Abstract
Increasing evidence indicates that the gut peptide ghrelin facilitates learning behavior and memory tasks. The present study demonstrates a cellular signaling mechanism of ghrelin in the hippocampus. Ghrelin stimulated CREB (cAMP response-element binding protein) through the activation of cAMP, protein kinase A (PKA), and PKA-dependent phosphorylation of NR1 subunit of the NMDA receptor. Ghrelin increased phalloidin-binding to F-actin suggesting CREB-induced gene expression might include reorganization of cytoskeletal proteins. The effect was blocked by the antagonist of the ghrelin receptor in spite of the receptor’s primary coupling to Gq proteins. We also discovered inhibitory effect of endocannabinoids on ghrelin-induced NR1 phosphorylation and CREB activity. 2-arachidonoylglycerol (2-AG) exerted its inhibitory effect in the Type1 cannabinoid receptor (CB1R)-dependent manner, while anandamide’s inhibitory effect persisted in the presence of antagonists of CB1R and the vanilloid receptor, suggesting that anandamide might directly inhibit NMDA receptor/channels. Our findings may explain how ghrelin and endocannabinoids regulate hippocampal appetitive learning and plasticity.
Keywords: CREB phosphorylation, NR1, PKA, anandamide, 2-AG, CB1, TRPV, F-actin, phalloidin, CA1, immunohistochemistry
1. INTRODUCTION
Ghrelin is a unique acylated 28 amino acid peptide that was first identified in rat stomach extracts as an endogenous ligand for the growth hormone secretagogue receptor (GHSR, or ghrelin receptor). Ghrelin initiates a release of growth hormone through the activation of Gq proteins (Kojima, 1999). In addition, ghrelin increases appetite and initiates a feeding behavior (Ferrini et al., 2009). The ghrelin receptor is localized in high concentrations in the hypothalamus (Harrold et al., 2008). However, the hypothalamus is not the only brain region that expresses the ghrelin receptor. The ghrelin receptor is also highly expressed in the hippocampus (Zigman et al., 2006). This evidence suggests an additional role of ghrelin, since the hippocampus is not considered as the primary brain area that controls appetite or the release of growth hormone. In the hippocampus, circulating ghrelin was reported to cross the blood-brain barrier and enhance long term potentiation (LTP)(Diano et al., 2006). A well-accepted key molecule in the induction and maintenance of hippocampal LTP is CREB. Indeed, the family of CREB transcription factors has been suggested to be involved in a variety of biological processes, including the development and plasticity of the nervous system (Mayr and Montminy, 2001). Nevertheless, it is not completely understood whether ghrelin stimulates CREB and activates its signaling in the hippocampus. We investigated the expression of phosphorylated CREB (pCREB) in response to ghrelin in the cultured hippocampus, since pCREB expression is a necessary step for the occurrence of functional and structural plasticity.
Endocannabinoid (eCB) and the type 1 cannabinoid receptor (CB1R) have been implicated as key molecules in modulating a feeding behavior. eCB and CB1R stimulate hypothalamic orexigenic neurons, enhance appetite, and facilitate feeding behavior (Jo et al., 2005). Interestingly, evidence suggests that ghrelin may exert its orexigenic effect by stimulating the production of eCB in the hypothalamus (Kola et al., 2008). However, to date, there is no evidence in the hippocampus that a similar interaction might occur between the ghrelin and endocannabinoid system. In the present study, we report a novel role of eCB on ghrelin-induced cellular signaling in CREB activation.
2. EXPERIMENTAL MATERIALS AND METHODS
2.1. Slice preparation and pharmacological treatment
The hippocampal slice culture was used because: 1) chemical effects of ghrelin and anandamide could be assessed directly on the expression of pCREB by eliminating potential neuron-circuit activities produced by synapses made by extrahippocampal neurons, which can cause secondary changes in CREB activities; and 2) a transient elevation of pCREB was reported as a possible result of decapitation and cardiac perfusion (O'Callaghan and Sriram, 2004).
Slice cultures were prepared from P6 postnatal male pups of Sprague-Dawley rats according to the method of Stoppini et al. (1991). Adequate measures were taken to minimize pain or discomfort. Experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). All protocols were approved by the University of Texas at Brownsville Institutional Animal Care and Use Committee. The slices were used for the experiments after being cultured for 1 wk in media that consisted of 50% MEM, 25% HBSS, 24% horse serum, 0.5% penicillin/streptomycin solution, 0.5% 50% glucose solution, and 25 mM HEPES. Ghrelin in an octanoylated form (Phoenix pharmaceutical, Burlingame, CA) was applied to the culture media at a concentration of 200 nM for 60 min (unless specified otherwise in the text). In some experiments, the following compounds were applied to culture media:100 µM L-Dys3-GHSR-6 (Phoenix pharmaceutical, Burlingame, CA), 5 µM ifenprodil, 50 µM Rp-cAMP, 5 µM capsazepine (all from Sigma Chemical, St. Luis, MO), 100 µM APV, 5 µM AM251, 10 nM iodoresiniferatoxin (IRTX), 4 µM WIN55,212-2, 10 µM 2-AG (all from Tocris, Ellisville, MO), and 100 nM JZL184 (Cayman Chemical, Ann Arbor, MI). We applied inhibitors and antagonists to our slice culture for 2 hours prior to the application of ghrelin, while agonists were applied for the identical duration of ghrelin application.
2.2. Immunohistochemistry
At the end of experiments, the slices were immersion-fixed with 4% paraformaldehyde in 1M PBS overnight, rinsed, and treated with 0.1% Triton X-100 and 10% goat (or donkey) serum. CREB phosphorylation was detected using a rabbit polyclonal antibody against pCREB (ser 133) (Cell Signaling, Danvers, MA). The ghrelin receptor was identified using a rabbit polyclonal antibody against GHSR (Phoenix Pharmaceutical, Burlingame, CA). pNR1 was detected using a goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The following fluorescently-tagged secondary antibodies were used: Alexa 488 for pCREB and pNR1, and Alexa 596 for the ghrelin receptor (all from Invitrogen, Carlsbad, CA). A control for immunohistochemistry consisted of several slices from each experimental condition that were incubated with a blocking peptide before the application of the primary antibody. In some cases, the primary antibody was omitted. Dendritic spines were visualized with phallotoxin-conjugated Alexa 488 (Invitrogen, Carlsbad, CA). Results were imaged at a single cell resolution using a confocal microscope (Fluoview, Olympus, Center Valley, PA) and results were quantified using IPLab imaging software (BD Bioscience, San Jose, CA). Relative changes in the fluorescence intensity for pCREB, pNR1, and phallotoxin were normalized among slices and the results were summarized as mean ± SEM (standard error of the mean). The results were tested for statistical significance with a student t-test or ANOVA (analysis of variance) by comparing a given experimental group with a control. Each experiment (i.e., a pharmacological treatment) was accompanied with its own control that was taken from the slices cultured together. P<0.05 was considered significant.
3. RESULTS
3.1. Ghrelin stimulated CREB activities
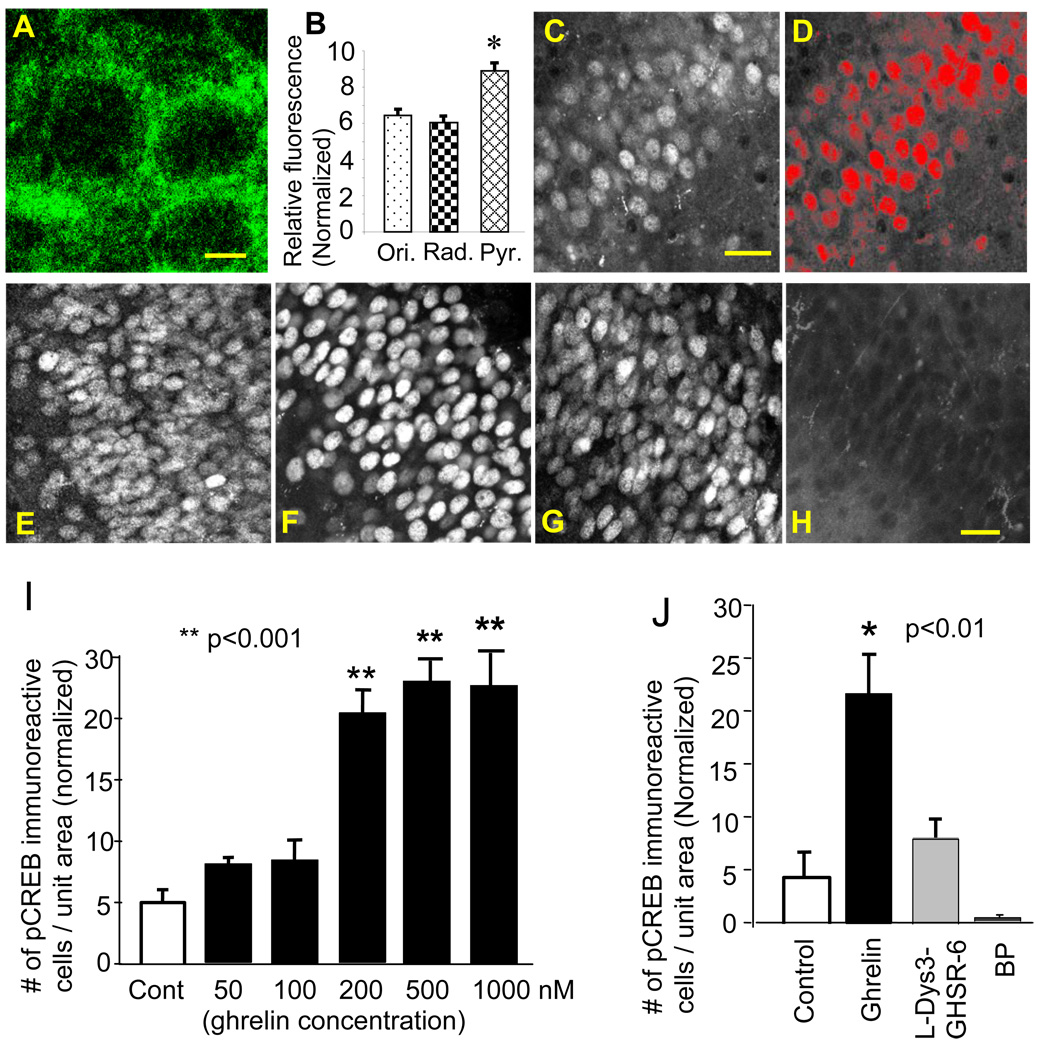
The ghrelin receptor was highly expressed in our cultured hippocampus. Immunohistochemical analysis showed that the ghrelin receptor had the highest concentration in the somatic region of the pyramidal cell and to a lesser extent in the apical and basal dendritic regions in the CA1 subfield (Fig. 1A and B). This observation is in agreement with a previous report on the ghrelin binding assay, which showed biotinylated ghrelin was scattered in cell bodies of the principal layer of the hippocampal formation (Diano et al., 2006).
Figure 1.
Immunofluorescent detection of the ghrelin receptor and pCREB. Ghrelin receptor immunoreactivity in the CA1 stratum pyramidale (A) and subfield distribution (B, *p<0.002, Ori: st. oriens, Rad: st. radiatum, Pyr: st. pyramidale). pCREB immunoreactive neurons (C) were selected and defined as a red-colored region of interest (ROIs)(D). Photomicrographs show pCREB immunoreactivity in control (E), in 200 nM ghrelin (F), in 200 nM ghrelin and 100 µM L-Dys3-GHSR-6 (G), and in a blocking peptide (BP) for pCREB (H). pCREB immunoreactivity in response to ascending concentrations of ghrelin (I) and its blockade by L-Dys3-GHSR-6 (J). The calibration:5 µm in A, 30 µm in C, and 20 µm in H.
The level of CREB activity was assessed with immunohistochemical identification of phosphorylated CREB (pCREB). Quantification of pCREB-immunoreactive neurons was conducted using an auto-segmentation tool (midpoint analysis) provided by IPLab imaging software (Fig. 1C and D). Ghrelin stimulated the expression of pCREB. Low concentrations of ghrelin in 50 and 100 nM did not have any effect on pCREB expression. However, when 200 nM of ghrelin was used, the expression of pCREB increased 4-fold compared to control (p<0.01)(Fig. 1I). We examined higher concentrations of ghrelin in 500 nM and 1 µM; however, the magnitude of pCREB expression did not increase any further in response to these concentrations. Magnitude of pCREB expression was not different among 200 nM and higher doses of ghrelin. Ghrelin binds to its receptor when octanoylated. The major circulating form of ghrelin is the des-acyl ghrelin, the biologically inactive form of ghrelin (at least on the GHS-R), and that very little is known about des-acyl ghrelin catabolism and ghrelin anabolism. Ghrelin can be degraded by both desoctanoylation and N-terminal proteolysis at several cleavage sites (De Vriese et al., 2004). Thus, a steep change in response to differing concentrations of ghrelin in our study may be explained by the unique process of ghrelin desoctanoylation. Finally, the effect of ghrelin was mediated by the ghrelin receptor, since the antagonist of the ghrelin receptor L-Dys3-GHSR-6 (100 µM) reduced the expression of pCREB (p<0.02)(Fig. 1J).
3.2. Effect of Rp-cAMP on ghrelin-induced pCREB expression
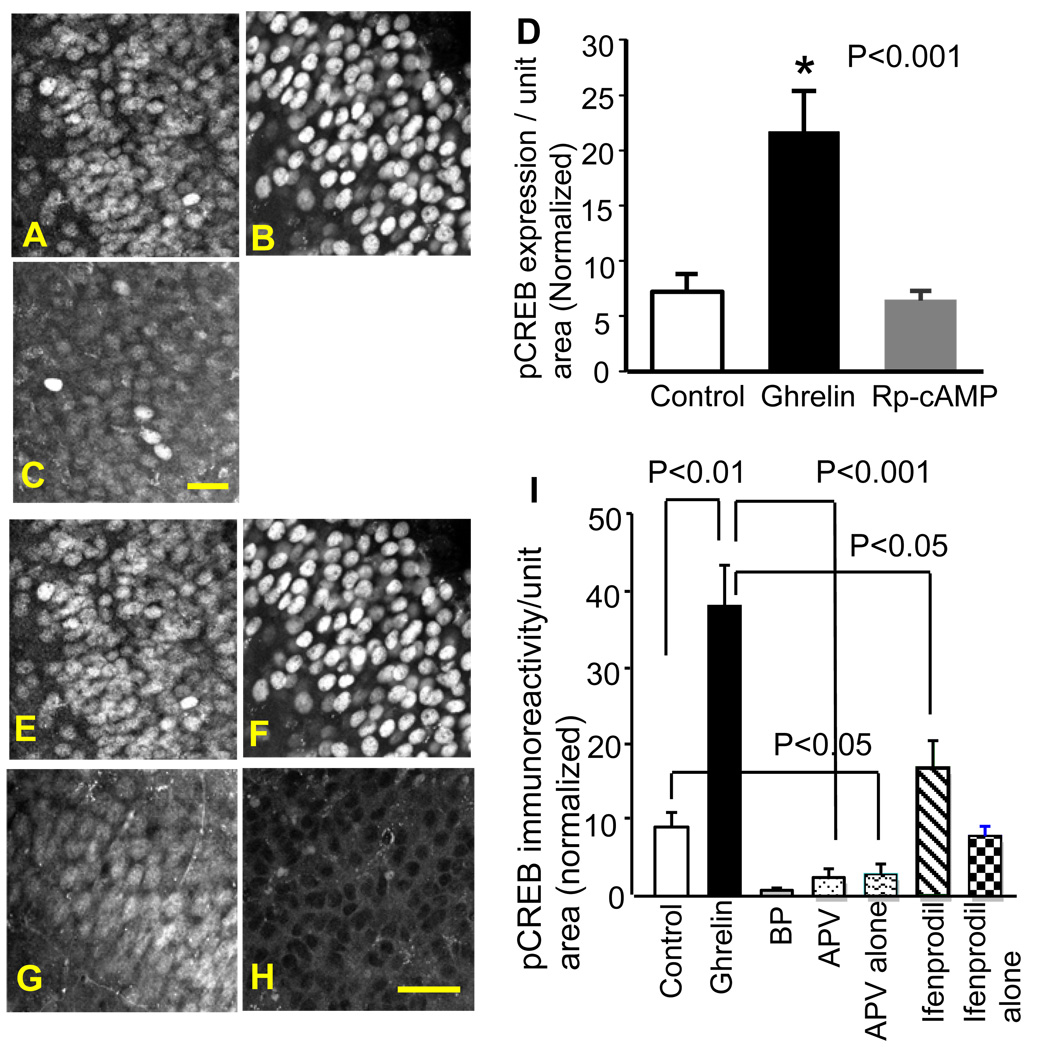
A primary molecular constituent for the ghrelin receptor is a Gq-protein. Although Gq is not linked to the cAMP/protein kinase A (PKA) signaling cascade that is necessary for CREB activation, the ghrelin receptor can cause a robust activation of CRE-mediated gene transcription (Holst et al., 2003). Therefore, we examined whether PKA was involved in the ghrelin-induced phosphorylation of CREB. Slices were incubated in the inhibitor of PKA (Rp-cAMP, 50 µM) before the application of ghrelin. Rp-cAMP blocked the ghrelin’s stimulatory effect on the expression of pCREB (Fig. 2A–D). This result suggested that cAMP-dependent activation of PKA was required in ghrelin-induced activation of CREB.
Figure 2.
Effects of Rp-cAMP, APV, and ifenprodil on ghrelin-induced pCREB expression. pCREB immunoreactivity in control (A), 200 nM ghrelin (B), and 200 nM ghrelin and 50 µM Rp-cAMP (C). Rp-cAMP blocked ghrelin-induced up-regulation of pCREB expression (D, *p<0.001). pCREB immunoreactivity in control (E), 200 nM ghrelin (F), 200 nM ghrelin and 5 µM ifenprodil (G), 200 nM ghrelin and 100 µM APV (H). APV and ifenprodil blocked ghrelin-induced up-regulation of pCREB expression (I). The calibration: 20 µm in C and 30 µm in H.
Gq activation can mobilize cytoplasmic calcium ([Ca2+]i) by translocating IP3 to the endoplasmic reticulum and initiate a release of Ca2+ from stores. Increase in cytosolic Ca2+ can stimulate cAMP production via the activation of Ca2+-dependent adenylate cyclases. Thus, we tested the possibility for whether the IP3 receptor was involved in the ghrelin-induced increase of pCREB in the present study. Pre-incubation of the hippocampal slices with 5 µM of Xestspongin-C, a specific antagonist of the IP3 receptor, for one hour before the application of ghrelin was not selective for inhibiting the ghrelin-induced up-regulation of pCREB immunoreactivity.
PKA has many well-characterized cAMP-dependent roles in cell physiology, which includes the phosphorylation of the NMDA receptor (Leonard and Hell, 1997). Phosphorylation potentiates NMDA receptor function and increases the receptor-mediated currents (Skeberdis et al., 2006). The increased current permits an enhanced Ca2+-permeation through the NMDA receptor and facilitates the induction of synaptic plasticity by promoting CREB signaling. Thus, we examined whether ghrelin-induced CREB expression depended on the activation of the NMDA receptor and whether NMDA receptor functions were amplified by ghrelin-induced amplification of PKA in a cAMP-dependent manner.
3.3. Effects of NMDA receptor antagonists on ghrelin-induced pCREB expression
The competitive antagonist of the NMDA receptor APV (100 µM) blocked ghrelin-induced expression of pCREB (p<0.001). Surprisingly, APV lowered the level of pCREB expression below the level of pCREB detected in our control slices (Fig. 2E–I). This finding suggests that CREB may have some constitutive activities in the hippocampal slice culture. In order to test this possibility, we applied APV alone without ghrelin. APV decreased the basal level of pCREB expression in our cultured slices (p<0.05, Fig. 2I), supporting our hypothesis that there is constitutive CREB activity in cultured hippocampal slices. A homeostatic level of CREB activity in the hippocampal slice culture may be the result of spontaneously active NMDA receptors.
One of the NMDA receptor subunit, NR2B, is critically involved in the facilitation of learning consolidation and synaptic plasticity induced by caloric restriction (Fontan-Lozano et al., 2007). Caloric restriction can increase plasma ghrelin levels up to a 4-fold (Luque et al., 2007). Thus, we examined whether the NR2B subunit was involved in ghrelin-induced expression of pCREB. The application of ifenprodil (5 µM), a specific antagonist of NR2B, inhibited ghrelin-mediated up-regulation of pCREB (Fig. 2I). The magnitude of pCREB expression in the presence of ifenprodil was similar to the level of the control. The application of ifenprodil alone did not have any effect on the basal (homeostatic) level of pCREB (p=0.112, Fig. 2I). These results suggested that the NR2B subunit may have preferential regulation on ghrelin-induced CREB activity while having little effect on the constitutive activity of CREB.
3.4. CREB-induced gene expression might include reorganization of cytoskeletal proteins
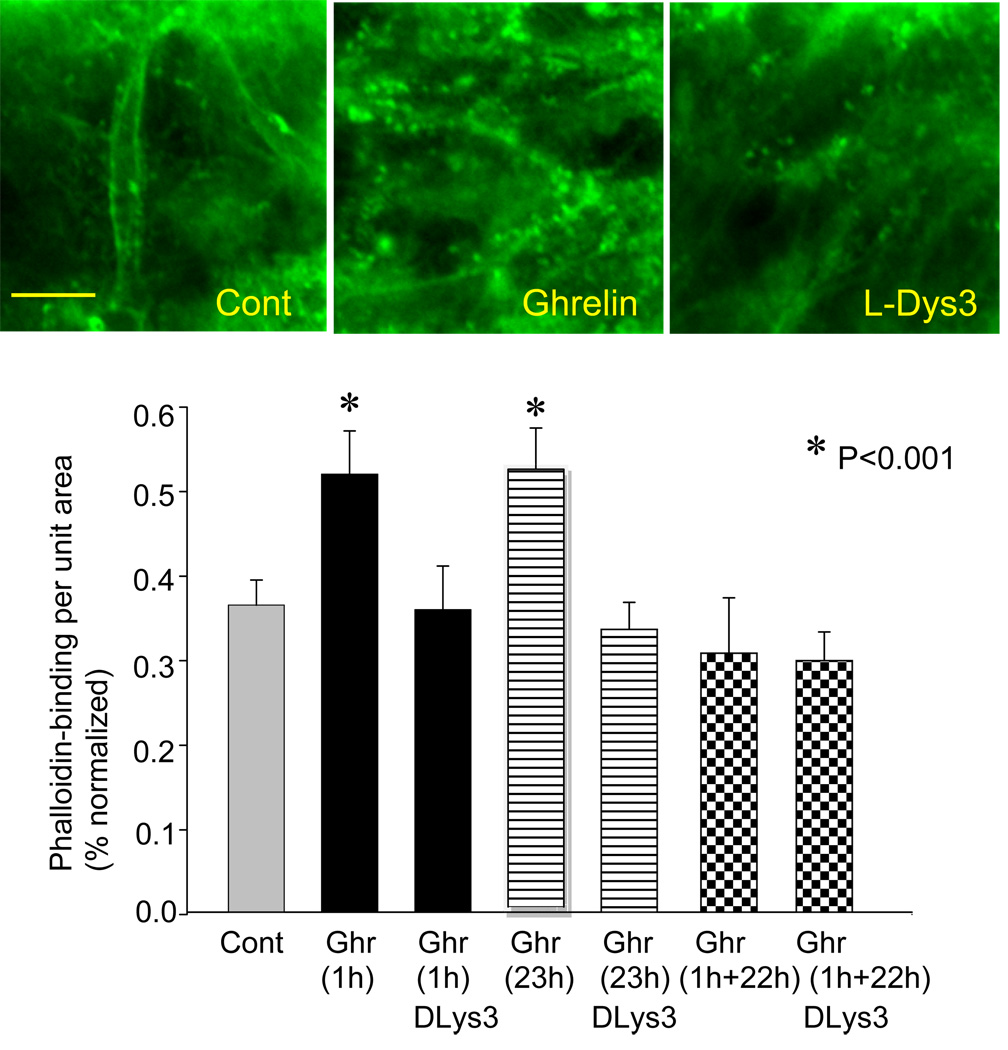
Ghrelin increased phalloidin-binding to F-actin. Phalloidin is a mushroom toxin that has a high affinity to polymerized F-actin. Photomicrographs in Fig. 3 show localization of Alexa488-conjugated phalloidin in dendrites and spines of hippocampal CA1 neurons in control, ghrelin-treated, and D-Lys-3-GHSR-6-treated slices. These data suggest CREB-induced gene expression might include reorganization of cytoskeletal proteins. As summarized in the graph in Fig. 3, an increase in phalloidin-binding (thus reorganization of F-actin) occurred relatively quickly (within an hour) in response to ghrelin application. When slices were incubated in ghrelin for a longer period of 23 hours, phalloidin-binding remained elevated for the entire duration of ghrelin-application (i.e., 23 h). In contrast, when slices were incubated in ghrelin for 1 hour and kept alive for the subsequent 22 hours without ghrelin before fixation, phalloidin-binding returned to a level similar to control and D-Lys3-GHRS-6-treated group. This result suggested the maintenance of ghrelin-induced F-actin-reorganization may require the presence of ghrelin.
Figure 3.
Effect of ghrelin on phalloindin-binding. Photomicrographs show phalloidin-based fluorescence representing polymerized F-actin in dendrites and spines in control, ghrelin, and D-Lys-3-GHSR-6. The graph depicts phalloidin-binding in response to ghrelin-application for 1 hour and 23 hours. The slices were fixed at the end of ghrelin-application. Some slices were kept alive before fixation for 22 hours in the absence of ghrelin after 1 hour of ghrelin-incubation (labeled as 1h+22h; see text for details). The calibration in Cont (10 µm) is shared by all three photomicrographs. Cont: Control, Ghr: Ghrelin, DLys3: D-Lys3-GHSR-6. Asterisks indicate p<0.01.
Finally, we tested the possibility whether CREB phosphorylation was stimulated due to an increased neuronal activity instead of ghrelin-induced cellular signaling cascade that we presented above. Ghrelin has been reported to increase neuron excitability in a selected group of hypothalamic neurons (Cowley et al., 2003). Thus, in the present study, some slices were treated with tetrodotoxin (TTX 1 µM) for 10 min before the application of ghrelin. The NMDA receptor agonist, NMDA (10 µM) was added in order to activate the NMDA receptor independent of the generation of action potentials. Magnesium was removed from culture media in order to remove the voltage-dependent magnesium block of the NMDA receptor channel. At the end of 1h application of ghrelin and NMDA, slices were fixed and processed for pCREB immunohistochemistry. In the presence of TTX, pCREB expression induced by ghrelin did not show any difference from those treated with ghrelin in the absence of TTX (P= 0.829). Namely, pCREB expression increased to 38.9% ± 2.6 SEM in normalized intensity in the presence of TTX, which was significantly higher compared with pCREB expression in control slices that were also incubated in TTX and NMDA (11.3% ± 3.5 SEM, p<0.01). Although there is evidence to indicate that ghrelin increased neuronal excitability in the hypothalamus (Cowley et al., 2003) and neuron firing enhanced CREB activation, the present result demonstrated in the hippocampus that the contribution of neuron firing as a result of ghrelin application, if any, is negligent to the increase of pCREB expression. Indeed, there is a report to show that intensive neuron firing reduced pCREB expression in the hippocampus (Nishimura et al., 2008). This report supports our interpretation that ghrelin-induced increase in CREB activity is not due to a secondary effect as a result of ghrelin-induced increase in neuronal firing.
3.5. Effects of endocannabinoids on ghrelin-induced expression of pCREB
Anandamide and 2 arachidonoylglycerol (2-AG) are representative endocannabinoids (eCBs) and agonists of the Type 1 cannabinoid receptor (CB1R). CB1R is a Gi-coupled receptor and highly expressed in the hippocampus. While synergistic involvement of eCBs and CB1R is suggested in the increased CREB activity and orexigenic effect of ghrelin in the hypothalamus, the contribution of eCB and CB1R in several forms of short- and long-term hippocampal plasticity has been explained independently of ghrelin. We examined a potential interaction between the endocannabinoid system and ghrelin-induced hippocampal plasticity.
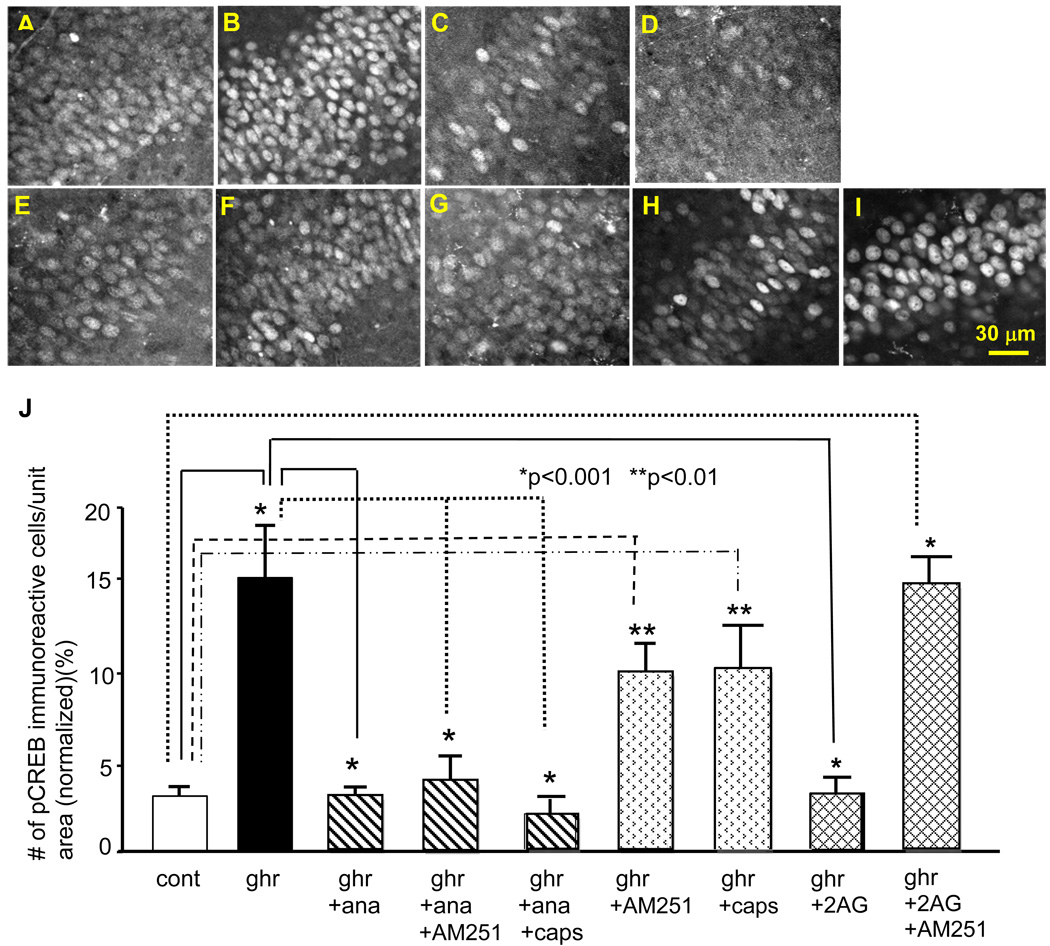
We first examined the effect of anandamide on ghrelin-induced pCREB expression. A low concentration (20 nM) of R(+)-methanandamide, a non-hydrolyzing form of anandamide, inhibited ghrelin-induced increase in pCREB expression (Fig. 4C). Surprisingly, this inhibitory effect of R(+)-methanandamide was not blocked by the CB1R antagonist AM251 (Fig. 4D). This result suggests that the action of R(+)-methanandamide may be independent of the CB1R cannabinoid receptor. Furthermore, incubation of slices in AM251 alone did not block a ghrelin-induced increase in pCREB expression. Indeed, 200 nM of ghrelin significantly increased the expression of pCREB in the presence of AM251 (p<0.01, Fig. 4F and J). These results suggest that CB1R is neither involved in the R(+)-methanandamide-mediated inhibition of ghrelin-induced pCREB expression nor the ghrelin’s effect of stimulating the phosphorylation of CREB.
Figure 4.
Effects of endocannabinoids on ghrelin-induced pCREB expression. Photomicrographs show pCREB immunoreactivity in the control (A), 200 nM ghrelin (B), 200 nM ghrelin and 20 nM R(+)-methanandamide (C), 200 nM ghrelin, 20 nM R(+)-methanandamide and 5µM AM251 (D), 200 nM ghrelin, 20 nM R(+)-methanandamide and 5 µM capsazepine (E), 200 nM ghrelin and 5 µM AM251 (F), 200 nM ghrelin and 5 µM capsazepine (G), 200 nM ghrelin and 10 µM 2-AG(H), 200 nM ghrelin, 10 µM 2-AG and 5 µM AM251 (I). The graph in J summarizes the results. The calibration in I: 30 µm.
Anandamide is an endogenous agonist for the vanilloid receptor (TRPV). We examined whether R(+)-methanandamide inhibited ghrelin-induced expression of pCREB through the activation of TRPV. As shown in Figure 4E, capsazepine (5 µM), a TRPV antagonist, did not have any effect on the R(+)-methanandamide-mediated inhibition of ghrelin-induced pCREB expression, suggesting that the inhibitory effect of R(+)-methanandamide was independent of TRPV. In addition, capsazepine did not have any effect on ghrelin-induced expression of pCREB when it was applied alone (Fig. 4G). Together these resulted suggest that TRPV was not involved in the inhibitory effect of R(+)-methanandamide on ghrelin-induced expression of pCREB. The result also suggested that TRPV had no contribution to ghrelin-induced expression of pCREB.
R(+)-methanandamide has been reported to induce CREB activity (Isokawa, 2009). The concentration of anandamide, reported effective in stimulating CREB activity, was 200 nM, which was a 10-fold higher concentration than what was used in the present study. Nevertheless, we tested whether 20 nM of R(+)-methanandamide induced CREB activity in the absence of ghrelin. As expected, the application of R(+)-methanandamide in 20 nM did not have any effect on the expression of pCREB.
We next examined the effect of 2-AG, another representative and well-investigated endocannabinoid in the hippocampus, on the ghrelin-induced expression of pCREB. 2-AG (10 µM) inhibited ghrelin-induced increase in pCREB expression (Fig. 4H) when it was applied to the culture media together with ghrelin. However, in contrast to anandamide, the inhibitory effect of 2-AG was blocked by the Type 1 cannabinoid receptor (CB1R) antagonist AM251 (Fig. 4I and J). This result suggested the action of 2-AG on the ghrelin-induced expression of pCREB was mediated by CB1R. In summary, the above results suggest that 2-AG likely exerts its inhibitory effect on the ghrelin-induced expression of pCREB through the activation of CB1R, while R(+)-methanandamide may exert its inhibitory effect in a receptor-independent manner.
A potential target of endocannabinoids may be the NMDA receptor, since the ghrelin’s stimulatory effect on the expression of pCREB was inhibited by antagonists of the NMDA receptor and the NR2B subunit (Fig. 2). NR2B-like immunoreactivity was reported to be co-expressed with that of NR1, suggesting that these two subunits co-assemble in the hippocampus (Thompson et al., 2002). Co-distribution of NR1 and NR2B mRNA is also reported (Monyer et al., 1994). NR1 is the pore-forming obligatory subunit. Channel function of the NMDA receptor is primarily regulated by the phosphorylation of the NR1 subunit at C-terminal serine residues (Zou et al., 2000), and NR2B affects channel gating to increase NMDA receptor mediated currents (Takasu et al., 2002). Finally, the magnitude of NR1 phosphorylation was reported to parallel the magnitude of the NMDA current as well as the magnitude of CREB activation (Choea et al., 2005). Therefore, we examined the effect of endocannabinoids on the phosphorylation of the NR1 subunit (pNR1).
3.6. Ghrelin-induced increase in pNR1 and its inhibition by anandamide
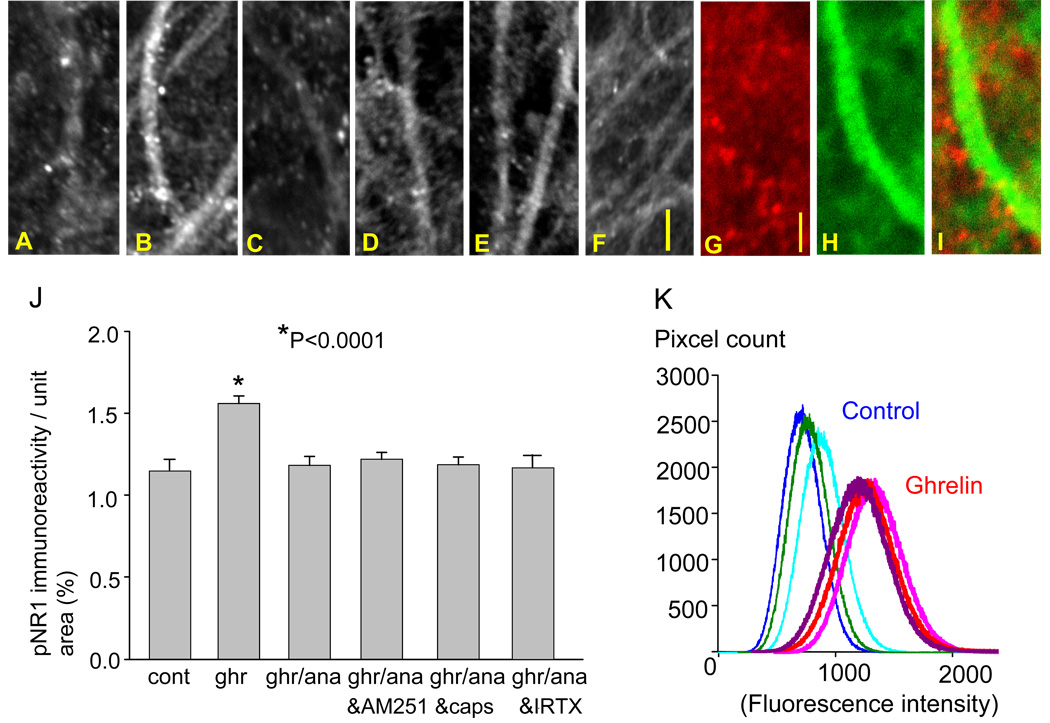
pNR1 immunoreactivity was observed as small and discrete puncta, primarily on the dendrites of the pyramidal neuron (Fig. 5A). The number of these immunopositive puncta markedly increased in response to an application of ghrelin (Fig. 5B). This finding was further supported by the pixel count of fluorescence intensity in the dendritic field of CA1 pyramidal neurons (Fig. 5K). The intensity of pNR1 immunofluorescence was greater in the CA1 stratum radiatum of the ghrelin-treated hippocampal slices compared to control slices.
Figure 5.
Effects of ghrelin and R(+)-methanandamide on pNR1 expression. Photomicrographs show pNR1 immunoreactivity in the control (A), 200 nM ghrelin (B), 200 nM ghrelin and 20 nM R(+)-methanandamide (C), 200 nM ghrelin, 20 nM R(+)-methanandamide and 5 µM AM251 (D), 200 nM ghrelin, 20nM R(+)-methanandamide and 5 µM capsazepine (E), 200 nM ghrelin, 20nM R(+)-methanandamide and 10 nM iodoresiniferatoxin (IRTX)(F). Dual imaging of pNR1 (red) with phalloidin (green)(G, H, and I). The graph summarizes the results (J). The intensity of pNR1 immunofluorescence in the CA1 stratum radiatum was greater in the ghrelin-treated hippocampal slices compared with control slices (K). The calibration in F (10 µm) is shared by photo-micrographs in A–F, and the calibration in G (2 µm) is shared by photomicrographs in G–I.
When the slices were incubated in 20 nM of R(+)-methanandamide together with ghrelin for 60 min, the effect of ghrelin on the pNR1 immunoreactivity was negated, and the average number of pNR1 immunoreactive puncta remained unchanged when compared to the control (p=0.939, Fig. 5C and J). The inhibitory effect of R(+)-methanandamide was not blocked by either AM251 (Fig. 5D and J), capsazepine (Fig. 5E and J), or iodoresiniferatoxin (IRTX)(Fig. 5F and J) suggesting that the effect of R(+)-methanandamide on ghrelin-mediated phosphorylation of NR1 is independent of the cannabinoid receptor and the vanilloid receptor. Dual labeling of pNR1 (red in Fig. 5G) with F-actin using a fluorescently-tagged phalloidin (Alexa 488 phalloidin, green in Fig. 5H) revealed the presence of pNR1-immunoreactivity along dendritic shafts and suggested that pNR1 immunoreactivity likely occurred on dendritic spines.
3.7. Ghrelin-induced increase in pNR1 and its inhibition by 2-arachidonoylglycerol (2-AG)
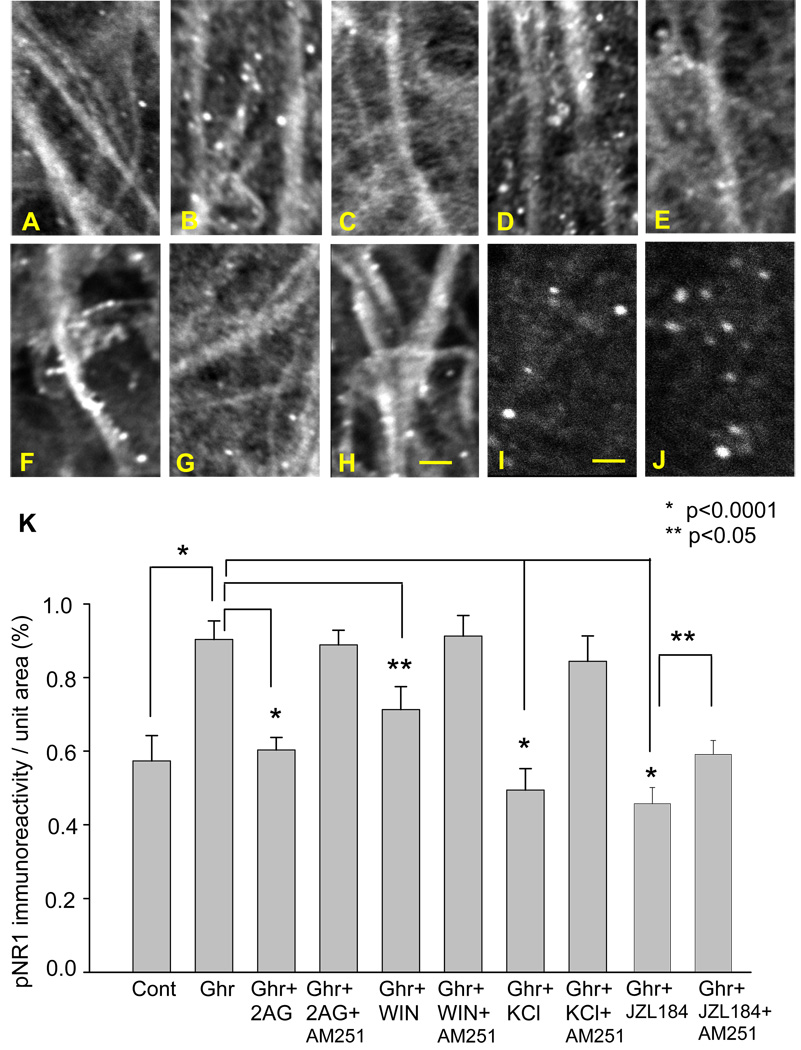
Co-application of 2-AG with ghrelin for 60 min negated the ghrelin’s stimulating effect on pNR1 expression. The number of pNR1 immunoreactive puncta decreased significantly (Fig. 6C, p<0.0001) to the level similar to the control. The inhibitory effect of 2-AG was blocked by the CB1R antagonist, AM251 (Fig. 6D and I), suggesting that the effect of 2-AG was receptor-dependent. Based on the finding of the involvement of CB1R, we tested the effect of WIN 55,212, an agonist of CB1R. Similarly to 2-AG, WIN 55, 212 blocked the stimulating effect of ghrelin on the pNR1 expression (Fig. 6E and I), which was also negated by the CB1R antagonist, AM251 (Fig. 6F and I).
Figure 6.
Effects of ghrelin and 2-arachydonoylglycerol (2-AG) on pNR1 expression. Photomicrographs show pNR1 immunoreactivity in the control (A), 200 nM ghrelin (B), 200 nM ghrelin and 10 µM 2-AG (C), 200 nM ghrelin, 10 µM 2-AG and 5 µM AM251 (D), 200 nM ghrelin and 4 µM WIN55,212-2 (E), 200 nM ghrelin, 4 µM WIN and 5 µM AM251 (F), 200 nM ghrelin and 150 mM KCl (10 s application) (G), 200 nM ghrelin, 150 mM KCl (10 s application) and 5 µM AM251 (H), 200 nM ghrelin and 100 nM JZL184 (I), 200 nM ghrelin, 100 nM JZL184 and 5 µM AM251(J). The graph summarizes the results (K). The calibration in H (10 µm) is shared by photomicrographs in A–H. The calibration in I (6 µm) is shared by photomicrographs in I and J.
We examined the effect of endogenous 2-AG. Endogenous 2-AG can be synthesized and released in response to strong neuronal depolarization. Thus, we applied 150 mM KCl for 10 seconds at the end of ghrelin application. The slices were fixed immediately after the application of KCl. KCl negatively regulated the stimulating effect of ghrelin on the pNR1 expression (Fig. 6G and I). The effect of KCl was blocked by AM251. The average number of pNR1-immunopositive puncta recovered to a level similar to that of ghrelin application (Fig. 6H and I). This experiment suggests neuronal depolarization and firing negatively contribute to ghrelin-induced phosphorylation of NR1 via the release of endogenous 2-AG. This finding is in agreement with our previous observation made on the effect of TTX on ghrelin-induced pCREB expression (Section 3.4.), which demonstrated that ghrelin-induced activation of CREB was not dependent on neuron excitation. We also tested the effect of MAGL inhibitor, JZL184. In this experiment, we did not use any experimental manipulation to induce time-locked synthesis and release of 2-AG. Thus, our hypothesis was that JZL184 could increase the concentration of ambient 2-AG in our cultured hippocampus. The hippocampal CA1 region exhibited a reduction in the pNR1 expression in the presence of JZL184 (100 nM, p<0.0001). AM251, antagonist of the cannabinoid receptor, reversed the effect of JZL184 (P<0.05)(Figure 6I, J, and K).
4. DISCUSSION
The present study demonstrates in CA1 pyramidal cells of cultured hippocampus that: 1) exogenous application of ghrelin up-regulates CREB signaling by activating the ghrelin receptor and cAMP/PKA signaling pathway, 2) the cAMP-dependent amplification of PKA results in the enhancement of NMDA receptor function by increasing the number of phosphorylated NR1 subunits, and 3) endocannabinoids block stimulatory effects of ghrelin on the NMDA receptor function by inhibiting the phosphorylation of NR1 subunit in a receptor-dependent (2-AG) or independent (anandamide) manner; thus, negatively regulate ghrelin-induced CREB signaling.
The up-regulation of CREB by ghrelin has been reported in the hypothalamus (Petersen et al., 2009). However, the kinases involved in the activation of CREB in the hypothalamus appeared different, since the activation of PKCδ (Zhao, 2007) and calcium calmodulin-dependent kinase IV (Matthews et al., 1994) were required. In the hippocampus, the role of cAMP/PKA signaling in the induction and late (protein synthesis-dependent) phase of NMDA receptor-dependent LTP has attracted considerable attention, and a gating role of PKA has been proposed in the induction of hippocampal plasticity (Thompson et al., 2002). Thus, our present findings of ghrelin’s stimulatory effect on cAMP and PKA reveal a novel signaling pathway of ghrelin in CA1 pyramidal neurons. Furthermore, our findings suggest that ghrelin and LTP may share a similar molecular mechanism in the induction and maintenance of hippocampal plasticity, which is to cause amplification of cAMP/PKA and a resultant increase in NMDA receptor function for the activation of CREB.
NMDA receptors are not only expressed at synapses but are also present extrasynaptically. Extrasynaptic NMDA receptors are also largely comprised of NR1/NR2B heterodimers (Tovar and Westbrook, 1999). Although the synaptic NMDA receptor and NMDA receptor-mediated Ca2+-signaling in dendritic spines are well characterized to contribute to the induction of LTP, extrasynaptic NMDA receptors are thought to activate CREB dephosphorylation under certain disease conditions (Hardingham and Fukunaga, 2002). This evidence suggests that the location of the NMDA receptor and its functional relationship to synaptic transmission appears to be critical for determining the role of the NMDA receptor in CREB-dependent neuron plasticity. Whether ghrelin can activate extrasynaptic NMDA receptors and regulate their functions remains to be investigated.
An interesting discovery in the present study was that endocannabinoids inhibited ghrelin-induced CREB phosphorylation by negatively interacting with the NMDA receptor and decreasing the number of phosphorylated NR1 subunits. We further identified that the mechanism of inhibition was different depending on the kind of endocannabinoids. 2-AG exerted its inhibition through the activation of its receptor, CB1R, while anandamide did so independently of the cannabinoid and vanilloid receptors. Interestingly, anandamide has been reported to directly interact and inhibit voltage-gated calcium channels (Oz et al., 2000), sodium channels (Nicholson et al., 2003), various types of potassium channels (Oliver et al., 2004), and the nicotinic acetylcholine receptor channel (Spivak et al., 2007), all in a receptor-independent manner. Thus, our present finding identifies the NMDA subtype glutamate receptor as an additional molecular target of anandamide.
Current understanding for the neuronal regulation of ghrelin is elusive. In the central nervous system, the main site of ghrelin synthesis is the hypothalamus, although at markedly lower levels than the stomach (Ferrini et al., 2009). In the hypothalamus, ghrelin is suggested to act as a neurotransmitter and modulate neuron excitability and synaptic transmission through the activation of Type 1a ghrelin receptor. In contrast, in the hippocampus, in spite of the presence of functioning receptors in high concentrations, the mRNA or protein of ghrelin was not reported either in the cell body or processes of hippocampal neurons (Cowley et al., 2003). Circulating ghrelin may play a major role in the hippocampus in appetite-driven plasticity.
RESEARCH HIGHLIGHTS.
Ghrelin can initiate a cellular signaling mechanism for hippocampal plasticity by activating CREB.
Ghrelin activates cAMP, PKA, and PKA-dependent phosphorylation of NMDA NR1.
2-arachidonoylglycerol, receptor-dependently, inhibits ghrelin-induced pNR1 phosphorylation.
Anandamide, receptor-independently, inhibits ghrelin-induced pNR1 phosphorylation.
Endocannabinoids negatively regulates ghrelin-induced CREB phosphorylation.
Acknowledgements
This work is supported by NIH grants R15DA021683 and SC1DA029329 to MI. JNC is a recipient of the American Physiological Society Undergraduate Summer Research Fellowship in 2010.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Choea ES, Shin EH, Wang JQ. NR1 subunits in striatal neurons in vivo. Neurosci. Lett. 2005;384:38–43. doi: 10.1016/j.neulet.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin Degradation by Serum and Tissue Homogenates: Identification of the Cleavage Sites. Endocrinol. 2004;145:4997–5005. doi: 10.1210/en.2004-0569. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay WC, da Silvo I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7:37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Dominguez SA, Lopez-Lluch G, Delgado-Garcia JM, Carrion AM. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J. Neurosci. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Dovey T, Cai X-J, Halford JCG, Pinkney J. Autoradiographic analysis of ghrelin receptors in the rat hypothalamus. Brain Res. 2008;1196:59–64. doi: 10.1016/j.brainres.2007.12.055. [DOI] [PubMed] [Google Scholar]
- Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor-identification of a potent inverse agonist. Mol Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- Isokawa M. Time-dependent induction of CREB phosphorylation in the hippocampus by the endogenous cannabinoid. Neurosci. Lett. 2009;457:53–57. doi: 10.1016/j.neulet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y-H, Chen Y-JJ, Chua SC, Jr, Talmage DA, Role LW. Integration of Endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. ProsOne. 2008;3(3):e1979. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J. Biol Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotrophin-releasing hormone. Endocrinol. 2007;148:300–309. doi: 10.1210/en.2006-0592. [DOI] [PubMed] [Google Scholar]
- Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CRBE-dependent gene expression. Mol. Cell. Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nicholson RA, Liao C, Zheng J, David LS, Coyne L, Errington AC, Singh G, Lees G. Sodium channel inhibition by anandamide and synthetic cannabimimetics in brain. Brain Res. 2003;978:194–204. doi: 10.1016/s0006-8993(03)02808-7. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Owens J, Swann JW. Effects of chronic network hyperexcitability on the growth of hippocampal dendrites. Neurobiology of Disease. 2008;29:267–277. doi: 10.1016/j.nbd.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan LP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135:159–168. doi: 10.1016/j.jneumeth.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Faker B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–270. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- Oz M, Tchugunova YB, Dunn SM. Endogenous cannabinoid anandamide directly inhibits voltage-dependent Ca2+ fluxes in rabbit T-tubule membranes. Eur J. Pharmacol. 2000;404:13–20. doi: 10.1016/s0014-2999(00)00396-4. [DOI] [PubMed] [Google Scholar]
- Petersen PS, Woldbye DPD, Madsen AN, Egerod KL, Jin C, Lang M, Rasmussen M, Beck-Sickinger AG, Holst B. In Vivo Characterization of High Basal Signaling from the Ghrelin Receptor. Endocrinol. 2009;150:4920–4930. doi: 10.1210/en.2008-1638. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. Epub 2006 Mar 12. [DOI] [PubMed] [Google Scholar]
- Spivak CE, Lupica CR, Oz M. The endocannabinoid anandamide inhibits the function of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 2007;72:1024–1032. doi: 10.1124/mol.107.036939. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs P-A, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Meth. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Drewery DL, Atkins HD, Stephenson FA, Chazot PL. Immunohistochemical localization of N-methyl-D-aspartate receptor subunits in the adult murine hippocampal formation: evidence for a unique role of the NR1D subunit. Brain Res Mol Brain Res. 2002;102:55–61. doi: 10.1016/s0169-328x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampalsynapses in vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D. Protein kinase Cδ-mediated CREB activation regulates ghrelin-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colonic epithelial cells. J. Cell Biochem. 2007;102:1245–1255. doi: 10.1002/jcb.21355. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]