Abstract
Variability in TLR function influences susceptibility to infectious as well as immune-mediated diseases. Given the outbred nature of humans, identifying functional Toll-like receptor variability and its role in clinical disease requires such analysis to be conducted in large, often multi-centered cohorts. Yet the technically complex measurements involved in innate immune analysis benefit from centralized processing of samples. Centralization requires shipping of samples or prolonged storage, possibly even cryopreservation. Deviation from standard operating procedures (SOP) for sample procurement, storage and processing may alter the final innate immune read out. We here set out to define the impact of variables most likely to be encountered during large, multi-site studies: (i) the source of the sample, (ii) time between sample procurement to processing, and (iii) processing of fresh vs. cryopreserved samples. We found that all of these variables exert a profound impact on the final innate response to TLR stimulation. Specific innate responses appeared to be affected in response to specific TLR stimuli by a particular variable under study, proving it impossible to provide global generalizations. Based on our studies and other published work on this topic, we propose a minimal list of variables that have to be met for samples to be comparable within and across studies: a) timing between procurement and processing can not vary by more than 10%; b) all samples have to be stored the same, c) the source of samples needs to be the same. In summary, for innate immune analysis scrupulous adherence to standard operating proecdures is paramount.
Keywords: cryopreservation, Toll-like receptor, innate immunity, cohort study, cord blood, dendritic cell, Luminex, standard operating procedures
1. INTRODUCTION
The innate immune system integrates multiple sources of information from the environment into signals that not only activate immediate innate effector functions, but also initiate antigen processing, costimulatory molecule expression and cytokine secretion that direct the ensuing adaptive immune response (reviewed in (Akira et al., 2006)). The innate immune system gathers information about the environment via specialized sensors such as pattern recognition receptors (PRRs), which respond to conserved, pathogen-associated molecular patterns (PAMPs). Amongst the best-studied PRRs are the Toll-like receptors (TLRs). Given the environment-sensing function of the innate immune system in general, and the sentinel function of PRRs in particular, innate immune cells are specifically geared towards detecting minute changes in the environment. Such high level of sensitivity of the innate immune system is partly responsible for its ability to aid in protecting the host from microbial invasion (Turvey and Hawn, 2006; Barreiro et al., 2009). However, the same exquisite sensitivity also makes measuring innate immune responses prone to technical artifacts. We had previously described a high-throughput system to study innate immune responses that controlled technical artifacts during in vitro stimulation (Jansen et al., 2008). However, we had not delineated technical variables affecting biological samples prior to in vitro stimulation that could affect subsequent innate immune response measurements. Only with precise knowledge of all potential artifacts and their impact on the resulting innate immune measurements can experiments be adequately planned, controlled and data correctly interpreted. This knowledge is currently lacking. We here set out to systematically analyze the variables most pertinent to large, multi-site studies.
2. METHODS
2.1. Preparation of TLR stimulation plates
TLR stimulation plates were prepared as described previously (Jansen et al., 2008). Briefly, deep-96-well (VWR) source plates containing 1.3 ml of various TLR ligands at 10 times the desired concentration were prepared using sterile procedures under a laminar airflow hood. The following TLR ligands were used: PAM3CSK4 (PAM; TLR2/1; EMC Microcollections; 10 μg/ml final concentration); 0111:B4 LPS (LPS; TLR4; Invivogen; 100 ng/ml); R848 (TLR7/8; Invivogen; 10 μM). For the 6-h intracellular cytokine staining (ICS) plates, Brefeldin A (BFA; Sigma-Aldrich) was added at a concentration of 100 μg/ml (10 times the desired final concentration of 10 μg/ml) to each well. BFA was not added to the 10× source plates for the plates that were used to obtain 24-h supernatants for Luminex cytokine quantification. Source plates were sealed with sterile aluminum plate sealers (USA Scientific), frozen at −80°C, and thawed before use. Twenty microliters from each well of the source plate was dispensed into each well of recipient 96-well round-bottom polystyrene plates (Corning) using the Evolution P3 Precision Pipetting Platform (PerkinElmer) under a laminar airflow hood using sterile procedures. Recipient plates were sealed with sterile aluminum plate sealers and frozen at −80°C until use.
2.2. Blood sample processing and in vitro stimulation
All studies were approved by the Research Ethics Board at the University of British Columbia and BC Children’s Hospital. Peripheral blood (7–10 ml per tube) or cord blood was drawn via sterile venipuncture into Vacutainers containing 143 USP units of sodium-heparin (Becton Dickinson (BD) Biosciences, catalog no. 8019839) using batches we had previously confirmed to be free of innate immune activating substance in assays performed as described elsewhere (Jansen et al., 2008). Blood samples were processed as described previously (Jansen et al., 2008). Mononuclear cells (MC) were isolated by Ficoll-Paque density gradient centrifugation. Whole blood (WB) was mixed 1:1 with sterile pre-warmed (37°C) RPMI-1640 medium (RPMI, Invitrogen). MC were cultured in RPMI supplemented with 100 units penicillin/ml, 100 mg streptomycin/ml (Invitrogen) and 10% human AB serum (Gemini Bio-Products). Two hundred microliters of MC suspension (2.5 × 106 MC/ml) or WB mixed 1:1 with RPMI was added to each well of the premade plates containing the specific TLR ligands. For the ICS assays, cells were incubated for 6 h at 37°C in 5% CO2. After culture, cells were treated with a final concentration of 2 mM EDTA for 15 min at 37°C, and then spun down. MC cultures were resuspended in 100 μl of 1x BD FACS Lysing Solution. For plates containing WB, the entire mixture was added to 1400 ml BD FACS Lysing Solution in deep 96-well plates (Nunc). Both WB and MC cultures were then sealed and stored frozen at −80°C until staining. An identical set of plates was incubated in parallel for 24 h without BFA; at 24 h, these plates were spun and 100 μl of supernatant was removed and frozen at −80°C for later Luminex analysis. We cryopreserved the remaining MC suspension using a published protocol (Maecker et al., 2005a; Disis et al., 2006). For this, the density-purified MC were slowly resuspended in pre-chilled freezing medium containing RPMI, 12.5 % serum (FCS unless indicated) and 12.5% DMSO, and 10 × 106 MC/ml. Controlled cooling of the cells to −80°C overnight was facilitated by a Mr. Frosty freezing chamber (Nalgene). The frozen MC were then transferred the next morning to a liquid nitrogen storage unit (vapor phase) for long-term maintenance of viability.
2.3. Staining, acquisition, and analysis
Preparation of the samples for flow cytometric analysis was performed as described previously (Jansen et al., 2008; Kollmann et al., 2009; Blimkie et al., 2010). A detailed description of antibodies (Ab) (source, clone, and dilution), machine set up, and data acquisition compliant with the recently accepted MiFlowCyt reporting standards (Lee et al., 2008; Blimkie et al., 2010) can be found in the Supplementary Text. Briefly, frozen plates were thawed and spun, and pellets were resuspended in 200 μl of BD FACS Permeabilizing Solution and incubated at room temperature for 10 min. After one wash in PBS containing 0.5% BSA and 0.1% sodium azide (PBSAN), cells were stained in a final volume 100 μl of PBSAN for 30–60 min at room temperature. After two additional washes with PBSAN, cells were resuspended in PBS containing 1% paraformaldehyde and immediately analyzed on an LSRII Flow Cytometer (BD Biosciences) set up according to published guidelines (Perfetto et al., 2006; Jansen et al., 2008; Blimkie et al., 2010). Compensation beads (CompBeads; BD Biosciences) were used to standardize voltage settings and used as single-stain positive and negative controls as described previously (Maecker et al., 2005b; Jansen et al., 2008; Blimkie et al., 2010). A total of 200,000 events were acquired for MC and 1,000,000 for WB. Compensation was set in FlowJo (Tree Star) and samples were analyzed compensated. The fluorescence-minus-one principle was used to set gates, and changes in cell size (FSC) and granularity (SSC) were used to identify live vs. dead target cells (Lamoreaux et al., 2006; Maecker and Trotter, 2006). We positioned the unstimulated flow cytometric samples as a biological negative control; this has been identified as the most appropriate approach for flow cytometric analysis of stimulation experiments (Maecker and Trotter, 2006).
2.4. Assessment of cytokines in culture supernatant
Supernatants were thawed at room temperature, and then filtered through a 1.2-μm filter plate (Millipore) into a clean 96-well plate to remove any remaining cellular debris using a multi-screen HTS vacuum manifold (Millipore). The Luminex assay was performed using Millipore’s Milliplex system with an overnight incubation of the sample and bead mixture at 4°C. Samples were diluted 1-to-1 (and, if needed to fall within the standard curve, 10- or 20-fold) with RPMI 1640 supplemented with 10% human AB serum. Beadlytes, biotin, and streptavidin-phycoerythrin, were used at half the manufacturer’s recommended concentrations. Assays were read using Luminex 200 Total System (Luminex) running the MasterPlex (MiraiBio) software, and the downstream analysis was performed using Excel (Microsoft) and an in-house database. The level of cytokine production detected in culture supernatants of unstimulated samples was not subtracted from the stimulated samples but shown side by side as this data is possibly biologically relevant (Marchini et al., 2000; Schultz et al., 2002; Romero et al., 2006).
2.5. Statistical and data analysis
Graphs were prepared and analyzed (e.g. standard error mean, SEM) using Excel (Microsoft). The pass-fail analysis to model the effect of blood processing delay on innate immune response is detailed in the Supplementary material.
3. RESULTS
We structured our study according to the most commonly encountered obstacles in studies involving human samples: processing time, storage considerations, and sample source.
3.1. Processing time
From our own experience as well as that of others, the most difficult aspect to standardize in any large, multi-site cohort study is the time between sample procurement and processing. We thus chose to address this variable first. We initiated our evaluation focused on whole blood (WB), as WB requires the least manipulation, i.e. allows the most direct assessment of only a delay in stimulation without any other confounding artifacts. To this end, we obtained blood from healthy adult volunteers and initiated the TLR stimulation immediately after venipuncture (i.e. at 15 minutes, as this was the earliest time point feasible; from hereon, we label this time point 0 h), or after storing the WB at room temperature for an additional 4, 8, 12, 24 or 48 hours prior to stimulation. Whole blood (WB) was then either left unstimulated, or stimulated with PAM3CSK4 (TLR2/1 ligand), LPS (TLR4 ligand) or R848 (TLR7/8 ligand), with cytokine production at the single-cell level assessed after 6 hours of stimulation via intracellular cytokine staining (ICS), or globally after 24 h of stimulation via determination of cytokines secreted into the culture supernatant using multiplexed assays (Luminex).
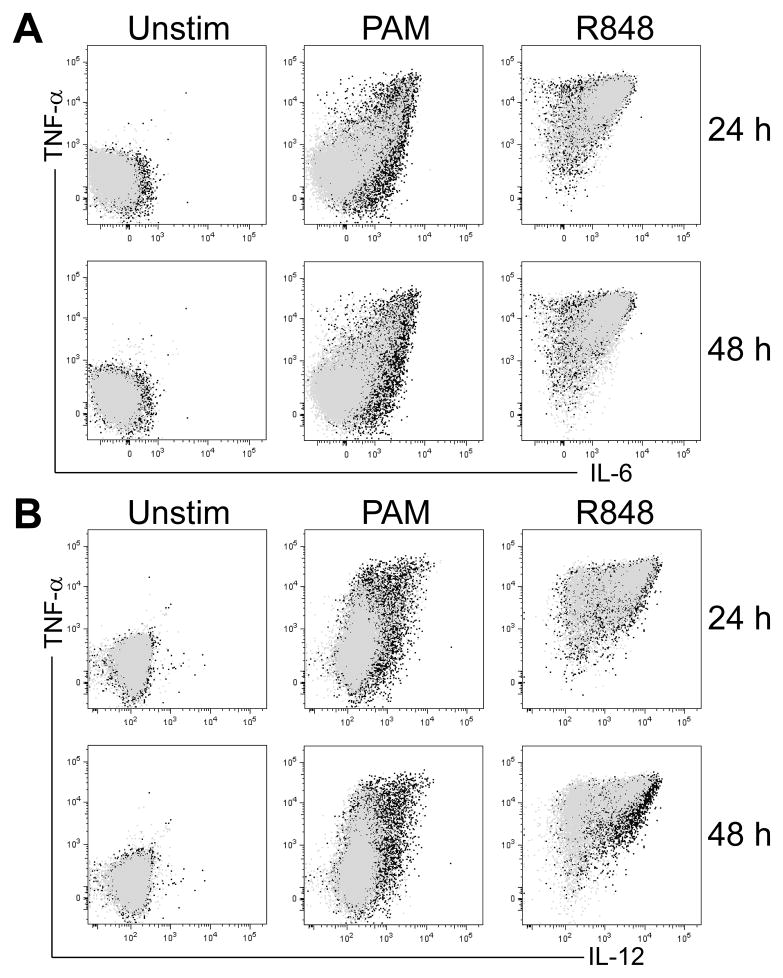
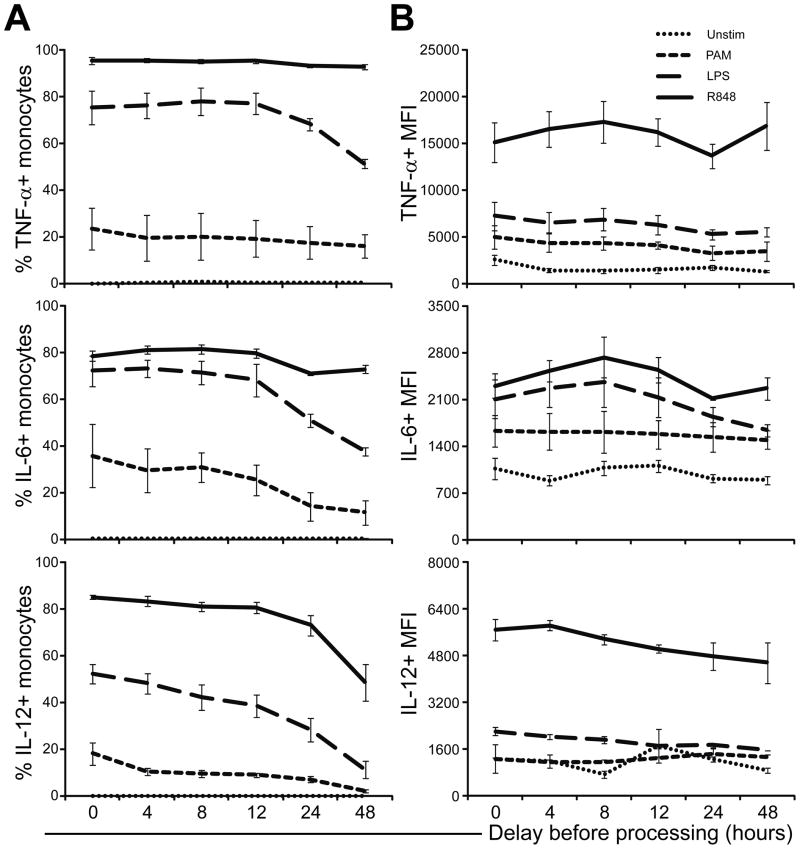
The delay in processing did not lead to a significant change in cell viability based on either trypan-blue exclusion or flow cytometric FSC/SSC assessment, nor a detectable change in cell composition based on flow cytometric assessment of surface phenotype (data not shown). Using ICS, we analyzed monocytes, cDC, pDC and B cells separately (Supplementary Figure 1) with respect to intracellular TNF-α, IL6, IL-12/23p40 and IFN-α production. As we had previously described, B cells were found not to produce any of the 4 cytokines measured by ICS in response to any of the stimuli employed (Blimkie 2010); thus they are not depicted in any of the subsequent ICS-based figures. As an example of this approach to ICS, Figure 1 shows TNF-α, IL6 and IL-12/23p40 production by monocytes in whole blood in response to PAM or R848 stimulation contrasting the 0 h time point to the 24 h and 48 h time points. Monocytes were not found to produce IFN-α to any of the stimuli employed. For each of our single-cell innate targets, we analyzed both the fraction producing a given cytokine (i.e. the % of cells scored as positive for cytokine expression), as well as the level of cytokine production per cell, i.e. the mean fluorescence intensity (MFI). The response of monocytes over the complete time course in delaying stimulation with PAM, LPS or R848 is depicted in Figure 2 for each of the 3 cytokines detectable in monocytes, i.e. TNF-α, IL-6 and IL-12/23p40. It shows that there is little change in intracellular expression of any of the 3 cytokines in the unstimulated sample over the entire time period. However, the ability of monocytes to produce IL-6 and IL-12/23p40 in response to PAM stimulation declined steadily over the measured interval of delay in processing. TNF-α production of monocytes in response to PAM or R848 appeared unaffected by the delay; however, TNF-α was produced less by monocytes in response to LPS in WB stored for 48 h. Similarly, IL-12/23p40 was produced less by monocytes in response to R848 in WB stored for 48 h. The MFI of monocytes overall showed a similar trend for each of the cytokines as the respective percent-positive data. Analyzing the impact of a delay in stimulation on monocytes’ ability to produce the 3 measured cytokines simultaneously, i.e. their polyfunctionality, revealed a shift in specific polyfunctional cytokine combinations following all three stimuli (Supplementary Figure 2). The analysis of polyfunctionality also identified a steady decline in the total response (i.e. height of the bars) from as early as 4 h delay in the response to PAM, but only after 24 h in response to LPS, and not at all in response to R848.
Figure 1. An example of intracellular cytokine staining illustrating the extent of change in cytokine expression after TLR stimulation in adult whole blood monocytes with delayed stimulation.
An overlay is used to compare the freshly stimulated sample (0 h; black) with the sample stimulated after a delay (grey) of either 24 h (top) or 48 h (bottom) row. A) shows TNF-α vs. IL-6 intracellular expression; B) shows TNF-α vs. IL-12/23p40 intracellular expression after stimulation with either a TLR2/1 ligand (PAM, 10 μg/ml), or TLR7/8 ligand (R848; 10 μM).
Figure 2. Delay of stimulation impacts whole blood monocyte cytokine responses to TLR stimulation in a stimulus- and cytokine-dependent manner.
Whole blood samples from healthy adults (n = 3) were stimulated for 6 hours with nothing (Unstim), a TLR2/1 ligand (PAM, 10 μg/ml), a TLR4 ligand (LPS, 100 ng/ml), or a TLR7/8 ligand (R848; 10 μM) either fresh (0) or a delay of 4–48 hours. The intracellular expression of TNF-α, IL-6 and IL-12/23p40 by monocytes was determined by polychromatic flow cytometry. (A) shows the percent of cells expressing TNF-α (top), IL-6 (middle), or IL-12/23p40 (bottom); B) shows the corresponding mean fluorescence intensity values. Means for each population were derived using the FlowJo software; error bars indicate SEM.
The impact of time on the response of cDC and pDC was assessed in a similarly comprehensive manner. While overall similarly affected as compared to monocytes, we found that cDC appeared even more sensitive to the delay in stimulation, as their ability to produce TNF-α in response to PAM and LPS dropped off with a 48 h delay, and even very strong stimuli such as R848 were not able to induce cytokine production comparable to the 0 h time point beyond a 24 h delay in stimulation (Supplementary Figure 3A, 3B). This was also reflected in the analysis of polyfunctionality of cDC (Supplemental Figure 3C). pDC only responded to R848, and exhibited a rapid decline in their ability to produce TNF-α and IFN-α with as early as 4 h delay in stimulation, followed by a steady decline with increasing time (Supplementary Figure 4). This was reflected in the analysis of the fraction of pDC expressing TNF-α or IFN-α (Supplementary Figure 4A), their MFI (Supplementary Figure 4B) or their ability to express both TNF-α and IFN-α (Supplementary Figure 4C). Surprisingly, the ability of pDC to produce only TNF-α alone and not IFN-α alone appeared to increase again with increasing delay of stimulation (Supplemental Figure 4C).
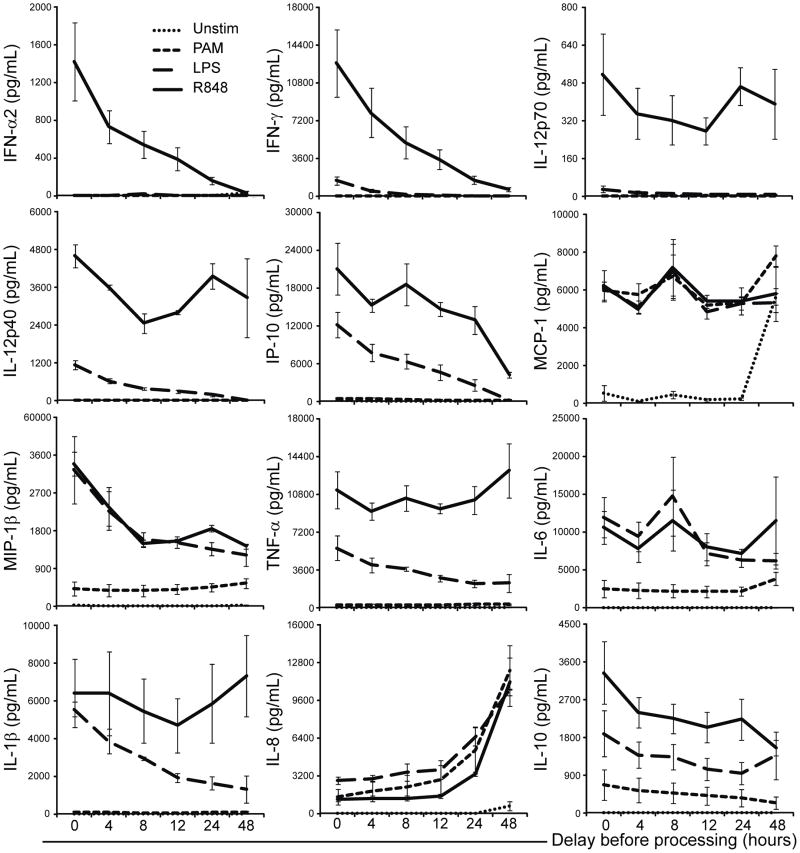
We also assessed the impact of processing delay on global WB cytokine responses by determining the concentration of cytokines secreted in the culture supernatant after 24 h of stimulation (Figure 3). With an increase in delay of stimulation of WB with R848 we observed a rapid decline in IFN-α or IFN-γ response already after processing delays of only 4 h; this decline continued with increasing delay in stimulation. Similar rapid declines were noted for IL-10, MIP-1β, and IP-10 in response to PAM and LPS, and for IL-12/23p40, TNF-α, IL-1β in response to LPS. IL-8 production displayed a striking increase with increasing processing delay but only after stimulation, while MCP-1 showed a relatively steady response following stimulation but also an increase in production even in the unstimulated samples with increasing delay prior to stimulation.
Figure 3. Bulk measurement of secreted cytokine production in whole blood after processing delay in response to TLR stimulation.
Cytokines secreted by 100 μl of adult whole blood (n = 3) into tissue culture media containing the indicated TLR ligands were measured by Luminex’s xMAP cytokine assays. The cultures were stimulated for 24 h at 37°C, 5% CO2, after which the supernatants were harvested and frozen at −80°C until time of assay. The y-axis represents the mean cytokine concentration in pg/ml; x-axis indicates the delay in stimulation measured in hours; error bars indicate SEM.
In Table 1 we calculated a simple model to assess the impact of variability in processing delay time on innate immune responses to TLR stimulation of whole blood samples. A detailed description of this model is provided in the Supplementary material. The question we asked was: What impact does variability in processing delay have on the final measured values? We assigned variability that resulted in measured changes of < 1 SEM a ‘pass’ grade. We found that variability in processing delay by only 10% – e.g., a 6-minute delay in processing for samples processed at 1 hour of procurement; a 24-minute delay for samples processed at 4 hours; or 288-minute delay for samples processed at 48 hours – resulted in a drop in pass rates of just below 10% (8.8%). Not surprisingly, with increasing variability in time to processing, fewer and fewer measurements passed. While variation in time to processing of < 10% resulted in failures of < 10%, variation in time of > 10% resulted in failures of > 10% of read-outs. This clearly emphasizes the importance of controlling for the time between venipuncture and blood processing when evaluating innate responses to TLR stimulation.
Table 1.
Estimating the impact of variability in time to processing on the measured innate immune response.a
| Percent change in time | Percentage passed |
|---|---|
| 1% | 100.00% |
| 5% | 97.22% |
| 10% | 92.22% |
| 15% | 83.89% |
| 20% | 76.11% |
| 30% | 57.78% |
| 40% | 44.44% |
| 50% | 40.00% |
| 60% | 32.78% |
| 70% | 26.67% |
| 80% | 22.78% |
The test is defined as: Does a given variability in the time of processing result in an estimated value that is within 1 SEM of the value at the particular time of processing. Percentage ‘passed’ reflects the proportion of tests that hold true (i.e., the delay in time resulted in calculated change in innate immune response that is within 1 SEM of the desired time point). Estimates are based on a piece-wise approximation of a curve of the percent change in concentration from the base of zero hours (see Supplementary material for detailed description of the method employed). The test was performed for each cytokine for each ligand for a point between each successive pair of measured time points (n = 180 tests).
Peripheral blood mononuclear cells (PBMC) are also a frequently targeted source of innate cells for TLR stimulation assays. We thus subjected PBMC to the same rigorous analysis using single-cell specific and global culture supernatant cytokine detection as described above for WB, and depicted the PBMC ICS and Luminex results in Supplementary Figures 5 and 6, respectively. To assess the impact of stimulation delay on PBMC, we either purified PBMC from WB immediately after venipuncture, or after 4, 8, 12, 24, and 48 h of storing the WB at room temperature. The process of PBMC purification from WB takes approximately 2 hours, such that the final delay of PBMC stimulation was in fact 2, 6, 10, 14, 26 and 50 h from venipuncture. Because of quantitative differences in innate cellular composition of WB and PBMC, a direct comparison of cytokine levels secreted into the supernatant between WB and PBMC was not feasible (see below for a cytometry-based, single-cell comparison of WB to PBMC in Section 3.3 ‘Source of the innate cells’). However, comparing the qualitative Luminex trends in Figure 3 and Supplementary Figure 6 for WB or for PBMC, we identified an overall similar pattern for PBMC as for WB. Only few specific comments need to be made in comparing the effect of delayed processing on either sample type. With increasing delay of stimulation, production of MIP-1β, TNF-α and IL-6 in response to PAM were all decreased in PBMC but not in WB. TNF-α, IL-6, IL-12p70 and IL-12/23p40 were also produced at lower levels in response to R848 with increasing delay for PBMC, while they seemed unaffected by a delay for WB stimulation. IL-12/23p40 production by PBMC in response to PAM and LPS appeared unaffected, while it was reduced in WB. Delays in stimulation lead to reduced IL-10 in response to PAM and LPS in PBMC but not in WB. On the other hand, and contrary to WB, IL-8 production of PBMC in response to any of the stimuli appeared unaffected by a delay. Overall, differences in time from venipuncture to stimulation with TLR from as little as 4 h to as long as 48 h altered the cytokine response of innate cells in blood, and did so in a time-, stimulus- and cytokine-dependent fashion.
3.2. Storage considerations
Given the difficulty of controlling delays between e.g. venipuncture and blood sample stimulation, storing the samples prior to stimulation is often described in the published literature.
While storage at room temperature (RT) did not result in a difference in cell viability or innate cell subset composition (see above), storage of samples at 4°C affected both cell viability and innate cell composition (data not shown). This has previously been recognized (Syme et al., 2002), and to our knowledge no study has since been published that analyzes innate immune response to TLR stimulation using blood samples stored at 4°C. However, several studies have been published analyzing innate immune responses in cryopreserved PBMC samples. Hence we opted to focus our analysis on the impact of cryopreservation on innate immune responses to TLR stimulation.
To this end, we purified PBMC from WB immediately following venipuncture, and either stimulated the samples immediately, or cryopreserved the cells according to published SOPs (Maecker et al., 2005a; Disis et al., 2006). Frozen samples were stored in liquid nitrogen for 1 week prior to stimulation. We found that all of the batches of low-endotoxin human serum albumin we tested contained innate immune activating substances that led to a high level of activation even on unstimulated samples (data not shown). This precluded their use for our purpose. We therefore restricted our detailed analysis on samples frozen with endotoxin-free FCS only. All samples, i.e. the fresh or frozen samples were subjected to the same rigorous quantitative analysis of single-cell and global cytokine production following PAM, LPS or R848 stimulation as described above for WB and PBMC. Importantly, to minimize artifacts during analysis, samples stimulated fresh were frozen after stimulation and analyzed in the same batch as those stimulated 1 week after cryopreservation. Viability as assessed by trypan blue was: 97% (±2%) in fresh samples and 95% (± 4%) in cryopreserved samples; there was no difference in innate cell composition between fresh or cryopreserved samples (data not shown).
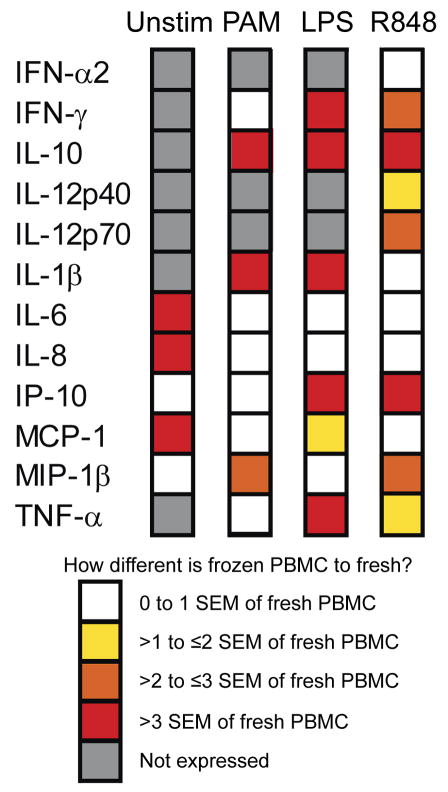
Supplementary Figure 7 shows the innate immune responses of fresh and frozen PBMC as measured by ICS. The impact of freezing on the percentage of cytokine-producing cells in the 6-h stimulation was not as dramatic as the differences seen in the 24-h stimulation assay measuring global cytokine production. However, it is worth noting that with R848 stimulation the percentage of cDC and pDC producing IL-12 and IFN-α, respectively, were lower in frozen PBMC as compared to fresh. Figure 4 shows the qualitative summary of our findings comparing the global innate cytokine response of cryopreserved PBMC to those of PBMC stimulated freshly. The response of every cytokine was affected by cryopreservation. However, there was no global pattern (i.e. all less or more reactive). Freezing alone, without subsequent stimulation with a TLR agonist, led to a large change in the expression of IL-6, IL-8 and MCP-1 compared to fresh PBMC (greater than 3× the SEM). The production of IL-10 by frozen PBMC after stimulation with each of the three TLR agonist differed from fresh PBMC greater than 3 times the SEM for the cytokine production made by fresh PBMC. It seems that the more potent the TLR agonist (R848 and LPS), the more significant is the difference between fresh and frozen PBMC innate immune responses. Cryopreservation thus clearly altered the cytokine response of innate cells in blood in a stimulus-dependent fashion.
Figure 4. Qualitative summary of the impact of cryopreservation on TLR stimulation.
PBMC samples – either fresh or after cryopreservation – from the same healthy adults (n = 3) were stimulated for 24 h with nothing (Unstim), a TLR2/1 ligand (PAM, 10 μg/ml), a TLR4 ligand (LPS, 100 ng/ml), or a TLR7/8 ligand (R848; 10 uM). The culture supernatants were harvested to measure the bulk cytokine production using Luminex platform.
3.3. Source of the innate cells
3.3.1. WB vs. PBMC
Already mentioned above are qualitative differences in the impact of delaying stimulation with TLR ligands between innate immune responses in WB vs. those in PBMC. A direct quantitative comparison of cytokines secreted into the supernatant of WB and PBMC is not feasible, given the differences in cellular composition of both sources. A direct quantitative comparison of WB to PBMC using a single-cell ICS platform on the other hand is permissible, as they reflect relative changes within a subset of cells. To compare PBMC to WB, we split our blood sample in half, and stored one half (WB) for 2 h at room temperature, during which time we processed the other half to purify PBMC. Both samples were then stimulated simultaneously under the same conditions. Supplementary Figure 8 summarizes the trends we observed, namely that IL-12/23p40 production is reduced in PBMC samples as compared to WB samples after stimulation with either PAM, LPS or R848, in both monocytes and cDC (pDC were found not to produce any IL-12/23p40). And while monocytes in PBMC samples also were less capable than WB samples of producing IL-6 and TNF-α following LPS or R848 stimulation, we found no difference in the ability to produce either cytokine in response to PAM stimulation between WB and PBMC. cDC contained in PBMC followed the same trend as monocytes, except they produced less IL-6 and TNF-α in response to PAM, and only less TNF-α but equivalent IL-6 in response to R848. Importantly, both monocytes and cDC in PBMC displayed an increase in IL-6 and TNF-α production in unstimulated samples albeit at low levels. pDC in both samples only responded to R848 producing only IFN-α and TNF-α; production of both of these were found reduced in pDC of PBMC as compared to WB. Clearly, innate responses to TLR stimulatin differed between PBMC and WB.
3.3.2. Impact of mode of delivery
Several groups, including ours, have compared neonatal to adult innate responses to TLR stimulation. Most of the published reports specifically state that samples were either from unlabored, elective Caesarian section deliveries (CSD) or labored normal spontaneous vaginal deliveries (NSVD). However, several studies pooled data derived from cord blood samples obtained from newborns born either via CSD or NSVD. To address the issue of impact of mode of delivery, we compared innate immune responses in mononuclear cells derived from cord blood obtained after elective CSD vs. NSVD. Our findings have to be interpreted with caution, as we are comparing different individuals, not simply different modes of delivery. To reduce the possible impact of different factors contained in plasma (Levy et al., 2006; Levy, 2007), and focus on the differential cellular response, we opted to compare purified cord blood mononuclear cells (CBMC) instead of WB obtained via different modes of delivery.
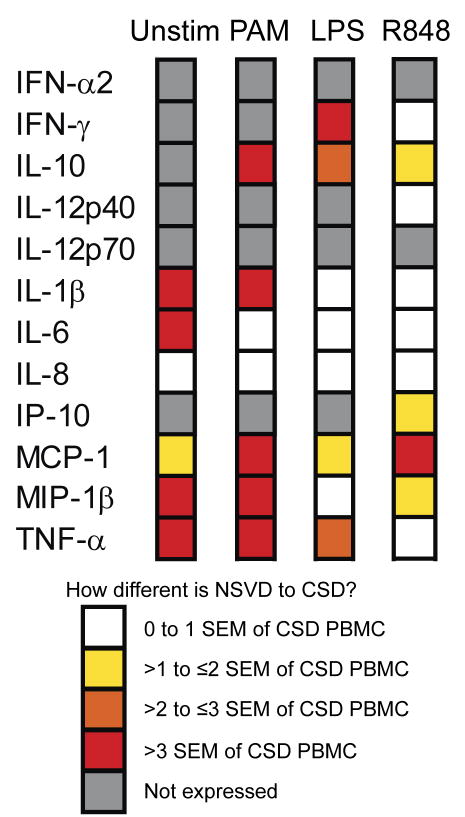
We again analyzed these samples in the same comprehensive fashion as outlined above, i.e. single-cell and global cytokine production after stimulation with various TLR ligands. Figure 5 and Supplementary Figure 9 depict the trends we observed summarizing findings from Luminex and ICS analysis platforms, respectively. Cell viability and cell composition of CBMC did not differ between CSD or NSVD (data not shown). Importantly, both TNF-α and IL-6 production were increased in unstimulated CBMC samples of NSVD as compared to CSD derived samples; this is evident in both the 6-h ICS assay and the 24-h Luminex assay. Other cytokines that differed greatly between NSVD and CSD CBMC even in the absence of TLR stimulation are IL-1β, MIP-1β and MCP-1 (the first two by more than 3× the SEM of the amount produced by CBMC from CSD births). Interestingly, stimulation with the highly potent R848 led to the least differential cytokine production between CBMC from NSVD and CSD.
Figure 5. Qualitative contrast of the impact of delivery mode on the cord blood responses to TLR stimulation.
Mononuclear cells obtained from cord blood from either unlabored elective Caesarian sections (CSD) (n = 10) or from labored normal spontaneous vaginal deliveries (NSVD) (n = 8) were stimulated overnight with nothing (Unstim), a TLR2/1 ligand (PAM, 10 μg/ml), a TLR4 ligand (LPS, 100 ng/ml), or a TLR7/8 ligand (R848; 10 μM). The culture supernatants were harvested after 24 h to measure the bulk cytokine production using Luminex platform
Overall, the source of innate cells – be that WB vs. PBMC, or CBMC from NSVD vs. CSD – displayed differences in their cytokine response which appeared stimulus- and cytokine-dependent.
4. DISCUSSION
The recognition of the role of the innate immune system in health and disease has lead to an explosion of published manuscripts on this topic. Specifically, the role of PRRs such as TLRs has been investigated extensively (Akira et al., 2006; Turvey and Hawn, 2006; Levy, 2007; Barreiro et al., 2009). Given the ever more detailed analysis, relevant potential artifacts need to be excluded to allow the findings to be comparable across different publications. We had previously described a robust SOP for the experimental set up of in vitro stimulation of innate cells in blood with TLR ligands (Jansen et al., 2008). We here set out to delineate the most relevant parameters that need to be controlled in the steps prior to in vitro stimulation. We found that depending on the source of blood (e.g. WB vs. PBMC) or mode of delivery (CSD vs. NSVD), cytokine production by innate cells such as monocytes, cDC and pDC can markedly differ. We further determined that depending on the stimulus and the target cytokine, even a short delay between procuring a blood sample and initiating the in vitro stimulation, can result in vastly differing results. And lastly, we found that cryopreservation of samples in liquid nitrogen prior to stimulation can have a profound impact on the innate cytokine response, again in a stimulus- and readout-dependent fashion.
Our findings presented here clearly indicate that the source of blood needs to be rigorously standardized within studies, and that studies using either e.g. PBMC or WB, or CBMC from CSD or NSVD cannot be compared outside of parameters shown not to differ. We had previously published a report analyzing the TLR response using whole blood or purified mononuclear cells (Kollmann et al., 2009). However, we had never directly contrasted the WB vs. PBMC response in the same sample. It is not possible to directly compare cytokine production in culture supernatant of PBMC to WB, as the actual cell content is clearly not the same. Thus a comparison of innate response to TLR stimulation between WB and PBMC could only be conducted using ICS. Our findings in this current study identified several differences, with some responses augmented in PBMC, others suppressed, and yet another set not affected at all. Ida et al (Ida et al., 2006) had contrasted PBMC to WB as well, and given the above outlined limitations also focused this comparison on single-cell, flow-based analysis; however they restricted their analysis to IFN-α and TNF-α in response to TLR7/8 only, and did not include IL-6 nor IL-12 in their analysis, nor LPS or PAM as stimuli as we did. Similar to our findings Ida et al. identified an increased spontaneous (i.e. in unstimulated samples) production of TNF-α in PBMC as compared to WB (Ida et al., 2006). Importantly, our findings confirm their observation that TLR7/8 stimulation results in a statistically significantly higher % of TNF-α expressing cDC and pDC in PBMC as compared to WB, as well as a trend towards higher but not statistically significant % of IFN-α expressing pDC. While it is unclear what specifically leads to the different response patterns in WB vs. PBMC, our findings as well as those of Ida et al. (Ida et al., 2006) emphasize that innate responses measured in WB differ from those in PBMC in a cytokine and TLR stimulus dependent manner.
We had previously published a report comparing newborn to adult responses (Kollmann et al., 2009). All of our studies had been conducted using samples obtained via elective CSD before the onset of labor. Most other studies analyzing neonatal innate TLR responses also specify in their sample description the type of delivery cord blood samples were obtained from. However, we are aware of only few previous studies that directly compared CSD to NSVD. For example, Levy et al. (Levy et al., 2004) analyzed only the concentration of TNF-α in culture supernatant in response to the same stimuli we employed (PAM, LPS, R848), and similar to us found striking differences in TNF-α production between CSD and NSVD only for PAM, but not for LPS and R848. Brown et al. (Brown et al., 2003) analyzed the concentrations of IFN-γ and IL-12/23p40 in culture supernatant after stimulation of CSD vs. NSVD CBMC samples with LPS at high dose (10 μg/ml) for 24 h; similar to us they found no spontaneous production of IFN-γ or IL-12/23p40, but a similar production of IL-12/23p40 after stimulation but a higher IFN-γ production in response to LPS. Shen et al. (Shen et al., 2009) identified that CBMC from CSD express lower levels of TLR2 & 4 on the surface than NSVD, despite similar mRNA concentrations, and suggest that processes associated with NSVD such as labor induce surface expression of at least some TLRs. Taken together our data and those of the other studies mentioned clearly emphasize that CBMC innate responses to TLR stimulation are not the same between samples obtained after CSD or NSVD. Thus, data obtained with different mode of delivery cannot be pooled or readily compared. This also implies that studies describing innate responses to TLR stimulation in CBMC need to specify their delivery source of cord blood. Unfortunately, some older studies did not provide this information (Hunt et al., 1994).
Larger clinical trials often require shipping of samples which – even if temperature controlled (see below) – results in delay of processing and stimulation. Given the sentinel function of the innate immune system, it is possible that such differences in processing may affect the final result. From the data presented here, it is evident that even a short delay in time to stimulation can have profound impact on the results of TLR stimulation; however this impact was cytokine-, stimulus- and time-dependent. The impact of a delay in stimulation had been investigated previously, but not in the same comprehensive manner we have reported here. For example, Deering and Orange (2006) measured only TNF-α in culture supernatant of WB stored at room temperature. Similar to our findings, they report that significantly less TNF is produced with increasing delay of stimulation with PAM. However, they conclude that ‘a delay for up to 30 h is ok without affecting read out’. A more extensive analysis was conducted by Meier et al. (2008), who only analyzed the impact of stimulation delay from 0, 6, 12, and 24 h on PBMC (not on WB or up to 48 h as we did); furthermore, they only used flow cytometric analysis, not coupled with a global culture supernatant analysis as we did. Our data on pDC and cDC are entirely in agreement with theirs, namely that the ability of pDC to produce IFN-α in response to TLR7/8 and TLR9 stimulation most strikingly decreases between 0 and 6 h, but then remains relatively stable up to 24 h; and that the ability to produce TNF-α decreases more slowly but steadily continues to decrease up to 24 h. Our study confirms their findings in that the ability of cDC to produce TNF-α and IL-12/23p40 in response to TLR7/8 decreased and steadily continues to decrease up to 24 h. And lastly, similar to us, they also found the ability of monocytes to produce TNF-α in response to TLR7/8 to decrease rapidly between 0 and 6 h, further by 12 h but then remaining stable for up to 24 h of a delay.
We had previously published reports stating that all of our samples were processed within < 4 h of procurement (Kollmann et al., 2009). Similar statements were made by others (Drohan et al., 2004; Nguyen et al., 2010), with some studies indicating an even longer time frame, e.g. ‘blood was processed within 24 h of birth’ (Hunt et al., 1994; Ly et al., 2006; Caron et al., 2009). Importantly, none provide any indication if some samples were processed, e.g., within 1 h, and others after 19 h. Furthermore, some published studies do not specify at all within what time frame or with what variability samples were processed and stimulated (e.g. (Biasin et al.,; Panda et al.,; De Wit et al., 2003; van Duin et al., 2007; Belderbos et al., 2009). Our data clearly demonstrate that the innate immune response to TLR stimulation is affected by a delay between sample procurement and processing. At minimum, the time between processing of samples should not vary for more than 10% between samples to ensure fewer than 10% of read-outs vary for > 1 SEM due to the artifact of processing delay. The technically demanding nature of measuring the innate TLR response often precludes immediate processing of samples at study sites. Given the impact of delays in processing outlined above, cryopreservation of cells prior to simulation has been employed by several groups. Beyond ours, few other studies have analyzed the impact this procedure has on the innate response; and no other study has approached this topic in the comprehensive manner we present here. For example, Deering and Orange (2006) analyzed the secretion of only TNF-α in culture supernatant; they found a significant increase in TNF-α production following pI:C and zymosan stimulation and a significant decrease in cryopreserved vs. fresh samples following CpG stimulation. Ida et al (2006) who compared IFN-α and TNF-α production by ICS only, described a complete dropout of IFN-α production in previously cryopreserved pDC, while TNF-α production in cDC and pDC appeared unaffected. Both Ida et al. and our data are in complete agreement, but stand in direct conflict with that of Gold et al. (2006) who described that the IFN-α production of pDC in response to TLR9 stimulation produced ‘equivalent results’ for fresh and cryopreserved cells; however, they did not provide the detailed data to support this. Yet another group categorically states that there are no differences between fresh and cryopreserved cells (Upham et al., 2002); however, they did not provide data to support this conclusion, and reference reports as support that only compared T cell responses between fresh and cryopreserved samples, not innate immune responses to TLR stimulation (Upham et al., 1995). Clearly, our data, supported by that of others identify that cryopreservation affects all of the cytokines we analyzed, and does so in a TLR stimulus and cytokine specific manner.
Despite the concerns about the impact on the results of variation in operating procedures we describe here, remarkable agreement between studies (e.g. those focused on innate immune ontogeny) often exists. While this could be taken to argue that scrupulous adherence to standard operating proecdures for innate immune analysis is of less importance than we propose, such agreement more likely is the result of differences in innate immune response to TLR stimulation between groups (e.g. newborn and adult) that are more pronounced than the impact of variability in SOPs. And given that even minor variation in TLR function impacts health and disease on a continous spectrum (Turvey and Hawn, 2006; Barreiro et al., 2009), it is becoming increasingly important to striclty adhere to a standardized protocol when studying TLR function to produce biologically meaningful results. We here show that especially the handling of a blood sample prior to in vitro stimulation has to be strictly controlled. In order for data to be comparable, we suggest the following minimal guidelines in reporting:
SAMPLE SOURCE: The source of the sample, such as blood, needs to be standardized, as data obtained from e.g. PBMC cannot be pooled with data obtained with WB. At minimum, the source has to be clearly described.
SAMPLE PROCESSING: The time between sample procurement (e.g. venipuncture) and stimulation needs to be reported, and ideally be strictly controlled. We suggest that between samples pooled in the same analysis it should not vary for more than ~10% of the total elapsed time.
SAMPLE STORAGE: Whole blood has to be stored at room temperature prior to processing. If innate cells are to be stimulated after cryopreservation, the author/s would need to show that the particular read out is not altered by this process. At minimum, all samples within a study have to be treated the same way, and data cannot be pooled or compared between protocols that use different storage methods.
SUPPORTING DATA: If innate readouts other than those described in this report are to be published and any of the suggested standardized parameters not be followed, the authors need to provide supplementary data showing the lack of e.g. processing delay, storage such as cryopreservation, or different sample sources do not affect the particular readout.
Supplementary Material
Acknowledgments
This work was supported in part by: 1) National Institute of Allergy and Infectious Diseases, NIH; Grant number: N01 AI50023; 2) AllerGen NCE; Grant numbers: 07-A1A, 07-B2B; 3) Canadian Institute of Health Research (CIHR); 4) the Michael Smith Foundation for Health Research. TRK is supported in part by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, and by a CIHR Training Grant in Canadian Child Health Clinician Scientist Program, in partnership with SickKids Foundation, Child & Family Research Institute (BC), Women & Children’s Health Research Institute (Alberta), Manitoba Institute of Child Health. We thank Lisa Xu for exceptional flow cytometry assistance, and Francis Thommai, and Xiu Yu Wang for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Barreiro LB, Ben-Ali M, Quach H, Laval G, Patin E, Pickrell JK, Bouchier C, Tichit M, Neyrolles O, Gicquel B, Kidd JR, Kidd KK, Alcais A, Ragimbeau J, Pellegrini S, Abel L, Casanova JL, Quintana-Murci L. Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5:e1000562. doi: 10.1371/journal.pgen.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belderbos ME, Bleek GMv, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JLL, Bont L. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: Low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasin M, Piacentini L, Lo Caputo S, Naddeo V, Pierotti P, Borelli M, Trabattoni D, Mazzotta F, Shearer GM, Clerici M. TLR activation pathways in HIV-1-exposed seronegative individuals. J Immunol. 184:2710–7. doi: 10.4049/jimmunol.0902463. [DOI] [PubMed] [Google Scholar]
- Blimkie D, Fortuno ES, 3rd, Thommai F, Xu L, Fernandes E, Crabtree J, Rein-Weston A, Jansen K, Brinkman RR, Kollmann TR. Identification of B cells through negative gating-An example of the MIFlowCyt standard applied. Cytometry A. 2010;77:546–51. doi: 10.1002/cyto.a.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Rad PY, Halonen MJ. Method of birth alters interferon-gamma and interleukin-12 production by cord blood mononuclear cells. Pediatr Allergy Immunol. 2003;14:106–11. doi: 10.1034/j.1399-3038.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- Caron JE, La Pine TR, Augustine NH, Martins TB, Hill HR. Multiplex Analysis of Toll-Like Receptor-Stimulated Neonatal Cytokine Response. Neonatology. 2009;97:266–273. doi: 10.1159/000255165. [DOI] [PubMed] [Google Scholar]
- De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–81. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Deering RP, Orange JS. Development of a clinical assay to evaluate toll-like receptor function. Clin Vaccine Immunol. 2006;13:68–76. doi: 10.1128/CVI.13.1.68-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis ML, dela Rosa C, Goodell V, Kuan LY, Chang JCC, Kuus-Reichel K, Clay TM, Kim Lyerly H, Bhatia S, Ghanekar SA, Maino VC, Maecker HT. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–8. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Drohan L, Harding JJ, Holm B, Cordoba-Tongson E, Dekker CL, Holmes T, Maecker H, Mellins ED. Selective developmental defects of cord blood antigen-presenting cell subsets. Hum Immunol. 2004;65:1356–69. doi: 10.1016/j.humimm.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Gold MC, Donnelly E, Cook MS, Leclair CM, Lewinsohn DA. Purified neonatal plasmacytoid dendritic cells overcome intrinsic maturation defect with TLR agonist stimulation. Pediatr Res. 2006;60:34–7. doi: 10.1203/01.pdr.0000220352.13547.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 1994;84:4333–43. [PubMed] [Google Scholar]
- Ida JA, Shrestha N, Desai S, Pahwa S, Hanekom WA, Haslett PAJ. A whole blood assay to assess peripheral blood dendritic cell function in response to Toll-like receptor stimulation. J Immunol Methods. 2006;310:86–99. doi: 10.1016/j.jim.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Jansen K, Blimkie D, Furlong J, Hajjar A, Rein-Weston A, Crabtree J, Reikie B, Wilson C, Kollmann T. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J Immunol Methods. 2008;336:183–92. doi: 10.1016/j.jim.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal Innate TLR-Mediated Responses Are Distinct from Those of Adults. The Journal of Immunology. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- Lee JA, Spidlen J, Boyce K, Cai J, Crosbie N, Dalphin M, Furlong J, Gasparetto M, Goldberg M, Goralczyk EM, Hyun B, Jansen K, Kollmann T, Kong M, Leif R, McWeeney S, Moloshok TD, Moore W, Nolan G, Nolan J, Nikolich-Zugich J, Parrish D, Purcell B, Qian Y, Selvaraj B, Smith C, Tchuvatkina O, Wertheimer A, Wilkinson P, Wilson C, Wood J, Zigon R, Force ISfAoCDST, Scheuermann RH, Brinkman RR. MIFlowCyt: the minimum information about a Flow Cytometry Experiment. Cytometry. 2008;73:926–30. doi: 10.1002/cyto.a.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–66. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–34. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- Ly NP, Ruiz-Perez B, Onderdonk AB, Tzianabos AO, Litonjua AA, Liang C, Laskey D, Delaney ML, DuBois AM, Levy H, Gold DR, Ryan LM, Weiss ST, Celedon JC. Mode of delivery and cord blood cytokines: a birth cohort study. Clin Mol Allergy. 2006;4:13. doi: 10.1186/1476-7961-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HT, Moon J, Bhatia S, Ghanekar SA, Maino VC, Payne JK, Kuus-Reichel K, Chang JC, Summers A, Clay TM, Morse MA, Lyerly HK, DeLaRosa C, Ankerst DP, Disis ML. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005a;6:17. doi: 10.1186/1471-2172-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HT, Rinfret A, D’Souza P, Darden J, Roig E, Landry C, Hayes P, Birungi J, Anzala O, Garcia M, Harari A, Frank I, Baydo R, Baker M, Holbrook J, Ottinger J, Lamoreaux L, Epling CL, Sinclair E, Suni MA, Punt K, Calarota S, El-Bahi S, Alter G, Maila H, Kuta E, Cox J, Gray C, Altfeld M, Nougarede N, Boyer J, Tussey L, Tobery T, Bredt B, Roederer M, Koup R, Maino VC, Weinhold K, Pantaleo G, Gilmour J, Horton H, Sekaly RP. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005b;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–42. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- Marchini G, Berggren V, Djilali-Merzoug R, Hansson LO. The birth process initiates an acute phase reaction in the fetus-newborn infant. Acta Paediatr. 2000;89:1082–6. doi: 10.1080/713794557. [DOI] [PubMed] [Google Scholar]
- Meier A, Fisher A, Sidhu HK, Chang JJ, Wen TF, Streeck H, Alter G, Silvestri G, Altfeld M. Rapid loss of dendritic cell and monocyte responses to TLR ligands following venipuncture. J Immunol Methods. 2008;339:132–40. doi: 10.1016/j.jim.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 184:2518–27. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay P, Roederer M. Quality assurance for polychromatic flow cytometry. Nat Protoc. 2006;1:1522–1530. doi: 10.1038/nprot.2006.250. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Seminars in fetal & neonatal medicine. 2006;11:317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C, Rott C, Temming P, Schlenke P, Möller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatric Research. 2002;51:317–22. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Shen CM, Lin SC, Niu DM, Kou YR. Labour increases the surface expression of two Toll-like receptors in the cord blood monocytes of healthy term newborns. Acta Paediatr. 2009;98:959–62. doi: 10.1111/j.1651-2227.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- Syme R, Callaghan D, Duggan P, Bitner S, Kelly M, Wolff J, Stewart D, Gluck S. Storage of blood for in vitro generation of dendritic cells. Cytotherapy. 2002;4:271–6. doi: 10.1080/146532402320219781. [DOI] [PubMed] [Google Scholar]
- Turvey SE, Hawn TR. Towards subtlety: understanding the role of Toll-like receptor signaling in susceptibility to human infections. Clin Immunol. 2006;120:1–9. doi: 10.1016/j.clim.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Upham JW, Holt BJ, Baron-Hay MJ, Yabuhara A, Hales BJ, Thomas WR, Loh RK, O’Keeffe PT, Palmer L, Le Souef PN, et al. Inhalant allergen-specific T-cell reactivity is detectable in close to 100% of atopic and normal individuals: covert responses are unmasked by serum-free medium. Clin Exp Allergy. 1995;25:634–42. doi: 10.1111/j.1365-2222.1995.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Upham JW, Lee PT, Holt BJ, Heaton T, Prescott SL, Sharp MJ, Sly PD, Holt PG. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002;70:6583–8. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–5. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.