Abstract
Objective
Studies in first episode psychosis samples about status of cardiovascular risk factors have shown discordant results. We aimed to determine the 10-year risk of developing coronary heart disease in a sample of first episode psychosis patients referred to an early intervention clinic and compared the same with age, gender, and race matched controls from the U.S. National Health and Nutrition Examination Survey (NHANES).
Method
We conducted a cross-sectional analysis of baseline data of 56 subjects enrolled in first episode psychosis clinic from April 2006 and January 2010. This sample was compared with age, gender, and race matched 145 individuals drawn from NHANES 2005-2006 database. Sociodemographic and clinical variables were collected. Physical examination including laboratory evaluation was used to screen for common medical illnesses. The 10-year risk of developing coronary heart disease was calculated by using a tool developed by National Cholesterol Education Program (NCEP-ATP III).
Results
There were elevated rates of smoking (46%) and hypertension (11%) albeit statistically significant differences from the control could not be demonstrated for these measures or weight, body mass index, or total or HDL cholesterol, fasting plasma glucose, status of diabetes and impaired fasting plasma glucose, HbA1C level. The 10-year median (range) risk of developing coronary heart disease in patients and controls was 1 (0-5) % and 0 (0-9) % respectively. The difference was not statistically significant.
Conclusions
First episode psychosis patients do not present with significantly higher cardiovascular risk than age and race-matched controls despite clinically significant prevalence of individual risk factors. This sample presents an opportunity for early intervention for primary prevention of cardiovascular morbidity and mortality.
Keywords: First episode psychosis, cardiovascular risk, cardiovascular mortality, critical period, early intervention
1. Introduction
Individuals with serious mental illness (SMI) die, on average, 25 years earlier than their peers (Colton and Manderscheid, 2006; Parks et al., 2006). While 30-40% of this premature mortality is attributable to suicide and accidental injury, cardiovascular disease accounts for the majority of early death. The single most common cause of death in patients with schizophrenia is cardiovascular disease (Osby et al., 2000; Capasso et al., 2009; Tiihonen et al., 2009). Patients with schizophrenia, relative to peers without SMI, experience a 3-fold increase in cardiovascular mortality between the ages of 18 and 49 and almost a 2-fold increase in mortality between the ages of 50 and 75 years (Osborn et al., 2007). They have a greater incidence of myocardial infarction than demographically similar persons without schizophrenia (Brown et al., 2000; Enger et al., 2004).
The causes of this increased cardiovascular burden in patients with schizophrenia are likely multi-factorial. Modifiable risk factors for cardiovascular disease include smoking, obesity, diabetes, dyslipidemia, and hypertension (Yusuf et al., 2004). When compared to age- and gender- matched controls, persons with chronic psychosis have higher rates of nicotine dependence (70-80% vs. 25-30%) (de Leon and Diaz, 2005), obesity (45-55% vs 31-39%) (De Hert et al., 2009; Meigs et al., 2003), diabetes (13% vs 3%) (Goff et al., 2005), dyslipidemia (25-69% vs 24-48%) (De Hert et al., 2009; Meigs et al., 2003) and hypertension (27% vs 17%) (Goff et al., 2005). The largest study comparing cardiovascular risk factors in chronic schizophrenia patients, drawn from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial, with age-, gender-, and race-matched controls from the U.S. National Health and Nutrition Examination Survey (NHANES) showed that patients had significantly higher 10-year coronary heart disease risk. This was due to higher rates of smoking, diabetes, and hypertension. Also, the mean (SD) duration of antipsychotic use in the CATIE study was 14.4 (10.7) years (Lieberman et al., 2005) and long-term use of antipsychotic medications may play an important role in the increased risk for cardiovascular diseases (Goff et al., 2005, Newcomer 2009). Antipsychotic medication use is associated with significant weight gain, dyslipidemia, and insulin resistance (Stahl et al., 2009).
In contrast to the consistent evidence across all measures of cardiovascular risk in chronic schizophrenia, studies of ‘first episode’ psychosis samples have been inconsistent. The first study comparing cardiovascular risk factors in drug-naïve first-episode schizophrenia patients with matched controls found that patients had significantly higher fasting plasma glucose levels. HDL cholesterol was not different, but total cholesterol was lower in patients (Ryan et al., 2003). These authors were unable to replicate these findings with a different sample, and, in a second study, reported that first-episode schizophrenia patients, their first degree relatives, and matched controls did not differ with respect to fasting plasma glucose levels (Spelman et al., 2007). Another study of drug-naïve first-episode psychosis patients compared to age, gender, and race matched controls showed that patients had a significantly higher prevalence of diabetes but lower frequencies of obesity and total and LDL cholesterol (Verma et al., 2009). A study of 38 first-episode psychosis patients compared to age, gender, and race matched controls did not find significant differences in fasting plasma glucose levels, glucose tolerance, body mass index, waist circumference and pulse pressure (Sengupta et al., 2008). Another study of antipsychoticnaïve, first-episode schizophrenia patients compared to healthy controls did not show significant differences in fasting glucose and insulin resistance (Arranz et al., 2004)
The use of specialized early intervention services (EI) to reduce long term psychosocial morbidity in psychotic disorders has been substantiated by several high quality studies (Marshall and Rathbone, 2006). The traditional focus of EI has been to deliver best available treatments during a putative ‘critical period’ for psychosocial development wherein intensive early intervention is hypothesized to achieve disproportionately positive results on long term outcomes. We propose an analogous formulation for reducing cardiovascular morbidity and mortality. The existence of EI clinics around the world, which are redefining care for early psychosis patients, presents an opportunity for the study and development of primary and secondary prevention of cardiovascular disease in schizophrenia.
Given discrepancies in the reported prevalence of cardiovascular risk factors in early psychosis samples, the current study aimed to measure these risks again in a carefully characterized sample of patients referred to an early intervention clinic. Also, we used the best available risk calculator to formulate a 10 year risk estimate of developing coronary heart disease. We report a cross sectional comparison of these first-episode psychosis patients with age, gender, and race matched controls from the U. S. National Health and Nutrition Examination Survey (NHANES) (Centers for Disease Control and Prevention, 2005-2006).
2. Methods
Subjects for this analysis were drawn from an ongoing NIH-funded pragmatic randomized controlled trial titled Specialized Treatment Early in Psychosis (STEP). The broader goals of this trial are to determine the effectiveness and costs of a package of empirically supported treatments delivered within a U.S. community mental health center (Srihari et al., 2009). The subjects were consecutively enrolled in the study. The target sample of the NIH trial includes Connecticut residents between the ages 16-45 years, who are in the first five years since psychosis onset and willing to travel to New Haven for care. Subjects with co-morbid mental retardation or clear substance-induced psychosis are excluded from trial participation, but those with diagnostic uncertainty with respect to affective, substance-induced or medical etiologies are enrolled until these can be clarified over longitudinal follow-up.
We conducted a cross-sectional analysis of baseline data from 56 subjects enrolled in the trial between April 2006 and January 2010. Of 85 total enrollees in this period, 29 were not included, either because a complete profile of laboratory results was not yet available (n=21), or because they were diagnosed with non-schizophrenia spectrum disorders by 6 months follow-up (n=8). The 29 patients excluded from this analysis did not differ from those included with respect to demographic and clinical variables. We conducted a comparison of 56 trial subjects with 145 individuals drawn from the U.S. National Health and Nutrition Examination Survey (NHANES) (2005-2006) database. This is a probability sample of the civilian, non-institutionalized U.S. population and was designed to assess nutrition and health status of children and adults in the United States. The strength of this survey was the use of a combination of detailed interviews and physical examinations. (Centers for Disease Control and Prevention, 2005-2006) Along with medical morbidities, it also screens for the presence of anxiety, depression, eating disorders, and panic disorders. Although the survey does not screen for psychotic disorder, it does query for the use of any psychotropic medications. The controls for this analysis neither had psychiatric morbidities nor were on any psychotropic medications. We matched each STEP patient with respect to age, gender, and race with all available controls from the NHANES 2005-2006 database.
Socio-demographic data was collected with a semi-structured questionnaire. Structured Clinical Interview for DSM-IV Axis-I disorders-Patient Edition was used for the assessment of the diagnosis (First et al., 1995). Nicotine use was assessed with AUS/DUS scale (Mueser et al., 1995) and a structured medical history included questions about previous diagnoses of hypertension or diabetes, other medical illnesses and current medications. Physical examination including vital signs, and laboratory evaluation was used to screen for common medical illnesses. Patients were asked to return, when necessary, for fasting blood draws (>8hrs post-prandial).
Patients were categorized as having diabetes in NHANES according to standard clinical criteria (American diabetes association, 2005) with one caveat detailed below. These criteria included symptomatic hyperglycemia with a random plasma glucose level of ≥200mg/dl, or a previous diagnosis confirmed by current prescription of oral hypoglycemics or insulin. Additional ADA criteria includes use of fasting plasma glucose levels (FPG ≥126 mg/dl) or an oral glucose tolerance test (2 hours postload glucose of ≥200mg/dL) which have to be confirmed by repeat testing on a different day, but in NHANES this repeat measurement was not required to classify patients as diabetic. This classification is thus referred to as NHANES-defined diabetes in Table 1. Patients were categorized as having impaired fasting glucose for FPG between 100 to 125 mg/dl. Hypertension was defined as mean systolic blood pressure of 140 mm of Hg or greater and/or mean diastolic blood pressure of 90 mm of Hg or greater or a previous prescription for antihypertensive medications. However, unlike standard clinical criteria (Chobanian et al., 2003) repeat confirmatory measurements within 2 months were not required. Patients were classified as smokers if they had used more than 5 cigarettes in the previous week (Goff et al., 2005).
Table 1.
Comparison of cardiovascular risk factors between the two groups
| Variables | STEP Patients (n=56) | NHANES Controls (n=145) |
|---|---|---|
| Smokers2 | 26 (46%) | 52 (36%) |
| Weight (kgs)1 | 80.1 (14.2) | 81.5 (23.3) |
| Body Mass Index (kg/m2)1 | 25.8 (4.8) | 26.5 (7.0) |
| Systolic blood pressure1 | 126.1 (13.5) | 116.3 (10.5) |
| Diastolic blood pressure1 | 71.3 (1.6) | 63.9 (10.6) |
| NHANES-defined Diabetes2 | 0 (0%) | 2 (1%) |
| Impaired Fasting Glucose (FPG: 100-125mg/dl)2 | 9 (16%) | 18 (12%) |
| Total Cholesterol, mg /dl1 | 171.8 (28.1) | 164.4 (33.5) |
| HDL Cholesterol, mg /dl1 | 48.2 (11.3) | 51.2 (12.9) |
| Prevalence of Metabolic Syndrome, n (%) | 11 (19.6%) | 24 (16.6%) |
| 10-year risk for developing coronary heart disease, %3 | 1 (0-5) | 0 (0-9) |
mean (SD)
n (%)
median (range)
We also transformed the BMI of patients to their respective waist circumference (De Hert et al., 2006) to calculate the prevalence of metabolic syndrome in patients as well as controls (Expert Panel on Detection and Evaluation of Treatment of High Blood Cholesterol in Adults, 2001).
There are two commonly used methods to estimate 10-year risk of developing coronary heart disease. The Framingham coronary heart disease risk score estimates the risk for persons of age 30 years and above (Wilson et al., 1998); while a tool developed by National Cholesterol Education Program estimates the risk for age 20 and above (NCEP-ATP III National Cholesterol Education Program, 2003). We used the latter because most of our patients were between the ages 17-30 years. The NCEP-ATP III tool uses age, gender, total cholesterol, HDL-cholesterol, smoking status, systolic blood pressure and status of antihypertensive medications for the estimation of 10-year risk of developing coronary heart disease (heart attack/myocardial infarction). Risk was classified in three categories depending on the final score: 20% or more = ‘very high’; 10-19%= ‘moderate’; <10%=‘low’ (Grundy et al., 2004).
Computation was done by SPSS 17.0 software for windows (SPSS Inc., Chicago, IL). The independent samples t test and chi-square test was used for continuous and categorical variables, respectively for comparison of demographic variables, cardiovascular risk factors. We used the Wilcoxon rank-sum test to compare 10-year risk of developing coronary heart disease in two groups. We also used Pearsonian bivariate correlation (r) for the assessment of correlation between the NCEP-ATP III and Framingham's tool for assessing the 10-year risk score.
3. Results
The sample for this analysis were young (mean 22.5 years, SD 4.4), predominantly male (89%) early psychosis patients with a variety of preliminary diagnoses including Schizophrenia (35%), schizophreniform disorder (32%), schizoaffective disorder (13%), and psychotic disorder not otherwise specified (20%). The sample was ethnically and racially diverse with 28 (50%) African-American, 10 (17%) Hispanic, 17 (31%) Caucasians. The majority were prescribed risperidone (n=26), and others were on olanzapine (n=12), aripiprazole (n=4), haloperidol (n=3), quetiapine (n=2), ziprasidone (n=1). The median duration of illness was 29 weeks, with a range from <1 week to 163 weeks. The mean (SD) duration of treatment with antipsychotic medication was 2.97 (2.0) weeks, with a range from 0 to 6 weeks. No patients were prescribed either diabetes medications or antihypertensive medications (including propranolol for akathisia).
The comparison of cardiovascular risk factors between the two groups is described in table 1. There was no significant difference in weight, body mass index or total or HDL cholesterol, and the absolute differences between the groups were small and not of clinical significance. Rates of current smoking were quite high among STEP clients (46% of patients compared to 36% of controls), though this difference did not reach statistical significance. The two groups were not different with respect to the prevalence of diabetes (0% of STEP patients vs 1% of controls) or impaired fasting glucose (16% of STEP patients vs 12% of controls). Since previous studies have demonstrated a hierarchy of risk of weight gain and adverse metabolic effects among the second generation antipsychotic medications, with clozapine and olanzapine associated with the greatest risk, we compared patients who were on olanzapine (n=12) with those who were taking other antipsychotic medications (n=36). The mean (SD) fasting plasma glucose in patients who were on olanzapine was 97.8 (15.6) mg/dl, which was significantly higher than those on all other antipsychotic medications 91.0 (7.4) mg/dl [t=2.220; p=0.03]. Controls did not have psychiatric illnesses and they were not on any psychotropic medications.
The number of subjects with elevated systolic BP (>140 mm Hg) was 12 of which one patient also had elevated diastolic BP (>90 mm Hg) and an additional 4 patients had only elevated diastolic BP. None of the patients had previously been diagnosed with or prescribed medications for hypertension. The frequency of cross-sectional elevations in blood pressure in the STEP sample was thus 16 out of 56 (29%) compared to 3 (2%) in the control or NHANES sample, but neither reflect a true diagnosis of hypertension. Follow-up measurements within two months were available on 6 of these patients, all of whom met criteria for hypertension, resulting in an estimate of at least 11% in our sample. Since repeat measures were not done in NHANES, comparison to the matched cohort was not possible. We also assessed the glycosylated hemoglobin (HbA1C) level for patients. The mean (SD) HbA1C level was 5.6 (0.3), which ranges from 4.7 to 6.3. We were unable to compare it with controls because during NHANES-2005 to 2006, glycosylated hemoglobin (HbA1C) level was not collected. The prevalence of metabolic syndrome in STEP patients was 11 (19.6%) and 24 (16.6%) in controls. The difference between them was not statistically significant (χ2=0.04; p=0.83).
We calculated 10-year risk of developing coronary heart disease in 47 patients and 122 controls using the NCEP-ATP III risk calculator. The median (range) risk for patients and controls was 1 (0-5) % and 0 (0-9) % respectively. There was no significant difference between the two groups (Wilcoxon W=10 357; p=0.119). Both groups were in the ‘low risk’ (<10%) category. Males and females did not differ in any of the measured cardiovascular risk factors in our sample. There was significant correlation between the NCEP-ATP III and Framingham estimation scores of 10-year risk of developing coronary heart disease (r=0.953; p<0.001).
4. Discussion
The major finding of this study is that patients who are early in the course of a psychotic illness, do not yet significantly differ from their peers in terms of extant 10-year risk calculators for coronary heart disease. This contrasts with consistent reports of increased risk among patients later in the course of psychotic illnesses and supports the proposal that early intervention programs embrace a role in primary prevention of cardiovascular morbidity.
Two cardiovascular risk factors that emerged in our first episode sample were rates of smoking and hypertension. A significant fraction of our cohort were active smokers (46%) and although they were comparable to a matched non-psychotic sample (36%), this represents a significant opportunity for primary prevention. We found a higher incidence of cross-sectional elevations in blood pressure in our sample (29%) compared to NHANES (2%) and were able to confirm a diagnosis of hypertension by standard criteria in 11% of our sample, although this is likely an underestimate, as we do not yet have follow-up measurements on 10 of the 16 with initial elevations.
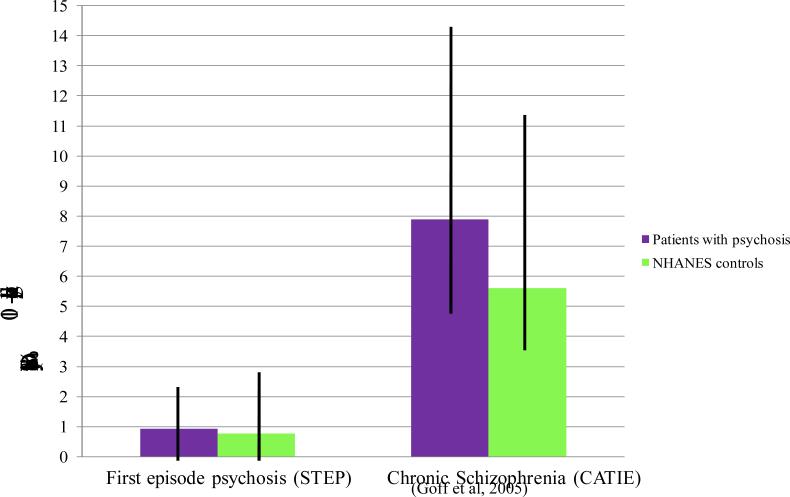
The contrast between our findings in a first episode sample and previous studies of samples with more chronic illness is striking. A comparison of cardiovascular risk factors in patients from the CATIE trial with age, gender and race matched controls from NHANES (Goff et al., 2005) revealed a significantly higher 10-year Framingham coronary heart disease risk score in male (9.4% vs 7.0%) and female (6.3% vs 4.2%) patients as compared to controls. Patients also had significantly higher rates of smoking (68% vs 35%), diabetes (13% vs 3%), and hypertension (27% vs 17%) and lower HDL cholesterol levels (43.7 mg% vs 49.3 mg %) compared to controls. Figure 1 shows the comparison of first episode psychosis (STEP) patients and chronic schizophrenia compared with age, gender and race matched controls from NHANES.
Figure 1.
Comparison of first episode psychosis (STEP) patients and chronic schizophrenia compared with age, gender and race matched controls from NHANES
Our findings are consistent with previous early illness studies and addresses limitations of previous studies (Sengupta et al., 2008; Arranz et al., 2004), by including a comparison with a control population evaluated for psychiatric morbidity and smoking status. To our knowledge, this is the first analysis to quantify and compare overall and longer term cardiovascular risk in an early psychosis sample, and we believe this provides a useful way to conceptualize and prioritize approaches toward prevention in this group of patients. Based on the results of this study, smoking may be the most important cardiovascular risk factor to address among first episode patients.
A limitation of this analysis was the use of a convenience or non-probability sample of early psychosis patients, which may not represent the true burden of risk factors in this population. There was also an over-representation of males (89%). Hence, the study may have been under-powered to detect differences. A second limitation is that some important cardiovascular risk factors were not evaluated in this study, either not measured because of feasibility issues (waist circumference and waist to hip ratio which are more reliable measures of abdominal obesity, insulin levels, glucose tolerance tests,) or comparisons could not be conducted because data was not collected in the NHANES comparison group (family history of coronary heart disease, true diagnoses of diabetes or hypertension) These additional variables, however, were not required in the risk prediction tools and, thus did not prevent the estimation of 10-year coronary heart disease risk.
It is important to acknowledge that, to date, no cardiovascular risk engine has been validated in a population with serious mental illness. It is quite possible, given the increased prevalence of CVD among persons with serious mental illness, that the existing risk engines (including the one used in this study), actually underestimate the true CVD risk among persons with mental illness. Future research should aim to validate current CVD risk engines, or develop or adapt one specific to this high risk population.
The best evidence to explain how relatively low risk samples like our first episode patients progress toward the high risk profile of the CATIE sample may be provided by studies that have looked at the effect of medication treatment in early samples. Weight gain occurs rapidly in the first few weeks of treatment with antipsychotics which continues during the following months in drug-naïve schizophrenia patients (Tarricone et al., 2010). After 9 months use of atypical antipsychotic medications, lean, drugenaïve, schizophrenia patients who were initially free of metabolic syndrome, had significant weight gain, and truncal fat accumulation associated with decreases in adiponectin and hyperbolic product with increased fasting glycaemia as well as impaired fasting glucose (Oriot et al., 2008). When early behavioral interventions (EBI) were given for 3 months to prevent antipsychotic induced weight gain, patients in EBI group gained significantly less weight as compared to treatment as usual group at the end of the interventon, but these differences were not durable at 12 month follow-up. Thus, weight management interventions may need to be offered for longer periods to maintain preventive effects (Alvarez-Jiminez et al., 2010).
Moreover, there are important differences among antipsychotic medications with respect to adverse metabolic effects, and these may be of even greater importance in the treatment of a first episode of psychosis. In a one year follow up report of a Comparison of Atypicals for First Episode (CAFE) study, authors reported that significant weight gain (≥7% from baseline) occurred in 80% of cases with olanzapine, 57.6% of cases with risperidone, and 50% of cases with quetiapine treatment (Patel et al., 2009). In a 2-year follow up study of first-episode patients, olanzapine (34 lbs) was associated with significantly more weight gain than haloperidol (16.5 lbs) (Zipursky et al., 2005). There is evidence that a significant proportion of weight gain occurs early in the course of antipsychotic treatment (Blin and Micallef, 2001). In a randomized, open label, prospective study of 12 weeks duration, olanzapine was associated with the maximum weight gain (7.5 kg) compared to risperidone (5.6 kg) and haloperidol (3.8 kg) (Perez-Iglesias et al., 2007). Interestingly, in longer term ( one year) follow-up of this sample, the difference in weight gain across medications disappeared: 10.9 kg for olanzapine, 8.9 kg for risperidone, and 9.7 kg for haloperidol (Perez-Iglesias, et al., 2008).
To the best of our knowledge, this is the first study where the 10-year risk of developing coronary heart disease was measured in first-episode psychosis patients and compared with age, gender, and race matched controls from a well-characterized sample assessed for smoking status. The evidence of the markedly increased coronary heart disease risk in chronic (and even relatively early treated) samples make a compelling case for risk reduction strategies, with a focus on smoking cessation and careful monitoring for other traditional risk factors, including hypertension. EI programs have an opportunity to implement and evaluate approaches to primary and secondary prevention of cardiovascular disease that will enrich the traditional focus on reducing the long-term psychosocial morbidity.
Acknowledgement
None
Role of funding source:
The Specialized Treatment Early in Psychosis (STEP) Program is supported by The Donaghue Foundation Grant number DF07-014 and by the National Institute of Health (NIH), Grant number 1RC1MH088971-01. Both The Donaghue Foundation and the NIH had no further role in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
All authors declare that they have no conflicts of interest
References
- 1.Alvarez-Jimenez M, Martinez-Garcia O, Perez-Iglesias R, Ramirez ML, Vazquez-Barquero JL, Crespo-Facorro B. Prevention of antipsychotic-induced weight gain with early behavioural intervention in first-episode psychosis: 2-year results of a randomized controlled trial. Schizophr Res. 2010;116(1):16–19. doi: 10.1016/j.schres.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl. 1):S37–S42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 3.Arranz B, Rosel P, Ramirez N, Duenas R, Fernandez P, Sanchez JM, Navarro MA, San L. Insulin resistance and increased leptin concentrations in noncompliant schizophrenia patients but not in antipsychotic-naive first-episode schizophrenia patients. J. Clin. Psychiatry. 2004;65(10):1335–1342. doi: 10.4088/jcp.v65n1007. [DOI] [PubMed] [Google Scholar]
- 4.Blin O, Micallef J. Antipsychotic-associated weight gain and clinical outcome parameters. J. Clin. Psychiatry. 2001;62(Suppl. 7):11–21. [PubMed] [Google Scholar]
- 5.Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br. J. Psychiatry. 2000;177:212–217. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, Himelhoch S, Fang B, Peterson E, Aquino PR, Keller W, Schizophrenia Patient Outcomes Research Team (PORT) The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr. Bull. 2010;36(1):71–793. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capasso RM, Lineberry TW, Bostwick JM, Decker PA, St Sauver J. Mortality in schizophrenia and schizoaffective disorder: an Olmsted County, Minnesota cohort: 1950-2005. Schizophr. Res. 2008;98(1-3):287–294. doi: 10.1016/j.schres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2005-2006. [January 26, 2010]. [ http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 10.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and cause of death among public mental health clients in eight states. Prev. Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 11.De Hert MA, van Winkel R, Van Eyck D, Hanssens L, Wampers M, Scheen A, Peuskens A. Prevalence of the metabolic syndrome in patients with schizophrenia treated with antipsychotic medication. Schizophr. Res. 2006;83(1):87–93. doi: 10.1016/j.schres.2005.12.855. [DOI] [PubMed] [Google Scholar]
- 12.De Hert M, Schreurs V, Vancampfort D, Winkel RV. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76(2-3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 14.de Leon J. Beyond the “hype” on the association between metabolic syndrome and atypical antipsychotics: the confounding effects of cohort, typical antipsychotics, severe mental illness, comedications, and comorbid substance use. J. Clin. Psychopharmacol. 2008;28(2):125–131. doi: 10.1097/JCP.0b013e318166f533. [DOI] [PubMed] [Google Scholar]
- 15.Druss BG, Bradford WD, Rosenheck RA, Radford MJ, Krumholz HM. Quality of medical care and excess mortality in older patients with mental disorders. Arch. Gen. Psychiatry. 2001;58(6):565–572. doi: 10.1001/archpsyc.58.6.565. [DOI] [PubMed] [Google Scholar]
- 16.Enger C, Weatherby L, Reynolds RF, Glasser DB, Walker AM. Serious cardiovascular events and mortality among patients with schizophrenia. J. Nerv. Ment. Dis. 2004;192(1):19–27. doi: 10.1097/01.nmd.0000105996.62105.07. [DOI] [PubMed] [Google Scholar]
- 17.Expert Panel on Detection and Evaluation of Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorder - Patient Edition (SCID-I/P), Version 2.0. Biometric Research, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 19.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D'Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr. Res. 2005;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ. (see endnote) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J. Am. Coll. Cardiol. 2004;44(3):720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 22.Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst. Rev. 2006;4:CD004718. doi: 10.1002/14651858.CD004718.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Meigs JB, Wilson PW, Nathan DM, D'Agostino RB, Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52(8):2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 24.Mueser KT, Drake RE, Clark RE, McHugo GJ, Mercer-McFadden C, Ackerson TH. A toolkit for evaluating substance abuse in persons with severe mental illness. Evaluation center at Human Services Research Institute; Cambridge, MA: 1995. [Google Scholar]
- 25. [January 26, 2010];National Cholesterol Education Program: Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) http://hp2010.nhlbihin.net/atpiii/calculator.asp?usertype=prof.
- 26.Newcomer JW. Comparing the safety and efficacy of atypical antipsychotics in psychiatric patients with co-morbid medical illnesses. J. Clin. Psychiatry. 2009;70(Suppl. 3):30–36. doi: 10.4088/JCP.7075su1c.05. [DOI] [PubMed] [Google Scholar]
- 27.Oriot P, Feys JL, Mertens de Wilmars S, Misson A, Ayache L, Fagnart O, Gruson D, Luts A, Jamart J, Hermans MP, Buysschaert M. Insulin sensitivity, adjusted beta-cell function and adiponectinaemia among lean drug-naive schizophrenic patients treated with atypical antipsychotic drugs: a nine-month prospective study. Diabetes Metab. 2008;34(5):490–496. doi: 10.1016/j.diabet.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom's General Practice Rsearch Database. Arch. Gen. Psychiatry. 2007;64(2):242–249. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- 29.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr. Res. 2000;45(1-2):21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 30.Padmavati R, McCreadie RG, Tirupati S. Low prevalence of obesity and metabolic syndrome in never-treated chronic schizophrenia. Schizophr. Res. 2010;121(1-3):199–202. doi: 10.1016/j.schres.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Parks J, Svendsen D, Singer P, Foti M. Morbidity and mortality in people with serious mental illness. [January 26, 2010];National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council. 2006 http://www.nasmhpd.org/general_files/publications/med_directors_pubs/Technical%20Report %20on%20Morbidity%20and%20Mortaility%20-%20Final%2011-06.pdf.
- 32.Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, Lieberman JA, CAFE Investigators Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr. Res. 2009;111(1-3):9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Iglesias R, Crespo-Facorro B, Amado JA, Garcia-Unzueta MT, Ramirez-Bonilla ML, Gonzalez-Blanch C, Martinez-Garcia O, Vazquez-Barquero JL. A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J. Clin. Psychiatry. 2007;68(11):1733–1740. doi: 10.4088/jcp.v68n1113. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O, Ramirez-Bonilla ML, Alvarez-Jimenez M, Pelayo-Teran JM, Garcia-Unzueta MT, Amado JA, Vazquez-Barquero JM. Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naive population. Schizophr. Res. 2008;99(1-3):13–22. doi: 10.1016/j.schres.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first episode, drug-naive patients with schizophrenia. Am. J. Psychiatry. 2003;160(2):284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- 36.Sengupta S, Parrilla-Escobar MA, Klink R, Fathalli F, Ng YK, Stip E, Baptista T, Malla A, Joober R. Are metabolic indices different between drug-naive, first-episode psychosis patients and healthy controls? Schizophr. Res. 2008;102(1-3):329–336. doi: 10.1016/j.schres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diab. Med. 2007;24(5):481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 38.Srihari VH, Breitborde NJK, Pollard J, Tek C, Hyman L, Frisman LK, McGlashan TH, Jacobs S, Woods SW. Public academic partnerships: early intervention for psychotic disorders in a community mental health center. Psychiatr. Serv. 2009;60(11):1426–1428. doi: 10.1176/appi.ps.60.11.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahl SM, Mignon L, Meyer JM. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr. Scand. 2009;119(3):171–179. doi: 10.1111/j.1600-0447.2008.01334.x. [DOI] [PubMed] [Google Scholar]
- 40.Tarricone I, Ferrari Gozzi B, Serretti A, Grieco D, Berardi D. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol. Med. 2010;40(2):187–200. doi: 10.1017/S0033291709990407. [DOI] [PubMed] [Google Scholar]
- 41.Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620–627. doi: 10.1016/S0140-6736(09)60742-X. [DOI] [PubMed] [Google Scholar]
- 42.Verma SK, Subramaniam M, Liew A, Poon LY. Metabolic risk factors in drug-naive patients with first-episode psychosis. J. Clin. Psychiatry. 2009;70(7):997–1000. doi: 10.4088/JCP.08m04508. [DOI] [PubMed] [Google Scholar]
- 43.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 44.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 45.Zipursky RB, Gu H, Green AI, Perkins DO, Tohen MF, McEvoy JP, Strakowski SM, Sharma T, Kahn RS, Gur RE, Tollefson GD, Lieberman JA. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br. J. Psychiatry. 2005;187:537–543. doi: 10.1192/bjp.187.6.537. [DOI] [PubMed] [Google Scholar]