from trafficking of these cells.
FIV infection in cats is a well-established model of HIV-1 infection in man and in recent years this model has been used to address significant concerns regarding the induction of encephalitis by HIV-1 (Podell et al., 2000; Meeker, 2007; Fletcher et al., 2008). A major goal in the treatment of HIV infection is the need to eliminate or significantly reduce the level of infection. Although this has been partly addressed with the advent of highly active anti-retroviral therapies (HAART) that decrease the severity of CNS disease (Lambotte et al., 2003; Fischer-Smith and Rappaport, 2005), the prevalence of CNS disease continues to increase (Ances and Ellis, 2007; Robertson et al., 2007). This is due, in part, to poor penetration of anti-retroviral compounds across the BBB that not only allows ongoing virus production in brain but may also establish an environment conducive to the development of anti-retroviral resistance.
Attempts to develop effective therapies require a better understanding of the control of virus, immune cell and drug delivery across the BBB. Addressing these issues will require the use of animal models of natural lentiviral infection that will facilitate experimental approaches to many of the critical issues surrounding infection and neuropathogenesis. Firstly, the mechanisms that underpin the critical early penetration of virus into the CNS can be investigated. At this time the majority of HIV-infected humans are clinically normal and thus access to useful samples from such patients such as blood, CSF, and brain tissue is minimal (Davis et al., 1992). With animal models it is possible to evaluate this phase of infection and develop and test theories on how virus enters the brain. Secondly, the subsequent interactions between virus and immune cells within the CNS can be investigated over the entire course of disease to determine how the various pathological processes evolve (Zink et al., 1997; Ryan et al., 2003, 2005).
Finally, the availability of well-characterised models of infection and pathogenesis will facilitate efforts to develop and test novel therapies. While still in their infancy in terms of use, animal models such as FIV infection in the cat will no doubt provide the necessary information that will facilitate the design and testing of therapies to combat CNS infection and its pathological consequences (Boche et al., 1996; Zenger et al., 1997; Fletcher et al., 2008). Furthermore, an understanding of the blood-brain and choroid plexus barriers will play a crucial role in these endeavours.
Early entry of FIV into the brain
As outlined previously, FIV is associated with neuropathology in both the early and late stages of infection (Dow et al., 1990; Boche et al., 1996; Ryan et al., 2003). FIVGL-8 enters the brain in the majority of animals following infection and, between 1 and 23 weeks later, is detectable in the brains of 9/12 animals (Ryan et al. 2003, 2005), highlighting that the acute phase of infection is a significant time during which virus invades the CNS. This entry is paralleled by the appearance of high levels of FIV in the CSF, a level that lags slightly behind the peak plasma viral titre (Liu et al., 2006a).
This acute phase of infection is also believed to represent the significant ‘time-window’ during which virus enters the brain following HIV-1 infection (Gray et al., 1996). Parallel histopathological studies using FIVGL-8 highlighted that in cats during this period of acute infection, there is prominent lymphocyte trafficking simultaneously through the BBB and into the choroid plexus (Ryan et al., 2005). Such cell populations were composed of CD79+ B and CD3+ T cells. The latter population contained a mixture of CD4+ and CD8+ T cells. These transient perivascular cell infiltrations in the meninges, choroid plexus and brain parenchyma were consistent findings suggesting early active cell migration from the bloodstream into the brain (Fig. 1). While these findings were in contrast to previous studies in which lesions in the meninges and choroid plexus were rarely observed (Hurtrel et al., 1992; Abramo et al., 1995; Boche et al., 1996; Poli et al., 1997), they are consistent with observations from HIV-infected patients (Falangola, et al., 1995), with the variation explained by differences in viral strains, time post-infection and sampling protocols.
Fig. 1.
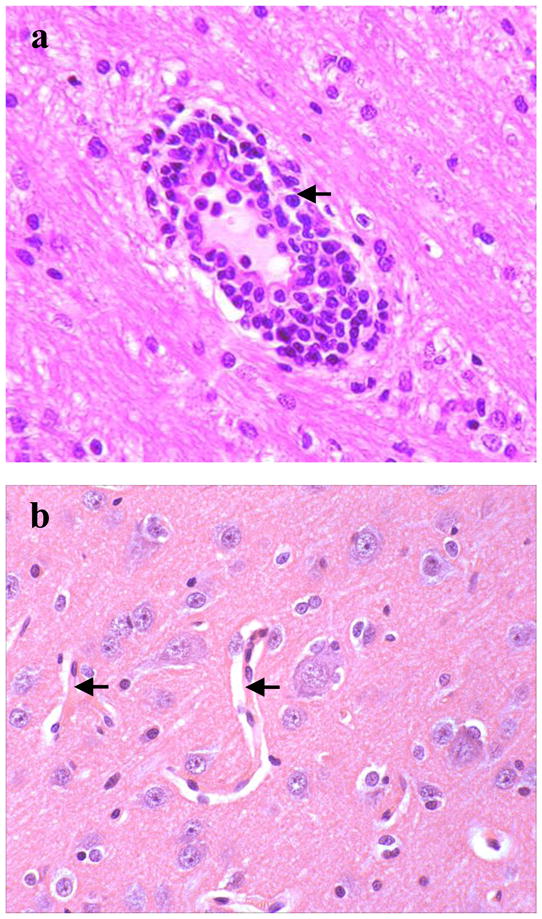
(a) Photomicrographs illustrating brain perivascular mononuclear cell infiltrations 10 weeks following IV Feline immunodeficiency virusGL8 infection (arrow), compared with (b) normal brain vasculature (arrows). Haematoxylin and eosin stain. Magnification factor × 200. Adapted from Ryan et al., 2003.
Studies investigating CNS infiltration in early-stage HIV-1 infection have reported predominantly CD8+ T cell-rich infiltrations, with fewer CD4+ T cells and B cells, around small blood vessels, particularly veins. These infiltrations were observed more frequently in the leptomeninges, in the sub-ependymal regions near the choroid plexus, in the deep white matter and in the basal ganglia (Gray et al., 1996; Anthony et al., 2003). CD8+ T cells and monocytes have also been reported to enter the brain during the acute phase of SIV infection. These cells were present in the perivascular spaces and parenchyma (Clay et al., 2007; Marcondes et al., 2007).
Whether FIV and HIV entered the brain through a disrupted BBB or blood-choroid plexus barrier, or whether entry of virus to the brain was facilitated by lymphocyte activation and migration, remained unclear from in vivo studies (Dallasta et al., 1999; Luabeya et al., 2000; Ryan et al., 2005). Furthermore, it was not clear if CNS infection was maintained by persistent immune cell trafficking. There is constant movement of immune cells in and out of the normal brain as part of natural immuno-surveillance. While little is known about how these migrations contribute to continual infection, it is clear from in vitro studies using HIV or FIVGL-8, that immune cells cross the BBB in similar numbers irrespective of whether they are infected or not (Fiala et al., 1997; Fletcher et al., 2009)
After the acute phase of infection, viral load and immune cell numbers decrease significantly and the individual enters a sub-clinical phase. Neural disease is thought to gradually progress during this period and is likely fuelled by a gradual, continuous influx of immune cells that give rise to the characteristic pathology (Gonzalez-Scarano and Martin-Garcia, 2005; Fischer-Smith et al., 2008). The extent of this trafficking and the control mechanisms at the BBB and blood-CSF barrier are not well understood but are important targets of therapies designed to halt early disease progression before it becomes clinically significant. As potential therapeutics are developed, animal models of subclinical disease will become increasingly important as this is the disease phase where treatment interventions are likely to be initiated in the clinical setting.
The blood-brain barrier and its role in viral entry to the brain
The BBB prevents free entry of hydrophilic compounds into the CNS. In vertebrates, this barrier is formed by tight junctions between brain capillary endothelial cells (Fig. 2), and these endothelial cells in turn are characterised by the absence of fenestrations and sparse pinocytosis (Ballabh et al., 2004). Astrocytes and pericytes are closely associated with the endothelial cells and contribute to the integrity of the barrier (Abbott et al., 1992; Hellstrom et al., 2001; Abbott et al., 2006). Lipophilic compounds and small molecules such as O2 and CO2 readily pass through the BBB by trans-cellular diffusion (Grieb et al., 1985). However, the transport of many substances is mediated by a number of surface transporters.
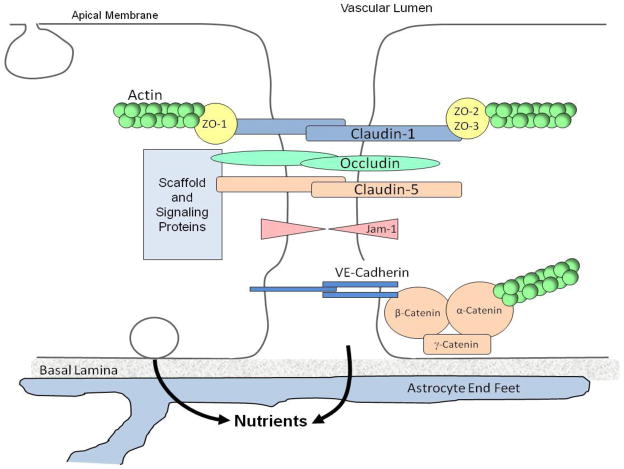
Fig. 2.
Diagram illustrating the location of tight junctions at the blood-brain barrier (BBB). Tight junctions between endothelial cells of the BBB limit the rate of paracellular transport of solutes between endothelial cells. These junctions are the most apical element of the junctional complex, and consist of several transmembrane and cytoplasmic proteins, including occludin, ZO-1, claudin-1 and -5, linked to an actin-based cytoskeleton. Tight junctions function as a boundary between the apical (blood) and baso-lateral (brain) side of the endothelium. Endothelial cells at the BBB are closely associated with astrocytic foot processes, which play a role in maintaining barrier integrity.
Receptor-mediated endocytosis is used by many substances, such as glucose, to cross the BBB. Carrier-mediated efflux such as that effected by the ATP-binding cassette transporter P-glycoprotein, is involved in the extrusion of drugs from the brain and is a major obstacle hindering the transfer of many pharmacological agents (Cordon-Cardo et al., 1989; Huber et al., 2001; Tambur and Roitberg, 2005). However, HIV, SIV and FIV are able to overcome the BBB and invade the brain, using mechanisms that are, as of yet, not clearly understood (Kramer-Hammerle et al., 2005). Three pathways by which HIV-1 enters the brain have been proposed and these are also likely to apply to FIV and SIV infections: (1) the passage of cell-free virus into the brain; (2) the carriage of virus into the brain by infected leucocytes (the ‘Trojan horse’ hypothesis); and (3) the direct infection of either brain endothelial cells or astrocytes, causing BBB breakdown and/or release of virus into the brain parenchyma (Albright et al., 2003; Kramer-Hammerle et al., 2005).
Details of how virus enters the brain have been gleaned mainly from clinical and pathological studies. However to elucidate the mechanism of viral entry, in vitro tissue culture-based models of the BBB and blood-choroid-plexus have been developed to facilitate study of lentiviral interactions with the BBB (Weiss et al., 1999; MacLean et al., 2004; Fletcher et al., 2006; Hudson et al., 2005). These models mimic specific characteristics of the BBB in vivo, including tight junction development, and have been used to study the interactions of neurovirulent viral strains and immune cells with the barrier (Fig. 3).
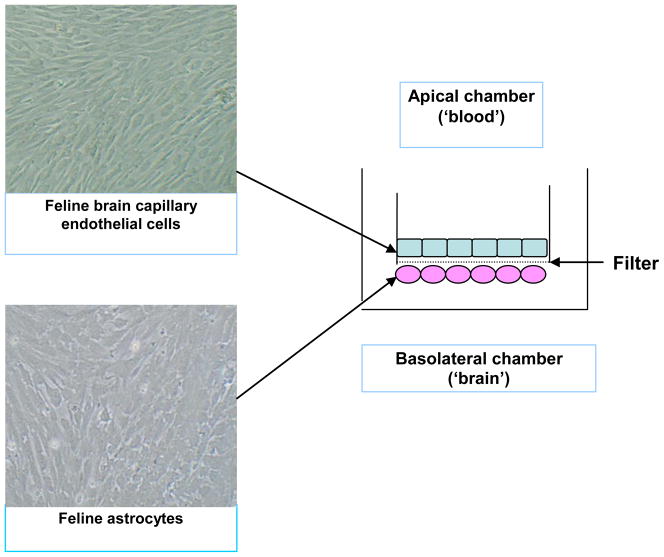
Fig. 3.
Construction of a feline in vitro blood-brain barrier (BBB) using primary cultures of feline brain endothelial cells (FBEC) and astrocytes. Cells were co-cultured on rat tail collagen and bovine fibronectin-coated filters with 1 μm pores. Astrocytes were seeded on the underside of the filters and cultured for 2–3 days, before the FBEC were seeded on the apical side of the filters. Tight junction formation was quantified using trans-endothelial electrical resistance and fluoresceinisothiocyanato-dextran (FD-4) para-cellular permeability assays. Original magnification of cell cultures x10.
Passage of cell-free virus into the brain
Migration of cell-free virus particles across the BBB from the blood may occur in either a para-cellular or trans-cellular manner, whereby virus migrates between brain endothelial cells (para-cellular) or is taken-up into vacuoles and released on the brain side of the barrier (trans-cellular). These mechanisms are not considered major routes of HIV or FIV entry into the brain early in infection (Kramer-Hammerle et al., 2005; Fletcher et al., 2009). Trans-cellular migration of HIV-1 across brain endothelial cells has been reported in vitro (Bobardt et al., 2004).
While brain endothelial cells have not been reported to express CD4 (Moses et al., 1993; Mukhtar et al., 2002), uptake of HIV particles occurs by a process termed absorptive endocytosis, induced by the viral envelope glycoprotein gp120. In this trans-cellular process which has been demonstrated in vitro, most of the virus is not released on the brain side of the endothelial cells, but is degraded or released back on the blood side of the endothelial cells (Banks et al., 2001, 2006), supporting the theory that direct viral migration across the BBB, while possible, is unlikely to be the primary mechanism of infection. Furthermore, in recent studies investigating the passage of FIVGL8 across an BBB model only 0.04% of virus had crossed the barrier after 24 h, and viral transmigration across the barrier not significantly increased in response to tumour necrosis factor (TNF)-α, a cytokine known to be up-regulated in the CNS in FIV and HIV infection (Fiala et al., 1997; Fletcher et al., 2009).
Trafficking of virus into the brain by infected leucocytes
The trafficking of virus into the brain by infected monocytes is currently the most favoured model of entry of HIV into the brain (Kramer-Hammerle et al., 2005). This ‘Trojan Horse’ hypothesis suggests that virus gains access to the brain by residing in monocytes and lymphocytes. Most studies of cell-mediated virus penetration of the CNS have focused on monocytes since HIV strains in this location are predominantly macrophage (M)-tropic, preferentially replicating in cells of monocyte lineage such as monocytes, macrophages and microglia) (Strizki et al., 1996; Gorry, et al., 2001; Peters et al., 2004).
Studies of monocyte trafficking across an in vitro BBB have indicated that invasion of activated monocytes is independent of infection (Persidsky et al., 1997). Studies of HIV-infected humans and SIV-infected macaques have observed that CD163+ and CD16+ subsets of monocytes preferentially traffic to the brain and harbour HIV/SIV infection (Fischer-Smith et al., 2001; Kim et al., 2006; Fischer-Smith et al., 2008). Although these observations suggest considerable selectivity in the cells that traffic across the BBB, the mechanisms that control this selectivity remain unknown.
Much less is known of the role of lymphocytes in the transport of virus across the BBB. The potential for lymphotropic HIV to be a source of CNS infection is compelling yet there is limited work on the potential mechanisms used by infected lymphocytes to enter the CNS (Katsetos et al., 1999; Albright et al., 2003). Although histopathological studies of early-stage CNS infection have obviously been limited, the major lesions observed are perivascular lymphocyte-rich infiltrations of the meninges, parenchyma and choroid plexus (Gray et al., 1996; Anthony et al., 2003). Lymphocytes also represent the predominant cell within perivascular infiltrations in children with HIV-1-associated encephalitis (Katsetos et al., 1999).
The role of infected lymphocytes is also a significant in the context of the primary and co-receptors used by HIV-1 (Berger et al., 1999). The majority of HIV strains use CD4 as the main receptor in combination with either chemokine co-receptors CCR5 or CXCR4. In any given host, it is believed that viral strains using CCR5 and strains using CXCR4 co-exist (Kramer-Hämmerle et al., 2005). The role of CCR5-using strains in HIV infection is supported by studies showing a reduced risk of HIV-1 infection and disease progression in individuals carrying a 32 base pair deletion in the CCR5 gene (CCR5 Delta32). Individuals heterozygous for CCR5 Delta 32 also have a reduced prevalence of HIV-associated dementia compared to controls (van Rij et al., 1999). Viruses utilising CCR5 preferentially infect macrophages/monocytes, whereas viruses using the CXCR4 receptor preferentially infect lymphocytes. These are known as the R5 and X4 strains, respectively.
CCR5 appears to be the co-receptor used by the majority of HIV strains early in infection, whereas CXCR4-using viruses are often observed with progression of disease (Scarlatti et al, 1997; Brumme et al., 2005), suggesting that infected lymphocytes circulate in all patients and may traffic into the CNS. In addition, in individuals participating in trials using CCR5 antagonists to suppress CCR5-using viruses, CXCR4 strains have emerged, suggesting a potential for these strains to become more prominent as treatment suppresses CCR5-using strains (Fätkenheuer et al., 2005). The potential role of X4-preferring virus and T cells in CNS infection and pathogenesis is also supported by studies showing that some highly M-tropic brain isolates infect microglia using the CXCR4 receptor (Gorry et al., 2001) and by the observation that CD16+ monocyte-derived macrophages achieve high levels of virus replication only after interaction with T cells (Ancuta, et al., 2006).
The ability of feline peripheral blood mononuclear cells and MYA-1 CD4+ T lymphocytes to cross in vitro models of the feline BBB have recently been investigated (Hudson et al., 2005, 2008; Fletcher et al., 2009). This work demonstrated that the presence of astrocytes increased monocyte, T cell and B cell migration across an in vitro BBB whereas microglial cells impeded migration. These findings suggest significant control of trafficking by brain parenchymal cells (Hudson et al., 2005), and have established astrocytes and microglia as important regulators of the BBB and thus potential pharmacological targets.
Further experiments have evaluated how prior exposure to virus may influence these observations and have indicated that exposure of astrocytes to FIVNCSU1 significantly increased transmigration of CD8+ T cells and monocytes (Hudson et al., 2005). Direct FIV interaction with endothelial cells was not sufficient to increase transmigration suggesting that astrocytes and microglia play a major role in the control of the BBB in FIV infection. While these results confirmed the interactions required between brain endothelial cells, astrocytes and microglia to support cell migration into the brain, they also highlighted that once infection is established, the internal control of the BBB is altered in ways not yet fully understood (Hudson et al., 2005).
Trafficking of a feline CD4+ T cell line (MYA-1), across the in vitro feline BBB has also been studied (Fletcher et al., 2009). In many ways the factors that influence monocyte migration across the BBB in HIV are similar to those that influence lymphocyte movement (Persidsky et al., 1997). MYA-1 cells are considered to be activated lymphocytes that readily migrate across an in vitro BBB, regardless of whether they harbour virus or not. This migration was significantly increased in response to TNF-α and/or the presence of infected lymphocytes on the brain-side of the BBB.
However, of greater interest was the fact that FIVGL-8-infected MYA-1 cells coupled with TNF-α in the brain compartment induced marked lymphocyte migration along with barrier disruption. While this migration coincided with increased adhesion molecule (VCAM-1) expression on brain endothelial cells, as found in studies on HIV and SIV, the mechanism has yet to be elucidated (Fletcher et al., 2009). This observation however has in vivo applications, as infected lymphocytes within the CNS, together with inflammatory cytokines, may increase trafficking of infected and activated lymphocytes into the CNS, accompanied by BBB disruption, which may ultimately increase the amount of virus present in the CNS during early infection.
Direct infection of cells of the blood brain barrier
Few studies have investigated whether FIV has the ability to infect brain endothelial cells and astrocytes of the BBB. While FIV Villefranche strain (a cell-adapted strain) has been reported to productively infect feline brain endothelial cell cultures 7 days post-infection (Steffan et al., 1994), other studies have failed infect feline brain endothelial cells with the Petaluma, FIV-2546 and Glasgow-8 strains (Dow et al., 1992; N.F. Fletcher et al., unpublished data). Given that these results are similar to those of studies investigating the ability of SIV and HIV to infect brain endothelial cells, evidence of such infection remains controversial and probably depends on the viral strain used (Kramer-Hammerle et al., 2005).
Some studies have reported non-productive infection of brain endothelial cells, and infection occurred in a CD4-independent manner (Moses et al., 1993; Moses and Nelson, 1994). However, using an in vitro BBB composed of simian brain endothelial cells co-cultured with human astrocytes, the endothelial cells were productively infected with SIV, and BBB breakdown did not occur (Strelow et al., 2002). Differences in the ability of different strains to infect simian brain endothelial cells was demonstrated by Mankowski et al. (1994), whereby a neurovirulent strain infected endothelial cells, but a non-neurovirulent strain did not. These results highlight the controversies that exist regarding the ability of SIV, HIV and FIV to infect brain endothelial cells and thus use this route to damage the BBB or migrate into the CNS. The role of endothelial cell infection is further obscured by the lack that as yet, there has been no definitive demonstration of clinically significant endothelial cell infection with HIV in vivo.
Although infection of primary feline astrocytes has been reported with FIV Petaluma (Dow et al., 1992; Zenger et al., 1997), other studies have failed to demonstrate astrocyte or astrocyte cell line (G355-5) infection (Zenger et al., 1997). Our studies with FIVGL8 and FIVNCSU1 have failed to show productive infection of astrocytes or G355-5 cells, although a reduction in cell viability over time, compared to uninfected controls, was observed (N.F. Fletcher et al., unpublished data). The suggestion that HIV-1 and SIV productively infect astrocytes is also controversial, given the varying capacities of different strains to infect these cells (Gonzalez-Scarano et al., 2005; Kramer-Hammerle et al., 2005).
It is generally accepted that HIV must overcome restrictions to entry and transcription in astrocytes (Gorry et al., 2001). However, studies by Gavrilin et al. (2002) using the FIVMD strain showed that productive infection of G355-5 cells could be established upon direct contact with infected lymphocytes. This observation suggests that cell-to-cell interactions may bypass traditional infection routes, a finding that may be significant in the context of lymphocyte trafficking into the CNS.
The blood-CSF barrier and its role in viral entry to the brain
The entry of virus through the choroid plexus is a further proposed route of CNS infection (Falangola et al., 1995; Chen et al., 2000; Bragg et al., 2002; Burkala et al., 2005) as well as a potential viral reservoir (Harouse et al., 1989; Hanly and Petito, 1998; Petito et al., 1999; Kim et al., 2006). Unlike the BBB, tight junctions are present between the cuboidal epithelial cells in the choroids plexus with no barrier at the endothelium (Fig. 4). Furthermore, the vasculature is separated from the epithelium by a stromal matrix that contains differentiated macrophages and dendritic cells which are often in abundance and are the targets of HIV, SIV or FIV infection.
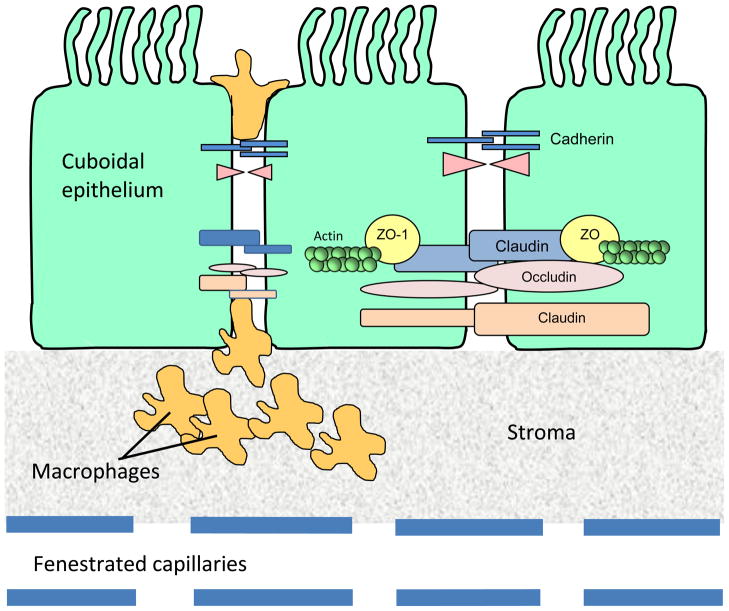
Fig. 4.
Diagram illustrating the location of tight junctions at the blood-cerebrospinal fluid (CSF) barrier. There are four choroid plexi in the brain, two in the lateral ventricles, one in the third and one in the fourth ventricle. The choroid plexus is lobulated with a single continuous layer of cells derived from the ependymal lining of the ventricles. These cells possess epithelial cell characteristics with a cuboidal morphology and are referred to as choroidal epithelial cells. Epithelial cells of the choroid plexus are linked by tight junctions, which contain trans-membrane and cytoplasmic proteins linked to an actin cytoskeleton. This forms what is termed the blood-CSF barrier.
Macrophages within the choroid plexus are thought to traffic into the cerebral ventricles where they become epiplexus cells (Ling, 1981) (Fig. 5). However, much less is known about the choroid plexus pathway than the BBB. To identify feline choroid plexus macrophages as targets of infection, Bragg et al. (2002) isolated purified choroid plexus macrophages and demonstrated a low level of productive infection with FIV. Importantly, choroid plexus macrophages exposed to FIVNCSU1, FIVMD or FIVPPR efficiently transferred virus to a feline T cell line, as assessed by quantification of viral P24 core antigen. Thus, although virus replication in the choroid plexus macrophages is not highly productive, these cells provide an efficient route for virus transfer/amplification at the blood-CSF interface.
Fig. 5.
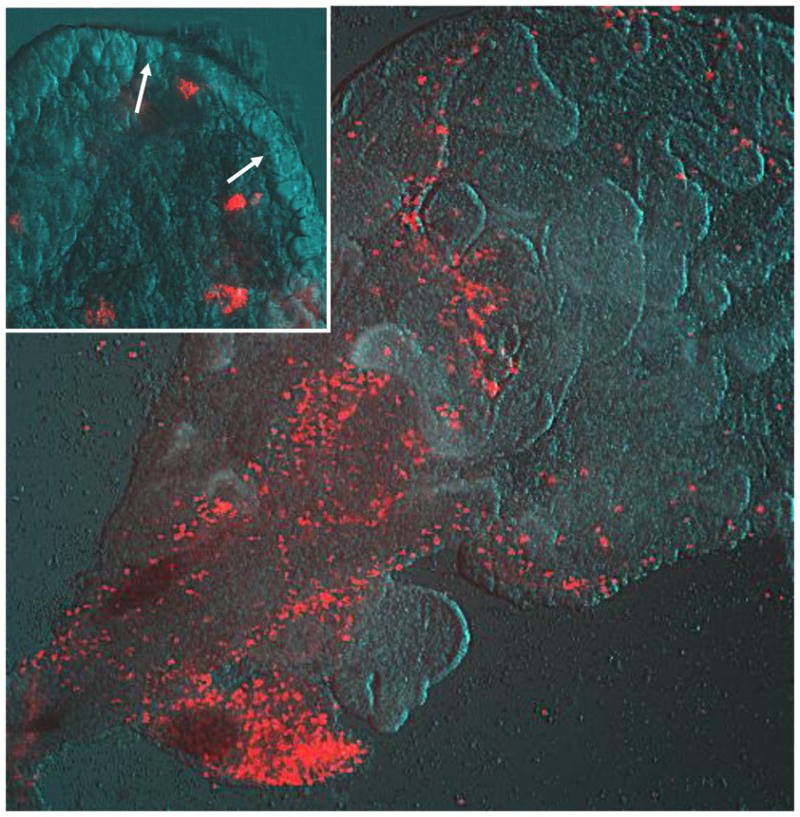
Choroid plexus macrophages stained with the red fluorescent compound DiI acetylated low density lipoprotein (DiI-Ac-LDL) in a live choroid plexus explant from the lateral ventricle of an uninfected fetal cat brain. The choroid plexus was removed and placed in culture with 2 μM DiI-Ac-LDL for 2 h. This dye is selectively phagocytosed by macrophages at this concentration resulting in bright red cells against the tissue background photographed with Hoffmann modulation contrast. Macrophages are densely clustered at the base of the choroid plexus and dispersed throughout the perivascular stroma (upper right quadrant of image). Magnification factor × 100. The inset shows labelled macrophages adjacent to the choroidal epithelium (arrows) where they are thought to transmigrate into the ventricle. Magnification factor × 400.
Although the role of immune cell trafficking in virus transfer across the blood-CSF barrier into the cerebral ventricles is not well understood, the rapid and efficient transfer of virus by this route has been well characterised (Chen et al., 2000; Gonzalez-Scarano et al., 2005). Studies of the early kinetics of infection following inoculation with FIV indicate that virus appears in the CSF just after the increase in plasma FIV. Concentrations in the CSF are typically 2–3 logs lower than in plasma. This slight lag and lack of equilibration with plasma FIV indicates that the virus is not transferred passively. Studies of HIV variants and turnover in humans have shown that much of the virus in CSF has a half-life of 1–3 days, consistent with T cells as the source of infection (Harrington et al., 2005).
Lymphocyte infiltration into the choroid plexus is prominent early in FIV (Ryan et al., 2003) as well as in HIV (Falangola et al., 1995) infection. The interactions between cells and virus within the choroid plexus gives rise to a mix of viral quasi-species with similarities to both peripheral tissue and brain virus, supporting the idea that the choroid plexus provides a unique environment that may support both neurotropism and drug resistance (Burkala et al., 2005). These observations highlight the potential importance of the blood-CSF interface in lentiviral infections. However, further study of immune function at the choroid plexus barrier is required.
Conclusions
FIV, like HIV-1 and SIV, enters the brain early following infection. Virus may enter via the BBB and/or the blood-CSF barrier at the choroid plexus. While monocytes are reported as the main cell type which carries HIV to the brain, our studies have demonstrated that both T and B lymphocytes traffic across the BBB. Although less well studied, entry of virus within CD4+ T lymphocytes also appears to be a significant mechanism of viral entry. Inflammatory cytokines such as TNF-α, secreted by astrocytes and microglia may play a significant role in the selection and trafficking of immune cells.
Entry of virus to the CNS results in the establishment of a viral reservoir that is protected from anti-retroviral drug therapy and which may re-seed peripheral tissues. The neuropathology of FIV infection is similar to that of HIV and SIV infection, and both viral and immune cell entry of the CNS contribute to the pathogenesis. Interactions of virus and immune cells with the BBB and the blood-CSF barrier are a crucial step in this process and remain a key a target of therapeutic intervention. FIV infection recapitulates important aspects of HIV infection and can facilitate the study of brain barrier function in the context of natural infection. Such an approach will provide essential information for the development of novel therapeutic strategies to control CNS infection and inflammation.
Acknowledgments
Drs. Fletcher and Callanan would like to acknowledge the National Neuroscience Network through the Programme for Third Level Institutions in Ireland, Science Foundation Ireland (BIM089), and the Royal Irish Academy for funding the studies described. Drs. Meeker and Hudson would like to acknowledge funding from National Institutes of Health (MH63646 and AI47749), National Science Foundation (IBN 9806128), and the State of North Carolina, USA. The authors would also like to acknowledge The Journal of Virology for granting permission to use the adapted figure (Fig 1).
Footnotes
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Revest PA, Romero IA. Astrocyte-endothelial interaction: physiology and pathology. Neuropathology and Applied Neurobiology. 1992;18:424–433. doi: 10.1111/j.1365-2990.1992.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abramo F, Bo S, Canese MG, Poli A. Regional distribution of lesions in the central nervous system of cats infected with feline immunodeficiency virus. AIDS Research and Human Retroviruses. 1995;11:1247–1253. doi: 10.1089/aid.1995.11.1247. [DOI] [PubMed] [Google Scholar]
- Ackley CD, Yamamoto JK, Levy N, Pedersen NC, Cooper MD. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. Journal of Virology. 1990;64:5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. Journal of Neurovirology. 2003;9:222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Seminars in Neurology. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–276. doi: 10.1016/j.virol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain. 2003;126:1058–1067. doi: 10.1093/brain/awg118. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiology of Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Banks WA, Freed EO, Wolf KM, Robinson SM, Franko M, Kumar VB. Transport of human immunodeficiency virus type 1 pseudoviruses across the blood-brain barrier: role of envelope proteins and adsorptive endocytosis. Journal of Virology. 2001;75:4681–4691. doi: 10.1128/JVI.75.10.4681-4691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Current HIV Research. 2006;4:259–266. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- Beebe AM, Dua N, Faith TG, Moore PF, Pedersen NC, Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. Journal of Virology. 1994;68:3080–3091. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clinical Microbiology Reviews. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annual Reviews in Immunology. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Bobardt MD, Salmon P, Wang L, Esko JD, Gabuzda D, Fiala M, Trono D, Van der Schueren B, David G, Gallay PA. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. Journal of Virology. 2004;78:6567–6584. doi: 10.1128/JVI.78.12.6567-6584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Hurtrel M, Gray F, Claessens-Maire MA, Ganiere JP, Montagnier L, Hurtrel B. Virus load and neuropathology in the FIV model. Journal of Neurovirology. 1996;2:377–387. doi: 10.3109/13550289609146903. [DOI] [PubMed] [Google Scholar]
- Bragg DC, Hudson LC, Liang YH, Tompkins MB, Fernandes A, Meeker RB. Choroid plexus macrophages proliferate and release toxic factors in response to feline immunodeficiency virus. Journal of Neurovirology. 2002;8:225–239. doi: 10.1080/13550280290049679. [DOI] [PubMed] [Google Scholar]
- Brown WC, Bissey L, Logan KS, Pedersen NC, Elder JH, Collisson EW. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. Journal of Virology. 1991;65:3359–3364. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumme ZL, Goodrich J, Mayer HB, Brumme CJ, Henrick BM, Wynhoven B, Asselin JJ, Cheung PK, Hogg RS, Montaner JS, Harrigan PR. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individuals. Journal of Infectious Disease. 2005;192:466–474. doi: 10.1086/431519. [DOI] [PubMed] [Google Scholar]
- Bucci JG, English RV, Jordan HL, Childers TA, Tompkins MB, Tompkins WA. Mucosally transmitted feline immunodeficiency virus induces a CD8+ antiviral response that correlates with reduction of cell-associated virus. Journal of Infectious Disease. 1998;177:18–25. doi: 10.1086/513822. [DOI] [PubMed] [Google Scholar]
- Burkala EJ, He J, West JT, Wood C, Petito CK. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. Aids. 2005;19:675–684. doi: 10.1097/01.aids.0000166090.31693.aa. [DOI] [PubMed] [Google Scholar]
- Callanan JJ, McCandlish IA, O’Neil B, Lawrence CE, Rigby M, Pacitti AM, Jarrett O. Lymphosarcoma in experimentally induced feline immunodeficiency virus infection [corrected] Veterinary Record. 1992;130:293–295. doi: 10.1136/vr.130.14.293. [DOI] [PubMed] [Google Scholar]
- Chen H, Wood C, Petito CK. Comparisons of HIV-1 viral sequences in brain, choroid plexus and spleen: potential role of choroid plexus in the pathogenesis of HIV encephalitis. Journal of Neurovirology. 2000;6:498–506. doi: 10.3109/13550280009091950. [DOI] [PubMed] [Google Scholar]
- Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA, Esser U. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. Journal of Virology. 2007;81:12040–12048. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proceedings of the National Academy of Science of the United States of America. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. American Journal of Pathology. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Diehl LJ, Mathiason-DuBard CK, O’Neil LL, Hoover EA. Longitudinal assessment of feline immunodeficiency virus kinetics in plasma by use of a quantitative competitive reverse transcriptase PCR. Journal of Virology. 1995;69:2328–2332. doi: 10.1128/jvi.69.4.2328-2332.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow SW, Poss ML, Hoover EA. Feline immunodeficiency virus: a neurotropic lentivirus. Journal of Acquired Immune Deficiency Syndromes. 1990;3:658–668. [PubMed] [Google Scholar]
- Dow SW, Dreitz MJ, Hoover EA. Feline immunodeficiency virus neurotropism: evidence that astrocytes and microglia are the primary target cells. Veterinary Immunology and Immunopathology. 1992;35:23–35. doi: 10.1016/0165-2427(92)90118-a. [DOI] [PubMed] [Google Scholar]
- Dow SW, Mathiason CK, Hoover EA. In vivo monocyte tropism of pathogenic feline immunodeficiency viruses. Journal of Virology. 1999;73:6852–6861. doi: 10.1128/jvi.73.8.6852-6861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egberink HF, De Clercq E, Van Vliet AL, Balzarini J, Bridger GJ, Henson G, Horzinek MC, Schols D. Bicyclams, selective antagonists of the human chemokine receptor CXCR4, potently inhibit feline immunodeficiency virus replication. Journal of Virology. 1999;73:6346–6352. doi: 10.1128/jvi.73.8.6346-6352.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JH, Phillips TR. Molecular properties of feline immunodeficiency virus (FIV) Infectious Agents and Disease. 1993;2:361–374. [PubMed] [Google Scholar]
- English RV, Nelson P, Johnson CM, Nasisse M, Tompkins WA, Tompkins MB. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. Journal of Infectious Disease. 1994;170:543–552. doi: 10.1093/infdis/170.3.543. [DOI] [PubMed] [Google Scholar]
- Falangola MF, Hanly A, Galvao-Castro B, Petito CK. HIV infection of human choroid plexus: a possible mechanism of viral entry into the CNS. Journal of Neuropathology and Experimental Neurology. 1995;54:497–503. doi: 10.1097/00005072-199507000-00003. [DOI] [PubMed] [Google Scholar]
- Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD, Rockstroh JK, Dezube BJ, Jenkins TM, Medhurst C, Sullivan JF, Ridgway C, Abel S, James IT, Youle M, van der Ryst E. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nature Medicine. 2005;11:1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Molecular Medicine. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. Journal of Neurovirology. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Reviews in Molecular Medicine. 2005;7:1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. Journal of Neurovirology. 2008;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher NF, Brayden DJ, Brankin B, Worrall S, Callanan JJ. Growth and characterisation of a cell culture model of the feline blood-brain barrier. Veterinary Immunology and Immunopathology. 2006;109:233–244. doi: 10.1016/j.vetimm.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Fletcher NF, Brayden DJ, Brankin B, Callanan JJ. Feline immunodeficiency virus infection: a valuable model to study HIV-1 associated encephalitis. Veterinary Immunology and Immunopathology. 2008;123:134–137. doi: 10.1016/j.vetimm.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Fletcher NF, Bexiga MG, Brayden DJ, Brankin B, Willett BJ, Hosie MJ, Jacque JM, Callanan JJ. Lymphocyte migration through the blood brain barrier (BBB) in feline immunodeficiency virus infection is significantly influenced by the pre-existence of virus and TNF-alpha within the CNS: studies using an in vitro feline BBB model. Neuropathology and Applied Neurobiology. 2009 doi: 10.1111/j.1365-2990.2009.01031.x. Epub: PMID: 19486302. [DOI] [PubMed] [Google Scholar]
- Fox HS, Phillips TR. FIV and neuroAIDS. Journal of Neurovirology. 2002;8:155–157. doi: 10.1080/13550280290049714. [DOI] [PubMed] [Google Scholar]
- Gavrilin MA, Mathes LE, Podell M. Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. Journal of Neurovirology. 2002;8:240–249. doi: 10.1080/13550280290049660. [DOI] [PubMed] [Google Scholar]
- Gebhard DH, Dow JL, Childers TA, Alvelo JI, Tompkins MB, Tompkins WA. Progressive expansion of an L-selectin-negative CD8 cell with anti-feline immunodeficiency virus (FIV) suppressor function in the circulation of FIV-infected cats. Journal of Infectious Disease. 1999;180:1503–1513. doi: 10.1086/315089. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Bristol G, Zack JA, Ritola K, Swanstrom R, Birch CJ, Bell JE, Bannert N, Crawford K, Wang H, Schols D, De Clercq E, Kunstman K, Wolinsky SM, Gabuzda D. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. Journal of Virology. 2001;75:10073–10089. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Bell J, Lantos P. Neuropathology of early HIV-1 infection. Brain Pathology. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Grieb P, Forster RE, Strome D, Goodwin CW, Pape PC. O2 exchange between blood and brain tissues studied with 18O2 indicator-dilution technique. Journal of Applied Physiology. 1985;58:1929–1941. doi: 10.1152/jappl.1985.58.6.1929. [DOI] [PubMed] [Google Scholar]
- Haas DW, Johnson BW, Spearman P, Raffanti S, Nicotera J, Schmidt D, Hulgan T, Shepard R, Fiscus SA. Two phases of HIV RNA decay in CSF during initial days of multidrug therapy. Neurology. 2003;61:1391–1396. doi: 10.1212/wnl.61.10.1391. [DOI] [PubMed] [Google Scholar]
- Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus: a potential reservoir of HIV in the central nervous system. Human Pathology. 1998;29:88–93. doi: 10.1016/s0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- Harouse JM, Wroblewska Z, Laughlin MA, Hickey WF, Schonwetter BS, Gonzalez-Scarano F. Human choroid plexus cells can be latently infected with human immunodeficiency virus. Annals of Neurology. 1989;25:406–411. doi: 10.1002/ana.410250414. [DOI] [PubMed] [Google Scholar]
- Harrington PR, Haas DW, Ritola K, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. Journal of Virology. 2005;79:7959–7966. doi: 10.1128/JVI.79.13.7959-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. Journal of Cell Biology. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends in Neuroscience. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Hudson LC, Bragg DC, Tompkins MB, Meeker RB. Astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells. Brain Research. 2005;1058:148–160. doi: 10.1016/j.brainres.2005.07.071. [DOI] [PubMed] [Google Scholar]
- Hudson LC, Tompkins MB, Meeker RB. Endothelial cell suppression of peripheral blood mononuclear cell trafficking in vitro during acute exposure to feline immunodeficiency virus. Cell and Tissue Research. 2008;334:55–65. doi: 10.1007/s00441-008-0623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtrel M, Ganiere JP, Guelfi JF, Chakrabarti L, Maire MA, Gray F, Montagnier L, Hurtrel B. Comparison of early and late feline immunodeficiency virus encephalopathies. Aids. 1992;6:399–406. doi: 10.1097/00002030-199204000-00007. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Tomonaga K, Kawaguchi Y, Kohmoto M, Inoshima Y, Tohya Y, Miyazawa T, Kai C, Mikami T. Feline immunodeficiency virus can infect a human cell line (MOLT-4) but establishes a state of latency in the cells. Journal of General Virology. 1996;77:1623–1630. doi: 10.1099/0022-1317-77-8-1623. [DOI] [PubMed] [Google Scholar]
- Johnston JB, Jiang Y, van Marle G, Mayne MB, Ni W, Holden J, McArthur JC, Power C. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. Journal of Virology. 2000;74:7211–7220. doi: 10.1128/jvi.74.16.7211-7220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Silva C, Hiebert T, Buist R, Dawood MR, Peeling J, Power C. Neurovirulence depends on virus input titer in brain in feline immunodeficiency virus infection: evidence for activation of innate immunity and neuronal injury. Journal of Neurovirology. 2002;8:420–431. doi: 10.1080/13550280260422721. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Fincke JE, Legido A, Lischner HW, de Chadarevian JP, Kaye EM, Platsoucas CD, Oleszak EL. Angiocentric CD3(+) T-cell infiltrates in human immunodeficiency virus type 1-associated central nervous system disease in children. Clinical and Diagnostic Laboratory Immunology. 1999;6:105–114. doi: 10.1128/cdli.6.1.105-114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, Williams K. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. American Journal of Pathology. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Research. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Dandekar S, Gardner MB. Neurobiology of simian and feline immunodeficiency virus infections. Brain Pathology. 1991;1:201–212. doi: 10.1111/j.1750-3639.1991.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Lambotte O, Deiva K, Tardieu M. HIV-1 persistence, viral reservoir, and the central nervous system in the HAART era. Brain Pathology. 2003;13:95–103. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling EA. Ultrastructure and mode of formation of epiplexus cells in the choroid plexus in the lateral ventricles of the monkey (Macaca fascicularis) Journal of Anatomy. 1981;133:555–569. [PMC free article] [PubMed] [Google Scholar]
- Liu P, Hudson LC, Tompkins MB, Vahlenkamp TW, Meeker RB. Compartmentalization and evolution of feline immunodeficiency virus between the central nervous system and periphery following intracerebroventricular or systemic inoculation. Journal of Neurovirology. 2006a;12:307–321. doi: 10.1080/13550280600889575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Hudson LC, Tompkins MB, Vahlenkamp TW, Colby B, Rundle C, Meeker RB. Cerebrospinal fluid is an efficient route for establishing brain infection with feline immunodeficiency virus and transferring infectious virus to the periphery. Journal of Neurovirology. 2006b;12:294–306. doi: 10.1080/13550280600889567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luabeya MK, Dallasta LM, Achim CL, Pauza CD, Hamilton RL. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathology and Applied Neurobiology. 2000;26:454–462. doi: 10.1046/j.1365-2990.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- MacLean AG, Rasmussen TA, Bieniemy D, Lackner AA. Activation of the blood-brain barrier by SIV (simian immunodeficiency virus) requires cell-associated virus and is not restricted to endothelial cell activation. Biochemical Society Transactions. 2004;32:750–752. doi: 10.1042/BST0320750. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Spelman JP, Ressetar HG, Strandberg JD, Laterra J, Carter DL, Clements JE, Zink MC. Neurovirulent simian immunodeficiency virus replicates productively in endothelial cells of the central nervous system in vivo and in vitro. Journal of Virology. 1994;68:8202–8208. doi: 10.1128/jvi.68.12.8202-8208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes MC, Burdo TH, Sopper S, Huitron-Resendiz S, Lanigan C, Watry D, Flynn C, Zandonatti M, Fox HS. Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. Journal of Immunology. 2007;178:5812–5819. doi: 10.4049/jimmunol.178.9.5812. [DOI] [PubMed] [Google Scholar]
- Mathiason-DuBard CK, Burkhard MJ, O’Neill JJ, Hoover EA. Infection and disease in cats infected with unpassaged FIV field isolates: a multi year longitudinal study. International Retrovirus Research Symposium; Glasgow, U.K.. 1998. p. 34. [Google Scholar]
- Meeker RB. Feline immunodeficiency virus neuropathogenesis: from cats to calcium. Journal of Neuroimmune Pharmacology. 2007;2:154–170. doi: 10.1007/s11481-006-9045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker RB, Thiede BA, Hall C, English R, Tompkins M. Cortical cell loss in asymptomatic cats experimentally infected with feline immunodeficiency virus. AIDS Research and Human Retroviruses. 1997;13:1131–1140. doi: 10.1089/aid.1997.13.1131. [DOI] [PubMed] [Google Scholar]
- Moses AV, Bloom FE, Pauza CD, Nelson JA. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proceedings of the National Academy of Science of the United States of America. 1993;90:10474–10478. doi: 10.1073/pnas.90.22.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses AV, Nelson JA. HIV infection of human brain capillary endothelial cells--implications for AIDS dementia. Advances in Neuroimmunology. 1994;4:239–247. doi: 10.1016/s0960-5428(06)80262-7. [DOI] [PubMed] [Google Scholar]
- Mukhtar M, Harley S, Chen P, BouHamdan M, Patel C, Acheampong E, Pomerantz RJ. Primary isolated human brain microvascular endothelial cells express diverse HIV/SIV-associated chemokine coreceptors and DC-SIGN and L-SIGN. Virology. 2002;297:78–88. doi: 10.1006/viro.2002.1376. [DOI] [PubMed] [Google Scholar]
- Olmsted RA, Hirsch VM, Purcell RH, Johnson PR. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proceedings of the National Academy of Science of the United States of America. 1989;86:8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil LL, Burkhard MJ, Diehl LJ, Hoover EA. Vertical transmission of feline immunodeficiency virus. AIDS research and human retroviruses. 1995;11:171–182. doi: 10.1089/aid.1995.11.171. [DOI] [PubMed] [Google Scholar]
- Pedersen NC, Ho EW, Brown ML, Yamamoto JK. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. Journal of Immunology. 1997;158:3499–3510. [PubMed] [Google Scholar]
- Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, Bell J, Simmonds P, Clapham PR. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. Journal of Virology. 2004;78:6915–6926. doi: 10.1128/JVI.78.13.6915-6926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Chen H, Mastri AR, Torres-Munoz J, Roberts B, Wood C. HIV infection of choroid plexus in AIDS and asymptomatic HIV-infected patients suggests that the choroid plexus may be a reservoir of productive infection. Journal of Neurovirology. 1999;5:670–677. doi: 10.3109/13550289909021295. [DOI] [PubMed] [Google Scholar]
- Phillips TR, Prospero-Garcia O, Puaoi DL, Lerner DL, Fox HS, Olmsted RA, Bloom FE, Henriksen SJ, Elder JH. Neurological abnormalities associated with feline immunodeficiency virus infection. Journal of General Virology. 1994;75:979–987. doi: 10.1099/0022-1317-75-5-979. [DOI] [PubMed] [Google Scholar]
- Phillips TR, Prospero-Garcia O, Wheeler DW, Wagaman PC, Lerner DL, Fox HS, Whalen LR, Bloom FE, Elder JH, Henriksen SJ. Neurologic dysfunctions caused by a molecular clone of feline immunodeficiency virus, FIV-PPR. Journal of Neurovirology. 1996;2:388–396. doi: 10.3109/13550289609146904. [DOI] [PubMed] [Google Scholar]
- Phillips TR, Billaud JN, Henriksen SJ. Methamphetamine and HIV-1: potential interactions and the use of the FIV/cat model. Journal of Psychopharmacology. 2000;14:244–250. doi: 10.1177/026988110001400309. [DOI] [PubMed] [Google Scholar]
- Podell M, Oglesbee M, Mathes L, Krakowka S, Olmstead R, Lafrado L. AIDS-associated encephalopathy with experimental feline immunodeficiency virus infection. Journal of Acquired Immune Deficiency Syndromes. 1993;6:758–771. [PubMed] [Google Scholar]
- Podell M, Hayes K, Oglesbee M, Mathes L. Progressive encephalopathy associated with CD4/CD8 inversion in adult FIV-infected cats. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;15:332–340. doi: 10.1097/00042560-199708150-00002. [DOI] [PubMed] [Google Scholar]
- Podell M, Maruyama K, Smith M, Hayes KA, Buck WR, Ruehlmann DS, Mathes LE. Frontal lobe neuronal injury correlates to altered function in FIV-infected cats. Journal of Acquired Immune Deficiency Syndromes. 1999;22:10–18. doi: 10.1097/00042560-199909010-00002. [DOI] [PubMed] [Google Scholar]
- Podell M, March PA, Buck WR, Mathes LE. The feline model of neuroAIDS: understanding the progression towards AIDS dementia. Journal of Psychopharmacology. 2000;14:205–213. doi: 10.1177/026988110001400303. [DOI] [PubMed] [Google Scholar]
- Poeschla EM, Looney DJ. CXCR4 is required by a nonprimate lentivirus: heterologous expression of feline immunodeficiency virus in human, rodent, and feline cells. Journal of Virology. 1998;72:6858–6866. doi: 10.1128/jvi.72.8.6858-6866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli A, Abramo F, Di Iorio C, Cantile C, Carli MA, Pollera C, Vago L, Tosoni A, Costanzi G. Neuropathology in cats experimentally infected with feline immunodeficiency virus: a morphological, immunocytochemical and morphometric study. Journal of Neurovirology. 1997;3:361–368. doi: 10.3109/13550289709030750. [DOI] [PubMed] [Google Scholar]
- Poli A, Pistello M, Carli MA, Abramo F, Mancuso G, Nicoletti E, Bendinelli M. Tumor necrosis factor-alpha and virus expression in the central nervous system of cats infected with feline immunodeficiency virus. Journal of Neurovirology. 1999;5:465–473. doi: 10.3109/13550289909045375. [DOI] [PubMed] [Google Scholar]
- Power C, Buist R, Johnston JB, Del Bigio MR, Ni W, Dawood MR, Peeling J. Neurovirulence in feline immunodeficiency virus-infected neonatal cats is viral strain specific and dependent on systemic immune suppression. Journal of Virology. 1998;72:9109–9115. doi: 10.1128/jvi.72.11.9109-9115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Zhang K, van Marle G. Comparative neurovirulence in lentiviral infections: The roles of viral molecular diversity and select proteases. Journal of Neurovirology. 2004;10:113–117. doi: 10.1080/753312762. [DOI] [PubMed] [Google Scholar]
- Prospero-Garcia O, Herold N, Phillips TR, Elder JH, Bloom FE, Henriksen SJ. Sleep patterns are disturbed in cats infected with feline immunodeficiency virus. Proceedings of the National Academy of Science of the United States of America. 1994;91:12947–12951. doi: 10.1073/pnas.91.26.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Ryan G, Klein D, Knapp E, Hosie MJ, Grimes T, Mabruk MJ, Jarrett O, Callanan JJ. Dynamics of viral and proviral loads of feline immunodeficiency virus within the feline central nervous system during the acute phase following intravenous infection. Journal of Virology. 2003;77:7477–7485. doi: 10.1128/JVI.77.13.7477-7485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G, Grimes T, Brankin B, Mabruk MJ, Hosie MJ, Jarrett O, Callanan JJ. Neuropathology associated with feline immunodeficiency virus infection highlights prominent lymphocyte trafficking through both the blood-brain and blood-choroid plexus barriers. Journal of Neurovirology. 2005;11:337–345. doi: 10.1080/13550280500186445. [DOI] [PubMed] [Google Scholar]
- Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng HK, Malnati MS, Plebani A, Siccardi AG, Littman DR, Fenyo EM, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nature Medicine. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- Shimojima M, Miyazawa T, Ikeda Y, McMonagle EL, Haining H, Akashi H, Takeuchi Y, Hosie MJ, Willett BJ. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–1195. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- Silvotti L, Corradi A, Brandi G, Cabassi A, Bendinelli M, Magnan M, Piedimonte G. FIV induced encephalopathy: early brain lesions in the absence of viral replication in monocyte/macrophages. A pathogenetic model. Veterinary Immunology and Immunopathology. 1997;55:263–271. doi: 10.1016/s0165-2427(96)05617-6. [DOI] [PubMed] [Google Scholar]
- Sodora DL, Shpaer EG, Kitchell BE, Dow SW, Hoover EA, Mullins JI. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. Journal of Virology. 1994;68:2230–2238. doi: 10.1128/jvi.68.4.2230-2238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora DL, Courcelle J, Brojatsch J, Berson A, Wang YC, Dow SW, Hoover EA, Mullins JI. Analysis of a feline immunodeficiency virus provirus reveals patterns of gene sequence conservation distinct from human immunodeficiency virus type 1. AIDS Research and Human Retroviruses. 1995;11:531–533. doi: 10.1089/aid.1995.11.531. [DOI] [PubMed] [Google Scholar]
- Steffan AM, Lafon ME, Gendrault JL, Koehren F, De Monte M, Royer C, Kirn A, Gut JP. Feline immunodeficiency virus can productively infect cultured endothelial cells from cat brain microvessels. Journal of General Virology. 1994;75:3647–3653. doi: 10.1099/0022-1317-75-12-3647. [DOI] [PubMed] [Google Scholar]
- Steigerwald ES, Sarter M, March P, Podell M. Effects of feline immunodeficiency virus on cognition and behavioral function in cats. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1999;20:411–419. doi: 10.1097/00042560-199904150-00001. [DOI] [PubMed] [Google Scholar]
- Strelow L, Janigro D, Nelson JA. Persistent SIV infection of a blood-brain barrier model. Journal of Neurovirology. 2002;8:270–280. doi: 10.1080/13550280290100734. [DOI] [PubMed] [Google Scholar]
- Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. Journal of Virology. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambur AR, Roitberg B. Immunology of the central nervous system. Neurology Research. 2005;27:675–678. doi: 10.1179/016164105X49544. [DOI] [PubMed] [Google Scholar]
- Tompkins MB, Nelson PD, English RV, Novotney C. Early events in the immunopathogenesis of feline retrovirus infections. Journal of the American Veterinary Medical Association. 1991;199:1311–1315. [PubMed] [Google Scholar]
- van Marle G, Power C. Human immunodeficiency virus type 1 genetic diversity in the nervous system: evolutionary epiphenomenon or disease determinant ? Journal of Neurovirology. 2005;11:107–128. doi: 10.1080/13550280590922838. [DOI] [PubMed] [Google Scholar]
- van Rij RP, Portegies P, Hallaby T, Lange JM, Visser J, de Roda Husman AM, van ‘t Wout AB, Schuitemaker H. Reduced prevalence of the CCR5 delta32 heterozygous genotype in human immunodeficiency virus-infected individuals with AIDS dementia complex. Journal of Infectious Disease. 1999;180:854–857. doi: 10.1086/314940. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. Journal of Immunology. 1999;163:2953–2959. [PubMed] [Google Scholar]
- Willett BJ, Hosie MJ, Callanan JJ, Neil JC, Jarrett O. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993;78:1–6. [PMC free article] [PubMed] [Google Scholar]
- Willett BJ, Flynn JN, Hosie MJ. FIV infection of the domestic cat: an animal model for AIDS. Immunology Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- Willett BJ, Adema K, Heveker N, Brelot A, Picard L, Alizon M, Turner JD, Hoxie JA, Peiper S, Neil JC, Hosie MJ. The second extracellular loop of CXCR4 determines its function as a receptor for feline immunodeficiency virus. Journal of Virology. 1998;72:6475–6481. doi: 10.1128/jvi.72.8.6475-6481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett BJ, Hosie MJ. Chemokine receptors and co-stimulatory molecules: unravelling feline immunodeficiency virus infection. Veterinary Immunology and Immunopathology. 2008;123:56–64. doi: 10.1016/j.vetimm.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenger E, Tiffany-Castiglioni E, Collisson EW. Cellular mechanisms of feline immunodeficiency virus (FIV)-induced neuropathogenesis. Frontiers in Biosciences. 1997;2:527–537. doi: 10.2741/a210. [DOI] [PubMed] [Google Scholar]
- Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. American Journal of Pathology. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]