Abstract
Emotion dysregulation is a key feature of mood and anxiety disorders. Many of these disorders also involve volumetric reductions in brain regions implicated in emotion regulation, including the dorsal anterior cingulate cortex (dACC). Investigating this relationship in healthy individuals may clarify the link between emotion regulation and volumetric reductions in this key brain region. High-resolution anatomical MRI images were used to calculate dACC volumes in 50 healthy female subjects. Trait measures of emotion regulation (cognitive reappraisal and expressive suppression) and negative affect were also obtained. As predicted, cognitive reappraisal was positively related to dACC volume, but not the volume of a control region, the ventral ACC. Expressive suppression, negative affect, and age were not related to dACC volume. These findings indicate that individual differences in cognitive reappraisal are related to individual differences in dACC volume in healthy participants.
Keywords: emotion regulation, reappraisal, volume, dorsal anterior cingulate, ROI
Introduction
Emotion dysregulation is a hallmark of mood and anxiety disorders (Campbell-Sills & Barlow, 2007; Gross & Thompson, 2007). These disorders also are associated with volumetric reductions in brain regions implicated in cognitive forms of emotion regulation, including the dorsal anterior cingulate cortex (dACC; Asami, et al., 2008; Vasic, et al., 2008; K. Yucel, et al., 2008). It is not clear, however, whether such regional volumetric reductions are causes or consequences of psychopathology. One way to address this issue is to examine cognitive emotion regulation and dACC volume in healthy individuals.
Emotion Regulation
Emotion regulation refers to the processes by which people seek to influence which emotions they have, when they have them, and how they experience and express these emotions (Gross, 1998). One particular target of research on emotion regulation has been cognitive reappraisal, which involves the effortful reinterpretation of an emotion-eliciting situation in a way that changes its emotional impact (Giuliani & Gross, 2009).
Laboratory studies have shown that reappraisal recruits cognitive control-related brain regions, including the dorsolateral prefrontal cortex (dlPFC) and dACC, which in turn modulate the response of brain regions associated with the generation of emotion, including the amygdala and insula (Ochsner & Gross, 2005). In particular, it has been shown that BOLD responses in the dACC predict success at employing cognitive reappraisal (Ochsner, et al., 2002). Individual-difference studies have shown that frequent use of reappraisal is related to greater experience and expression of positive mood, less experience and expression of negative mood, and fewer symptoms of depression (Gross & John, 2003; John & Gross, 2004).
Psychopathology, Emotion Dysregulation, and dACC Volume
Compared to healthy controls, individuals with mood and anxiety disorders display a range of difficulties managing their emotions (Campbell-Sills & Barlow, 2007). Indeed, there is a growing consensus that many of the clinical features of these disorders may result from failure to adaptively regulate unwanted negative emotion (Campbell-Sills & Barlow, 2007). This perspective is congruent with the central tenets of cognitive therapy, which involves the modification of dysfunctional negative appraisals about the self, the world, and the future (Campbell-Sills & Barlow, 2007).
Of the many brain regions that support emotion regulation, it is the dACC that has been most consistently implicated in studies assessing brain volume in psychological disorders characterized by dysregulated emotion, including panic disorder and major depression (Asami, et al., 2008; Vasic, et al., 2008). Compared with healthy controls, the volume of the dACC (corrected for whole brain volume) is consistently smaller in individuals with panic disorder (Asami, et al., 2008), depression (Vasic, et al., 2008), schizophrenia (Giuliani, et al., 2005), and anorexia (McCormick, et al., 2008). It is not clear, however, whether diminished dACC volume is a cause or consequence of psychopathology.
The Present Study
The goal of the present study was to examine the relation between cognitive reappraisal and dACC volume in a sample of healthy participants. We hypothesized that more frequent use of cognitive reappraisal would be associated with greater dACC volume, but not the volume of a control region, the ventral ACC (vACC). In addition, we hypothesized that this association would remain when controlling for another commonly used form of emotion regulation, expressive suppression, and for negative affect.
Methods and Materials
Participants
Fifty right-handed healthy female volunteers between the ages of 18 and 25 (mean: 21.9 years, SD: 2.4) participated after providing informed consent according to the guidelines of the Stanford University Institutional Review Board. All participants were recruited as part of a larger parent study, which was limited to females to control for known differences in regional brain volume and emotional responding (Bradley, et al., 2001; Chen, et al., 2007; M. Yucel, et al., 2001).
Participants were screened for psychiatric disorders using an interview based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID for DSM-IV; First, et al., 1995). Eligible participants did not meet criteria for any psychiatric disorder within the past year, or for lifetime posttraumatic stress, bipolar, obsessive-compulsive, or psychotic disorders, and were not currently taking psychotropic medications or had any health conditions that affect cerebral blood flow. Participants were paid for their participation.
Individual Difference Measures
Individual differences in cognitive reappraisal and expressive suppression were assessed using the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003)1 (reappraisal: M = 4.3, SD = 1.2, α = .87; suppression: M = 3.7, SD = 1.2, α = .81). Items were answered on a 1-to-7 Likert scale (1 = “strongly disagree,” 7 = “strongly agree”) and summed by subscale; higher scores indicate greater frequency of use of each strategy.
Trait negative affect was assessed using the Negative Affect (NA) subscale of the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) (M = 13.8, SD = 3.0, α = .76). This scale was chosen because it has been previously used as an index of general emotional experience (mood) in relation to the ERQ (Gross & John, 2003).
Image Acquisition
Subjects were scanned with a General Electric Signa 3.0T magnet (General Electric Medical Systems, Milwaukee, WI) with a quadrature head coil at Stanford University’s Lucas Center. Head movement was minimized using a bite bar and foam padding. A whole brain, high-resolution three-dimensional spoiled gradient recalled (SPGR) T1-weighted anatomical scan (.859×1.2mm, field of view = 22cm, frequency encoding = 256) was acquired for each subject. Functional scans were also gathered, but are not reported here.
Image Preprocessing and Analysis
Preprocessing was done using MATLAB (2008a, The MathWorks, Natick, MA), and consisted of reslicing images to 1mm3 voxels and aligning them into AC-PC space. Images were then converted to NIFTI format for local ROI drawing, and Analyze format for total brain volume (TBV) calculation.
Quantitative volumetric processing was conducted using the ITKGray software package, a variant on ITKSnap (Yushkevich, et al., 2006), which allows simultaneous 3D image viewing and outlining of the selected ROI in three orthogonal planes. ROI volumes were derived by summing the relevant consecutive slice volumes. Dorsal and ventral ACC borders were drawn using an empirically derived and replicated protocol (Killiany, et al., 2000), shown in Figure 1, and included the cingulate sulcus (superior), interhemispheric fissure (medial), and callosal sulcus (inferior). Mean raw left and right dACC and vACC volumes were 2965mm3 (SD: 976.3), 3229mm3 (SD: 947.8), 3403mm3 (SD: 1333.2), and 3210mm3 (SD: 1074.3) respectively. To ensure inter-rater reliability, ~20% of subjects (N=10) were rated independently by both raters. Cronbach’s alphas were acceptable, at > .75 for all ROIs.
Figure 1.
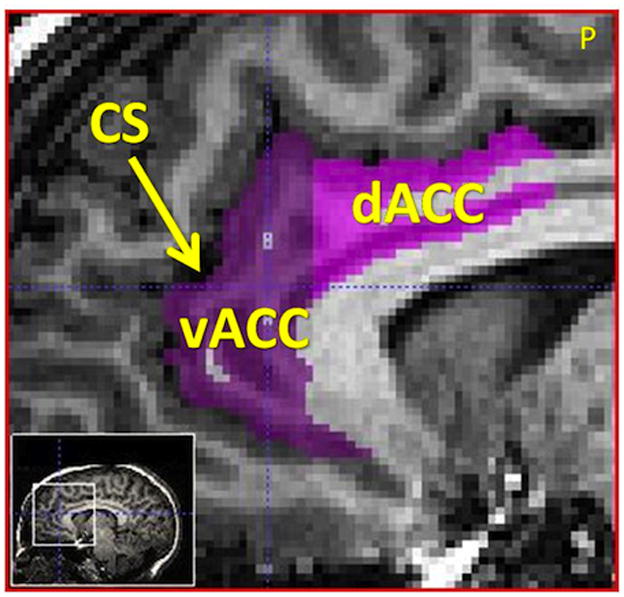
Sagittal slice showing the dACC and vACC borders, where the cingulate sulcus forms the superior border (dACC = dorsal Anterior Cingulate Cortex, vACC = ventral Anterior Cingulate Cortex, CS = Cingulate Sulcus, P = posterior). The cingulate sulcus formed the anterior and superior borders of the dACC and vACC. The medial boundary of both ROIs was the interhemispheric fissure and the inferior boundary was the corpus callosum. The posterior boundary of the dACC was determined in the coronal plane, as the slice containing the mammillary bodies, posterior to the fornix. The posterior boundary of the vACC was determined in the coronal view, as the last slice anterior to the joining of the corpus callosum across the interhemispheric fissure. Image is sliced in 1mm3 voxels for volumetric calculations.
To control for between-subject variation in intracranial size, each ROI was expressed as a ratio of TBV. TBV was calculated as the sum of gray matter, white matter, and cerebrospinal fluid, which were segmented using the VBM5.1 toolbox provided by Christian Gaser (http://dbm.neuro.uni-jena.de/vbm.html).
Statistical analyses were conducted using SPSS17.0 (SPSS Inc., Chicago, IL). Data were checked for normality using histograms and the Kolmogorov-Smirnov test. Kolmogorov-Smirnov tests indicated that the distribution of NA was significantly non-normal (p < .05). A log transformation of this variable improved the distribution (K-S p > .05). The remaining variables were not significantly different from normal (p > .1); raw data were therefore used.
Results
As hypothesized, cognitive reappraisal was positively associated with bilateral dACC volume (Figure 2: r = .35, p = .013). Both the left and right dACC ROIs were significantly correlated with each other and with reappraisal frequency (left and right dACC: r = .37; left dACC and reappraisal: r = .34; right dACC and reappraisal: r = .32; all p-values < .02). By contrast, neither suppression (r = −.14, n.s.) nor NA (r = −.04, n.s.) was correlated with bilateral dACC volume. Importantly, when we controlled for both NA and expressive suppression, the relation between reappraisal and dACC volume remained significant (β= .29, p = .024).
Figure 2.
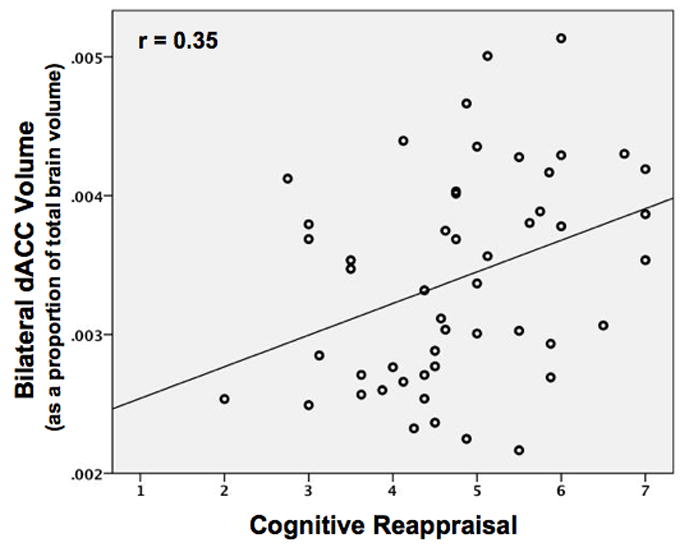
Scatterplot showing the positive relationship (r = 0.35, p = 0.013) between bilateral dACC volume and reappraisal frequency as reported on the ERQ.
Neither cognitive reappraisal nor dACC volume was significantly associated with age (p-values > .13). Reappraisal was also not significantly correlated with TBV (p = .93) or the volumes of the control region, the left and right vACC (p-values > .1). Reappraisal and NA were negatively but not significantly correlated (r = −.2, p = .15).
Discussion
Morphometric studies suggest that the volume of the dACC differs between clinical and non-clinical populations, with smaller volumes in clinical populations characterized by difficulties with emotion regulation. Functional neuroimaging studies with healthy participants have shown that the dACC plays a key role in cognitive forms of emotion regulation. In the present study, we examined the association between cognitive reappraisal and dACC volume in a sample of psychiatrically healthy participants. As predicted, cognitive reappraisal was positively related to dACC volume.
The results of this study are congruent with reports that individuals with psychiatric conditions characterized by emotion dysregulation have decreased dACC volumes (Asami, et al., 2008; McCormick, et al., 2008; Vasic, et al., 2008). Some authors have suggested that these alterations may be due to neurodevelopmental abnormalities that predispose the individual to develop mood disorders. It is also possible, however, that the decreased dACC volumes seen in individuals with mood and anxiety disorders may be due to infrequent use of adaptive cognitive emotion regulation strategies. This possibility is suggested by a small but robust literature showing use-dependent volume alterations. Correlates of learning and gray matter volume have been shown in different cognitive domains, including spatial navigation and the posterior hippocampus, and juggling and area hMT/V5 (Draganski & May, 2008). This is also supported by the finding in the current study that trait NA was not associated with reappraisal use or dACC volume, which supports the notion that the decreased dACC volumes seen in clinical populations may be due to decreased emotion regulation use and not chronically elevated levels of negative affect.
Although the present study focuses on the relationship between everyday emotion regulation behavior and the structure of the dACC, much work has been done on the function of this region. The dACC is regularly activated during cognitive reappraisal of emotional stimuli by healthy participants (Ochsner & Gross, 2005), and dACC activation during cognitive and emotional tasks is increased in clinical populations (Chechko, et al., 2009; Fonzo, et al., 2010). However, the relationship between structure and function is complex; past studies have found that brain structure does not necessarily predict brain function (Pezawas, et al., 2005). If increased reappraisal use leads both to greater efficiency and to increased dACC volume over time, it is possible that individuals with smaller dACC volumes might be less efficient at reappraisal, and show greater dACC activation to reach the same level of performance as peers with larger volumes.
The present study has several important limitations. First, we limited our sample to females to control for known sex differences in regional brain volume and emotional responding (Bradley, et al., 2001; Chen, et al., 2007). We chose females because they have been found to have greater symmetry of the ACC compared to males (M. Yucel, et al., 2001). It remains to be determined whether these results apply to males, and future research should also examine subjects of different ages, cultures and socioeconomic backgrounds. Second, we prioritized the assessment of one form of emotion regulation, cognitive reappraisal, and one control measure, expressive suppression, in order to capitalize on the known neuroimaging literature on these forms of regulation (Drabant, et al., 2009; Goldin, et al., 2008; Hajcak & Nieuwenhuis, 2006; Krompinger, Moser, & Simons, 2008; Ochsner, et al., 2002; Ochsner & Gross, 2005). In future work it will be important to assess a broader range of emotion regulatory processes using behavioral and self-report methods. A third limitation is that we did not assess cognitive control, which also recruits the dACC. Future work should include this control measure. Lastly, our cross-sectional design does not permit us to draw causal inferences, or assess the directionality of the association between dACC volume and emotion regulation. Future research should employ longitudinal or intervention designs to test the direction of this relationship. It is conceivable that teaching at-risk individuals how to successfully reappraise emotional stimuli might increase dACC volume, facilitating adaptive forms of emotion regulation.
Acknowledgments
This research was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award 34676 to Wiveka Ramel, and the National Institutes of Health (NIH) R01 Grant MH58147 awarded to James Gross.
The authors would like to thank Anthony Liatsis, Doc Edge, Wiveka Ramel, and members of the Stanford Psychophysiology Laboratory for their comments and help with this work.
Footnotes
We used an alternate form of the ERQ consisting of 8 reappraisal items and 8 suppression items.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asami T, Hayano F, Nakamura M, Yamasue H, Uehara K, Otsuka T, et al. Anterior cingulate volume reduction in patients with panic disorder. Psychiatry and Clinical Neurosciences. 2008;62(3):322–330. doi: 10.1111/j.1440-1819.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of Emotion Regulation. New York, NY: Guilford Press; 2007. pp. 542–559. [Google Scholar]
- Chechko N, Wehrle R, Erhardt A, Holsboer F, Czisch M, Samann PG. Unstable prefrontal response to emotional conflict and activation of lower limbic structures and brainstem in remitted panic disorder. PLoS One. 2009;4(5):e5537. doi: 10.1371/journal.pone.0005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sachdev PS, Wen W, Anstey KJ. Sex differences in regional gray matter in healthy individuals aged 44–48 years: a voxel-based morphometric study. Neuroimage. 2007;36(3):691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross J. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: 1995. [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Calhoun V, Pearlson G, Francis A, Buchanan RW. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophrenia Research. 2005;74(2–3):135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Gross JJ. Reappraisal. In: Sander D, Scherer KR, editors. Oxford Companion to the Affective Sciences. New York, NY: Oxford University Press; 2009. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. The Emerging Field of Emotion Regulation: An Integrative Review. Review of General Psychology. 1998;2:271–299. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion Regulation: Conceptual Foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York, NY: Guilford Press; 2007. p. 22. [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. Journal of Personality. 2004;72(6):1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s Disease. Annals of Neurology. 2000;47:430–439. [PubMed] [Google Scholar]
- Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8(1):132–137. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- McCormick LM, Keel PK, Brumm MC, Bowers W, Swayze V, Anderson A, et al. Implications of starvation-induced change in right dorsal anterior cingulate volume in anorexia nervosa. International Journal of Eating Disorders. 2008;41(7):602–610. doi: 10.1002/eat.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Höse A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. Journal of Affective Disorders. 2008;109(1–2):107–116. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Chahal R, Taylor VH, Macdonald K, Joffe R, et al. Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology. 2008;33(13):3157–3163. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]
- Yucel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cerebral Cortex. 2001;11(1):17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]


