Abstract
Profound depletion of follicular dendritic cells (FDCs) is a hallmark of sepsis-like syndrome, but the exact causes for the ensuing cell death are unknown. The cell death-driven depletion contributes to immunoparalysis and is responsible for most of the morbidity and mortality in sepsis. Here we have utilized immuno-spin trapping, a method for detection of free radical formation, to detect oxidative stress-induced protein and DNA radical adducts in FDCs isolated from the spleen of septic mice and human tonsil-derived HK cells, a subtype of germinal center FDCs, to study their role in FDC depletion. At 24 h post-LPS administration, protein radical formation and oxidation was significantly elevated in vivo and in HK cells as shown by ELISA and confocal microscopy. The xanthine oxidase inhibitor allopurinol and the iron chelator desferrioxamine significantly decreased the formation of protein radicals, suggesting the role of xanthine oxidase and Fenton-like chemistry in radical formation. Protein and DNA radical formation correlated mostly with apoptotic features at 24 h and necrotic morphology of all the cell types studied at 48 h with concomitant inhibition of caspase-3. The cytotoxity of FDCs resulted in decreased CD45R/CD138+ve plasma cell numbers, indicating a possible defect in B cell differentiation. In one such mechanism, radical formation initiated by xanthine oxidase formed protein and DNA radicals which may lead to cell death of germinal center FDCs.
Keywords: oxidative stress, follicular dendritic cell, caspase-3, xanthine oxidase, apoptosome, necrosis, plasma cell
Introduction
Sepsis-like syndrome is characterized by a severe hyperinflammatory stage followed by a protracted immunosuppressive phase that has been termed immunoparalysis. At least 25 clinical trials with new agents have failed, and it is understood that much still has to be learned about the patho-physiological mechanisms that drive sepsis (1). The loss of immunocompetence in the critically ill has been ascribed to the apoptotic deletion of cells of both the innate and adaptive immune systems (2). One of the principal components of the innate immunity-adaptive immunity crosstalk is the antigen-presenting cells of secondary lymphoid organs such as the spleen. Studies indicate that in septic spleens, there is a profound depletion of splenic dendritic cells, including follicular dendritic cells, due to apoptosis (3). This large scale depletion may severely compromise B- and T- cell function and impair the ability of the host to raise an appropriate immune response to secondary bacterial or viral infections, as is evidenced by the reactivation of the otherwise dormant cytomegalo virus and herpes simplex virus in critically ill individuals (4,5).
Studies in the cecal ligation and puncture model of sepsis have shown that there is a rapid expansion of FDC networks at 24–36 h followed by a rapid depletion, the cause being caspase-3- mediated apoptosis (3). The latter study assumes significance because follicular dendritic cell networks are crucial for the germinal center reactions in secondary lymphoid organs and present antigens to the B cell in the form of immunocomplexes or icocosomes. With HK cells, an FDC cell line that represents one of the subpopulations of FDCs and that are characterized by HJ2+GP93+3C8+DRC-1-KIM4-, it was established that GC B cells undergo complex interactions with FDC and T cells in the course of differentiation into memory B and plasma cells (6). Thus, it is justifiable to speculate that profound depletion of FDCs in sepsis can have serious consequences on the immune state of the critically ill. The scarcity of available evidence, both at clinics and in preclinical studies, about the distinct role of FDC depletion and the mechanism behind the events led us to investigate the mode of rapid depletion of FDCs.
One of the main causes that can lead to such rapid apoptotic cell death as proposed by Tinsley and colleagues, could be the involvement of reactive oxygen species (ROS) and reactive nitrogen species (3). Involvement of ROS in mechanisms of cell death has been well documented. Free radicals and their associated modifications of cellular processes, may disrupt signal transduction pathways, can be perceived as abnormal and, under some conditions, may trigger apoptosis (7). Oxygen metabolites such as superoxide anion and H2O2 in the presence of metal ions like iron and copper can form hydroxyl radical via Fenton chemistry (8; 9) and can have a major impact on cellular functions ranging from proliferation and differentiation to regulation of cell cycle events, apoptosis and, under extreme conditions, necrosis (7,10). Measurements of ROS, especially in free radical-mediated processes in cellular and in vivo systems, have been indirect and are generally presumed to cause cellular oxidative damage. Electron spin resonance spectroscopy remains the gold standard for measurement and identification of radical-mediated reactions. However, with the recent development of immuno-spin trapping technology, it is possible to detect free radical-mediated reactions on proteins in the form of protein radicals within cells (11–14), which provides strong evidence in real time of formation of reactive free radicals on protein amino acid residues and DNA bases.
Using this technique, we investigated the complex interplay between the involvement of ROS and profound FDC death associated with the acute systemic inflammatory settings in sepsis-like syndrome. In this study we provide evidence for the role of xanthine oxidase-derived superoxide and H2O2 and involvement of Fenton-like chemistry in forming protein-derived radicals in follicular dendritic cells of septic spleen and human tonsil-derived HK cells. The protein radical formation preceded apoptotic features in both in vitro and in vivo observations at 24 h and, interestingly, with a concomitant loss of caspase-3 activity at 48 h. Further, we identify nuclear accumulation of DNA-derived radical adducts that may signal late stage necrosis (15). This mode of cell death led to a significant decrease in plasma cell population in the spleen with no change of germinal center B cell numbers, suggesting a distinct effect of FDC death on the immune system, as correlates well with the immunoparalysis seen in sepsis.
Materials and Methods
Materials
LPS (Escherichia coli: Strain 55:B5), iron chelator deferrioxamine mesylate (desferrioxamine) , apocynin, catalase inhibitor aminotriazole (AT), Cytochrome P450 inhibitor 1-aminobenzotriazole (ABT) and allopurinol were obtained from Sigma Chemical Company (St. Louis, MO). The spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was obtained from Alexis Biochemicals (San Diego, CA). All other chemicals were of analytical grade and were purchased from Sigma Chemical Company or Roche Molecular Biochemicals (Mannheim, Germany). All aqueous solutions were prepared using water passed through a Picopure 2UV Plus system (Hydro Services and Supplies, Inc., RTP, NC) equipped with a 0.2 μm pore size filter.
Mice
Adult male, pathogen-free, 8–10-week-old C57BL6/J mice (Jackson Laboratories, Bar Harbor, Maine) weighing 23–27 g on arrival were used in these experiments. The animals were housed for 1 week, one to a cage, before any experimental use. Mice that contained the disrupted gp91phox ( gp91phox−/−; B6.129S6-Cybbtm1Din) (Jackson Laboratories) or disrupted p47 phox (B6.129S2-Ncf1tm1shl N14) (Taconic, Cranbury, NJ) genes were treated identically. In the case of NADPH oxidase, the control animals for knockout experiments were age-matched mice of C57BL6/J origin that had normal NADPH oxidase activity. Mice had ad libitum access to food and water and were housed in a temperature-controlled room at 23–24 °C with a 12-hour light/dark schedule. All animals were treated in strict accordance with the NIH Guide for the Humane Care and Use of Laboratory Animals, and the experiments were approved by the institutional review board.
LPS-induced systemic inflammation model
Systemic inflammation was induced in mice following LPS administration as described previously (16,17). Briefly, mice received a bolus infusion of LPS (12 mg/kg) (referred to as 0 h). A sham group was also included, where normal mice received saline in place of LPS. LPS was dissolved in pyrogen-free saline and administered through the intraperitoneal (i.p.) route. At +24 h , +48h and +72h, mice from the sham group and the LPS groups were sacrificed. For experiments that involved detection of protein radical adducts from tissue sections of mice spleen, DMPO was injected in two divided doses of 1 g/Kg. The spleens were collected and snap-frozen in liquid nitrogen.
Administration of allopurinol, apocynin and desferrioxamine
Allopurinol, a specific inhibitor of xanthine oxidase, was administered in a single bolus dose of 35 mg/kg through the i.p. route 30 minutes prior to LPS treatment. In other studies, desferrioxamine (50 mg/kg) was administered to mice 1 h prior to LPS injections (18), and apocynin (10 mg/kg) was administered to mice 1 h prior to LPS injections (19).
Isolation of CD14/CD21+ve follicular dendritic cells from mouse spleens
Because there is no specific protocol for isolation of splenic follicular dendritic cells, we chose to follow two distinct methodologies with modifications (20,21). Spleens from LPS and LPS+ allopurinol- or desferrioxamine-treated mice were dissected out and placed in 35 × 10 mm Petri dishes containing complete Dulbecco's Modified Eagles Medium on ice. The organs were then gently teased with a syringe piston and passed through a 75 micron cell strainer. The spleen cell suspension was then digested using a cocktail consisting of 1 ml of 8 mg/ml Collagenase D and 1 ml of 10 mg/ml DNase (Sigma, St. Louis, MO) plus 1 ml complete DMEM with 10% fetal bovine serum. The cells were digested for 1 h at 37 °C in a humidified incubator and given a 0.8% NH4CL treatment to remove the red blood cells. The cells were washed with DMEM and suspended in azide containing FACS buffer. Cells were stained with anti CD14-FITC and anti CD21-PE and sorted on a FACs Vantage SE flowcytometer (BD). The sorted cells were 80–90 % viable and did not proliferate under culture conditions. However, these cells were used for assays which involved short-term incubation (24–48h) with the spin trap DMPO. Isolated splenic FDCs stained positive for the mouse FDC marker FDC-M1.
Experiments using a human tonsil-derived follicular dendritic-like cell line, HK
An established FDC-like line (HK cells) was obtained from Dr. Y. S. Choi (Alton Ochsner Medical Foundation, New Orleans, LA) and maintained as described by Kim et al. (22). HK cells were maintained in antibiotic-supplemented RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. HK cells after 10–15 passages were used for various experiments. To study the effect of acute LPS treatment and formation of protein radicals in follicular dendritic cells, HK cells were incubated with 10 μg/ml LPS, 50 ng/ml TNF-α and 25 mM of DMPO for 24 h and 48 h, respectively. 10 μg/ml LPS has been found to up-regulate phosphor-IκB-α in FDCs more than lower doses, and thus this dose was selected for reproducing a sepsis-like environment (23). To see the effects of xanthine oxidase, NADPH oxidase, catalase and cytochrome p450 enzymes on protein radical formation, HK cells were co-incubated with either allopurinol (100 μM), AT (100μM), ABT (100μM), apocynin (100μM), DMPO (100mM) or the iron chelator desferrioxamine (200 μM).
Immuno-spin trapping of DMPO-protein radicals from cell lysates using ELISA
Cell lysates for use in ELISA for the detection of DMPO-protein nitrone adducts were prepared using a cell sonicator. Since buffers contain traces of metals and other contaminants that could form DMPO-protein radicals, we compared chelexed phosphate buffer (pH 7.4) containing 100 μM DTPA and RIPA buffer. Results from the pilot experiments found no significant difference in the chemiluminescence signal from these experiments, and all subsequent lysates were prepared in RIPA buffer (0.05 g sodium deoxycholate, 100 μL Triton X-100 and 10 μL of 10% SDS in 10 mL of 0.1 M PBS) containing protease inhibitors (Complete, Mini Protease Inhibitor Cocktail Tablets, Roche Applied Science, Indianapolis, IN). Following a 30 min incubation of the samples in ice, they were centrifuged at 20 000 × g for 20 min. The soluble material (supernatant) was stored at 4 °C until use.
DNA extraction from HK cells
Cell pellets were resuspunded in digestion buffer and proteinase K solution, and DNA extraction was carried out in our laboratory as outlined in Ramirez et al. (15,24). The extraction process maintains the nitrone adducts covalently bound to the DNA. DNA concentration and purity were measured from the absorbance at 260 and 280 nm. Purified DNA with low protein content will exhibit an A260/A280 ratio between 1.8 and 2.
Confocal laser scanning microscopy (Zeiss LSM 510 UV Meta)
Mice were administered LPS and one of the following: the xanthine oxidase inhibitor allopurinol, the catalase inhibitor 4-aminotriazole , or the iron chelator desferrioxamine. DMPO was injected in two doses of 1 g/kg at 2 h and 1h prior to sacrifice. Spleens were fixed in 10% neutral buffered formalin and soaked in 30% sucrose for 24 h. The frozen sections (10 micron) were cryocut using a frozen tissue processor (Leica Instruments, Bannockburn, IL) at the immunohistochemistry core facility at NIEHS. Tissue slices were then treated with 0.5% SDS (5 minutes) for antigen retrieval, permeabilised, and blocked (2% nonfat dry milk, Pierce Biomedical, Rockford, IL). In experiments where HK cells were used, 5×105 cells were plated with 10 μg/ml LPS and 50 ng/ml TNF-α with or without, allopurinol or desferrioxamine. Cells were harvested at 24 h and 48 h, respectively, fixed in 4% paraformaldehyde, and permeabilized with 0.01% Surfact Amps-X100 for 1 h. An antibody specific to DMPO nitrone adducts and Alexafluor 568 goat anti-rabbit antibody (Molecular Probes, now Invitrogen, Carlsbad, CA) were used as primary and secondary antibodies, respectively. Experiments that were performed to examine the xanthine oxidase levels in FDC-M1 positive cells in LPS-treated mouse spleen used anti-mouse xanthine oxidase (Abcam, Cambridge, MA) and Alexa 488 conjugated secondary antibodies (Invitrogen, Carlsbad CA). N-formylkynurenine, an oxidized product of tryptophan residues, was visualized using rabbit polyclonal antibody in both tissues and HK cells. Confocal images were taken on a Zeiss LSM510-UV meta (Carl Zeiss, Inc., Oberkochen, Germany) using a Plan-NeoFluor 40X/1.3/63X Oil DIC objective with different zoom levels. The 488 nm line from an argon laser was used for producing polarized light for a DIC image as well as fluorescence excitation of the Alexa 488 secondary antibody.
Western blot analysis
Cell lysates from FDCs isolated from mouse spleens were resolved in 4–10% Bis-Tris gels using SDS-PAGE and subjected to Western blot analysis. Antibodies used in these experiments were mouse monoclonal xanthine oxidase (1:1000 dilution, Abcam, Cambridge, MA), mouse monoclonal caspase-3 (32 kD), rat monoclonal to LAMP-2 (1:2000, Abcam, Cambridge, MA) and anti-mouse goat polyclonal p47 phox (Santa Cruz Biotechnology, Santa Cruz, CA) (1:2000). The immunocomplexed membranes were probed (1h at RT) with goat anti-mouse (1:5000, Milipore, Bellerica, MA) or anti-goat horseradish peroxidase-conjugated secondary antibodies. Immunoreactive proteins were detected using enhanced chemiluminescence (Immobilon TM Western Chemiluminescence HRP substrate, Millipore, Bellerica, MA). The images were subjected to densitometry analysis using LabImage 2006 ProfessionalTM 1D gel analysis software from KAPLEAN Bioimaging Solutions, Germany.
Apoptosis detection assay in HK cells
Treated cells were fixed using a 4% formaldehyde solution on Mattek uncoated glass-bottomed plates, permeabilized using 50 microliters of cytonin, and incubated for 20 minutes. The samples were washed twice in DNAse-free water, followed by terminal deoxynucleotidyl transferase (TdT) labeling as per the manufacturer’s protocol (TACS TdT in situ apoptosis detection kit, Fluorescein, R & D Systems, Minneapolis, MN). After labeling, the plates were immediately analyzed by fluorescence microscopy.
Caspase-3 activity assay
Caspase-3 activity was assayed using a fluorometric detection assay kit (Santa Cruz Biotechnology Santa Cruz, CA) following the manufacturer’s protocol. Briefly, HK cells at a density of 1 × 105 were treated with LPS/TNF-α with or without allopurinol, desferrioxamine, and apocynin in 96 well plates. For each reaction, 50 microliters of the cell lysate were added to each well of a 96-well plate. The reaction buffer was diluted and DTT was added to a final concentration of 10 mM prior to use. 200 μl of this buffer and 5 μl of DEVD-AFC substrate were added to each well containing cell lysate. The reaction mixtures were incubated for 1 hour at 37 °C, and the level of free AFC was measured using a plate reader with a 400 nm excitation filter and a 505 nm emission filter.
Spleen cell culture from LPS-treated mice
Single cell suspension from spleens of LPS- administered mice and mice treated with inhibitors of xanthine oxidase, catalase, and the iron chelator desferrioxamine were cultured after re-stimulating them with 10 ng/ml LPS for 24, 48, and 72 h, respectively. Spleen cells were harvested at these time points, stained with CD45R/B220-FITC and CD138-PE, and analyzed in a flow cytometer to examine the differentiation of B cells into plasma cells. Also, to ascertain the number of germinal center B cells, splenocytes were double-stained with CD45R/B220-FITC and PNA-PE. The supernatants from these time points were analyzed for T helper cell cytokines (TH1 and TH2) using a multiple array ELISA kit (Multi-Analyte ELISArray Kit, SABiosciences, Frederick, MD).
Statistical analyses
All in vivo experiments were repeated three times with 3 mice per group (N=3; data from each group of three mice was pooled). All in vitro experiments were repeated three times, and the statistical analysis was carried out by analysis of variance (ANOVA) followed by Kruskal-Wallis nonparametric test for intergroup comparisons. Quantitative data from Western blots as depicted by the relative intensity of the bands were analyzed by performing a Student’s t test. P<0.05 was considered statistically significant.
Results
LPS-induced sepsis-like syndrome forms cytosolic and nuclear protein radicals in germinal center FDCs and HK cells
Sepsis-like syndrome and other acute inflammatory insults result in copious generation of ROS, primarily in the form of superoxide radicals and non-radicals like H2O2 and peroxynitrite (14,25–28) . Though the initial generation of ROS contributes to the hyperinflammatory state, the resultant cellular stress may alter cell signaling, leading to cell death (29). FDCs, by functioning as accessory cells in the immune system, play a major role in germinal center reactions of the host. They also contribute significantly to B cell differentiation. ROS-related tissue damage can result in free radical-mediated damage to proteins.
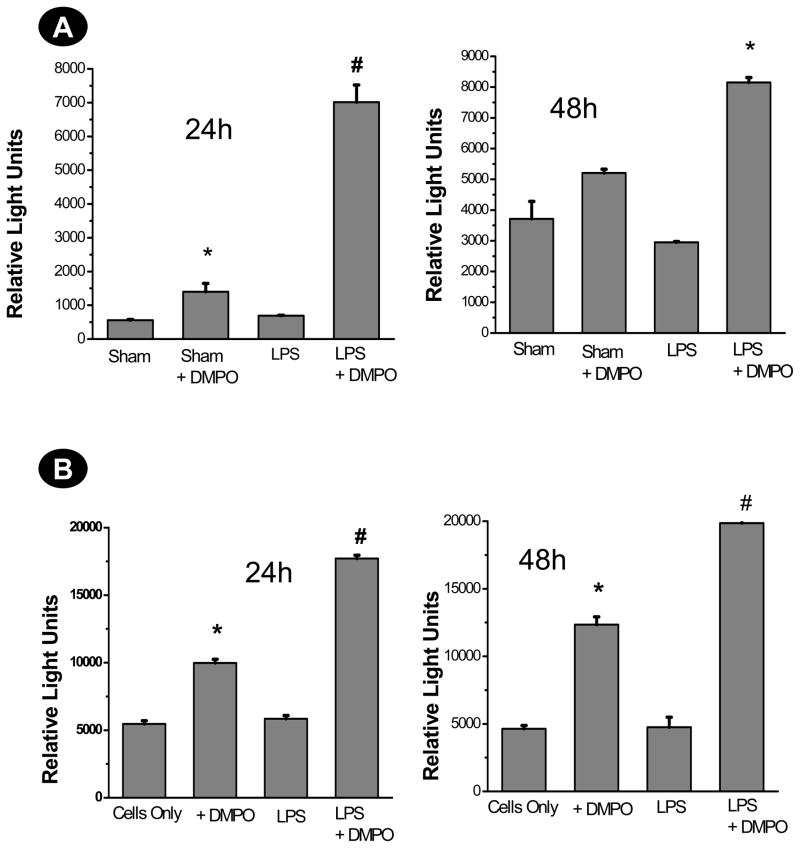
To study the ROS-induced deactivation of FDCs in sepsis-like syndrome, we used immuno-spin trapping (11,14) to quantify the generation of protein-derived radicals at 24 and 48 h post-LPS administration in FDCs isolated from LPS-treated mice and in HK cells. We observed a significant increase in protein radical adducts at 24 h in both FDCs isolated from LPS-treated septic spleens and HK cells treated with LPS and TNF-α, as detected by anti-DMPO ELISA (P<0.05) (Fig. 1A and 1B). In CD14/CD21+ve cells, a subset of FDCs isolated from septic spleens, there was a 7-fold increase in the protein radical adducts in FDCs isolated from the LPS-treated group co-incubated with the spin trap DMPO at 24 h (P<0.05). Experiments with HK cells co-stimulated with 10 μg of LPS and 50 ng/ml of TNF-α showed a significant increase in protein radical adducts at 24 and 48 h (Fig. 1B).
Fig. 1.
Protein radical adducts in FDCs from septic mice and HK cells.
A. CD14/CD21+ve cells were flow-sorted from spleen cell suspensions that were either from sham-treated or LPS-treated mice and cultured for 24 and 48 h using 10% fetal bovine serum-supplemented RPMI-1640 medium. Each subset of these cells was checked for viability. Cell populations with 80–90% viability were seeded at a density of 2×105/well with or without DMPO (25mM). At the end of each time point, cells were harvested and lysed. An anti-DMPO ELISA was performed using cell supernatants (2 μg protein/well) to detect DMPO-protein radical adducts. The results are shown as mean +/− SEM and are from 3 independent experiments.* P<0.05 compared to sham. # P<0.05 compared to sham+DMPO-treated mice. B. The human tonsil-derived follicular dendritic-like cell line HK was obtained from Ochsner Medical Foundation (Dr. Y.S. Choi) and cultured according to the protocol provided. Confluent cells between 7–16 passages were used for the assays. 5×105 cells were treated with either DMPO (25 mM) or LPS +DMPO for 24 and 48 h. TNF-α was supplemented to LPS-treated cells to mimic a septic environment. At the end of each time point, cells were harvested and lysed. A direct anti-DMPO ELISA was performed using cell supernatants to detect DMPO-protein radical adducts. The results are shown as mean +/− SEM and are from 3 independent experiments. *P<0.05 compared to cells only. # P<0.05 compared to the DMPO-only group.
To localize the site of radical formation in spleen tissues of LPS-treated mice, spleen sections were stained with the mouse FDC marker FDC-M1 and anti-DMPO antibody and visualized using confocal microscopy. Results indicated that protein radical adducts co-localized in FDC-M1 positive cells in the germinal center of the spleen and at 24 h were significantly higher than in sham-treated spleens where no germinal center formation was seen (Fig. 2A and 2B). As expected, we could not locate germinal center formation in sham-treated spleens by PNA+ve staining (data not shown). We could localize and identify several tingible body macrophages (TIBMs), a hallmark of germinal centers in the LPS-treated spleen (data not shown). A 63× magnified image of a tingible body macrophage with endocytosed FDC debris is shown in Fig. 2C. A control where no DMPO is administered to mice given LPS is shown in Fig. 2D. There was a gradual decline in the FDC-M1 positive cells at 48 h and 72 h when compared to 24 h, in agreement with the findings of Tinsley et al. (3), who showed depletion of FDCs in the septic spleen (Fig. 2E). Interestingly, we identified distinct co-localization of both FDC-M1 positive and anti-DMPO positive cellular debris within the TIBMs, suggesting engulfment of FDCs by these macrophages as early as 24 h (Fig. 2C). This co-localization may be of significance since the germinal center cells that undergo apoptosis are rapidly engulfed by TIBMs to prevent autoimmune reactions and possible inflammatory spillages. It also may explain the apoptotic mode of cell death in FDCs in early sepsis. The numbers of TIBMs in a germinal center may reflect the efficacy of removal of the cell debris arising from apoptosis. TIBM numbers were significantly reduced at 48 h in the septic spleen, indicating a change in the inflammatory microenvironment in the spleen (Fig. 2G).
Fig. 2. Localization of protein radical adducts in FDCs from septic mice and HK cells.
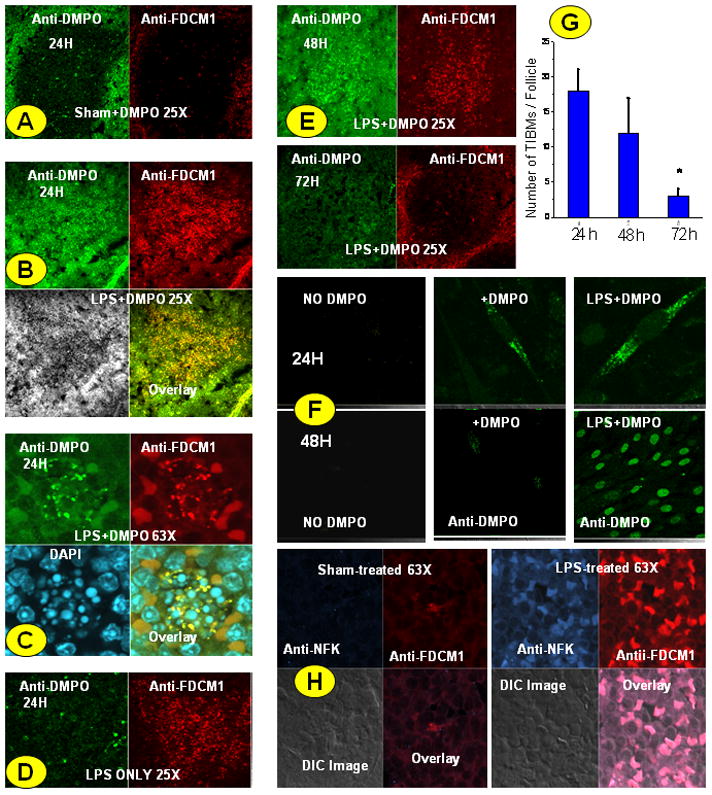
Spleens from sham+DMPO- and LPS+DMPO-treated mice were fixed in 10% neutral buffered formalin and rehydrated in sucrose solution. Cryocut sections were double-stained with anti-DMPO antibody and anti-FDCM1 antibody followed by secondary ALEXA 488- and ALEXA 567-tagged secondary antibodies. A. Sham-treated spleen section at 24h. B. LPS-treated, DMPO-labeled spleen section. Anti-DMPO staining co-localizes with FDC specific marker FDC-M1. C. 63X zoom view of a TIBM showing co-localization of protein adducts and FCM1 specific cell components within the cell boundary. D. LPS only control where no DMPO was injected into the animal. E. Spleen slices showing rapidly decreasing FDCM1-specific cells in the germinal center at 48 and 72 h post LPS administration. F. HK cells were treated with either DMPO only or LPS+DMPO for 24 and 48 h. Adherent cells were stained for protein radical adducts using anti-DMPO antibody and ALEXA 488-tagged secondary antibody and observed under confocal microscopy. G. The number of TIBMs that is a hallmark of the germinal center was counted with 40× magnification at 24, 48 and 72 hours. A minimum of 3 follicles from 3 separate experiments was analyzed per slide * P<0.05 when compared to the 24 h group. H. Tryptophan oxidation as a result of oxidative stress in FDCs of septic mice. Spleens at 36 h from sham- and LPS-treated mice were fixed in 10% neutral buffered formalin and rehydrated in sucrose solution. Cryocut sections were double-stained with anti-NFK antibody (blue in photomicrograph) and anti-FDCM1 antibody (red in photomicrograph) followed by secondary ALEXA 488- and ALEXA 567-tagged secondary antibodies. The sections were analyzed by confocal microscopy at 63× magnification. Confocal microscope images are representatives of a pool of images from at least 3 independent experiments.
To reproduce the inflammatory microenvironment of FDCs observed in the germinal centers of treated spleens of septic mice, we examined HK cells treated with LPS and TNF-α co-incubated with 25 mM DMPO. Confocal imaging showed two distinct and time-dependent localization patterns of DMPO nitrone adducts in HK cells (Fig. 2F). At 24 h incubation, protein radical adducts primarily co-localized in the cytosol, while at 48 h intense and punctate nuclear staining was observed (Fig. 2F).
N-formylkynurenine (NFK) and kynurenine are formed from the oxidation of tryptophan and tryptophan residues through a number of reactions (30, 31). We used a recently developed antiserum against NFK to detect NFK-containing proteins in splenic FDCs (31). FDCs that stained positive with FDCM1, a marker for mouse germinal center FDCs, had a significant increase in NFK immunoreactivity in LPS-treated spleens compared to sham-treated spleens at 36 h but not at 24 h as shown by confocal microscopy (Fig. 2H). A detailed time response experiment for generation of NFK adducts following LPS administration by using a low magnification confocal microscopy image of the germinal center was also conducted (Supplemental Fig.1).
The distinct punctate staining with anti-DMPO antibody within the nucleus of HK cells at 48 h prompted us to analyze the formation of DNA-derived radical adducts (15). The nuclear fraction was isolated by cell fractionation using a manufacturer’s protocol. The DNA was isolated and was analyzed for DNA-nitrone adducts by ELISA. Results indicated that there was a very significant increase in the formation of DNA-derived radicals in the nucleus of HK cells at 48 h (Fig. 3).
Fig. 3.
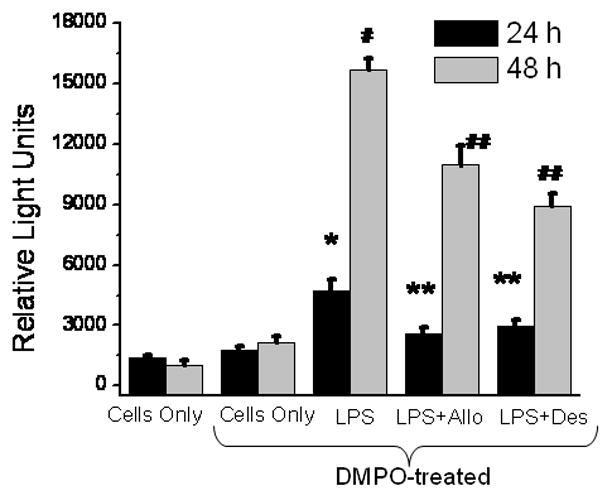
Nuclear DNA radical formation mediated by xanthine oxidase and Fenton-like chemistry in LPS-treated HK cells. HK cells were treated with LPS and TNF-α and harvested at 48 h. DNA was isolated from the nuclear fraction after cell fractionation. ELISA for detection of DNA-DMPO nitrone adducts was carried out as per Ramirez, D.C. et al. To see the involvement of xanthine oxidase and loosely bound iron, allopurinol (Allo) and desferrioxamine (Des) were co-incubated with LPS. The results are shown as mean +/− SEM and are from 3 independent experiments. *P<0.05 compared to cells only at 24 h. #P<0.05 compared to cells only at 48 h **P<0.05 compared to the LPS-treated group at 24 h. ##P<0.05 compared to the LPS-treated group at 48 h.
To assess the role of the lysosome as a possible organelle where the protein radicals and their stable oxidation products can be generated and/or mobilized, we used confocal laser scanning microscopy and fluroscence imaging to detect the DMPO-nitrone adducts. Results indicated that there were very few areas where punctate staining of both lysosomal marker LAMP-2 and DMPO-nitrone adducts could be identified or co-localization was evident in both 24 and 48 h (Supplemental Fig. 2).
Xanthine oxidase and Fenton-like chemistry are involved in protein radical formation in germinal center FDCs and HK cells
To begin to identify the source of radicals and H2O2 in LPS-treated mice and HK cells, we used the xanthine oxidase inhibitor allopurinol and the iron chelator desferrioxamine in experiments analyzing both DNA and protein radical formation. ELISA analysis showed that co-incubation with either allopurinol or desferrioxamine significantly decreased DNA-DMPO adduct formation in HK cells (Fig. 3). Similarly, analysis of protein radical accumulation in HK cells and septic mouse spleen cells also implicated both xanthine oxidase and Fenton chemistry in radical formation. Protein-DMPO adduct accumulation in HK cells was significantly reduced by both allopurinal and desferrioxamine at both 24 and 48 h. Pretreatment of mice with desferrioxamine significantly reduced the accumulation of protein-DMPO nitrone adducts in spleens of LPS induced septic mice at both 24 and 48 h. While allopurinol also attenuated the production of protein radicals at 24 h, this effect was attenuated by 48 h.
Confocal analysis was then used to confirm and expand the inhibitor experiments. DMPO adduct accumulation is reduced by both allopurinol and by desferrioxamine in both spleen (Fig. 5A) and HK (Fig. 5B) cells, paralleling the ELISA results (Fig. 4A and 4B). Confocal analysis also allowed the detection of an increase in xanthine oxidase protein in mouse spleen due to LPS treatment as well as accumulation of xanthine oxidase in HK cells (Fig. 5C). Western analysis of these cells corroborated the confocal images, showing higher amounts of xanthine oxidase in LPS-treated spleen and HK cells. The role of NADPH oxidase was ascertained by using gp91 phox knockout and p47 phox knockout mice and injecting apocynin to LPS-treated mice and co-incubating with HK cells. Apocynin injection or co-incubation did not result in a significant decrease in DMPO-nitrone adduct formation in either FDCs derived from the septic mice or in HK cells. Similarly, both p47 phox and gp91 phox knockout mice did not show a significant reduction in DMPO-nitrone adduct formation in FDCs from septic mice (4A and 4B). Further Western analysis of immunoblotted proteins for the p47 phox subunit of NADPH oxidase revealed no immunoreactivity for this protein. In addition, confocal analysis showed that spleens from LPS-treated NADPH oxidase knockout mice form amounts of DMPO adducts equal to the wild type. This experiment, however, also showed that these knockout spleens had low recruitment of FDCs to the germinal centers. Cells incubated with 100 mM DMPO showed significant decrease in DMPO-nitrone adducts (4A and 4B).
Fig.5.
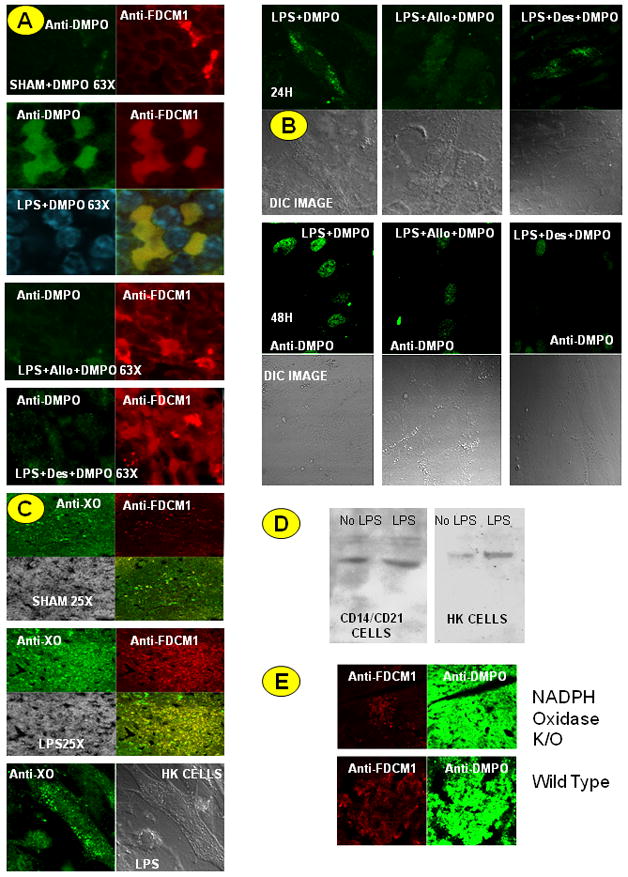
Oxidative stress in sepsis generates cytosolic and nuclear radical adducts in a time-dependant manner. A. Cryocut spleen sections from sham+DMPO, LPS +DMPO-treated, LPS+allopurinol (Allo)+DMPO-treated and LPS+desferrioxamine(Des)+DMPO-treated mice were double stained with anti-DMPO antibody and anti-FDCM1 antibody followed by secondary ALEXA 488- and ALEXA 567-tagged secondary antibodies. Sections were visualized under confocal microscopy. Confocal microscope images are representatives of a pool of images from at least 3 independent experiments. B. HK cells were co-incubated with DMPO only (data not shown), LPS+DMPO, LPS+Allo+DMPO or LPS+Des+DMPO for 24 and 48 h. Adherent cells were stained for protein radical adducts using anti-DMPO antibody and ALEXA 488-tagged secondary antibody and observed under confocal microscopy. C. Spleen sections from sham-, LPS-treated mice and LPS-treated HK cells were analyzed for xanthine oxidase localization using confocal microscopy. D. Western blot analysis of xanthine oxidase levels in CD14/CD21+ve and HK cells. E. Spleen sections from LPS-treated mice from wild-type and NADPH oxidase knockout mice were analyzed for protein radical adducts using confocal microscopy.
Fig. 4.
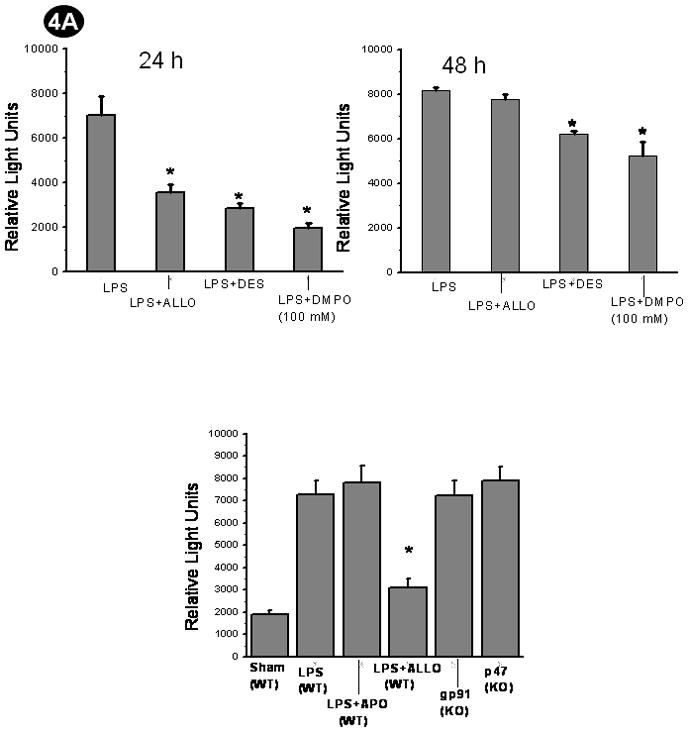
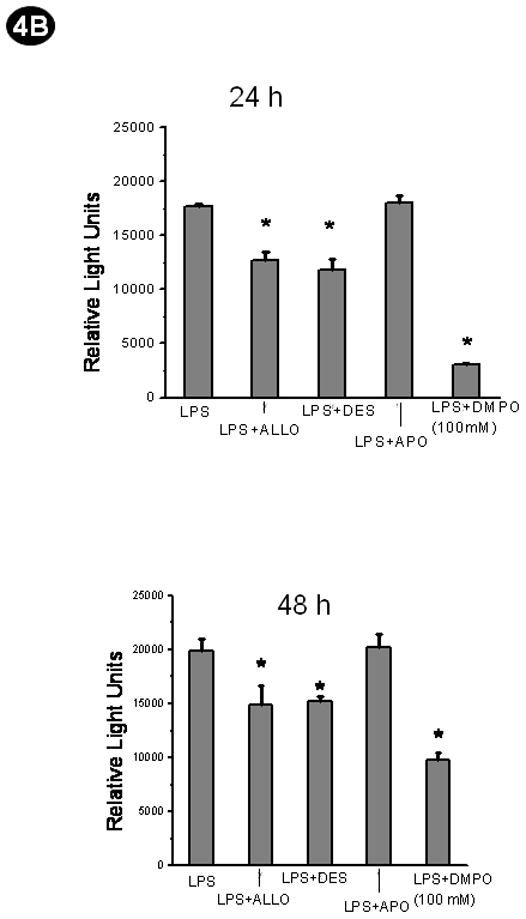
Protein radical adducts in LPS-treated FDCs are mediated by xanthine oxidase and occur through Fenton-like chemistry. A. Wild-type mice or p47 or gp91 phox knockout mice were administered LPS. Wild-type LPS administered mice received the xanthine oxidase inhibitor allopurinol (Allo), NADPH oxidase inhibitor apocynin (APO) or the iron chelator desferrioxamine (Des), or the cells were incubated with 100 mM DMPO (excess DMPO) as pretreatments. CD14/CD21+ve cells from these mice were flow sorted from spleen cell suspensions and cultured for 24 and 48 h using 10% fetal bovine serum-supplemented RPMI-1640 medium. A direct anti-DMPO ELISA was performed using cell lysates to detect DMPO-protein radical adducts. The results are shown as mean +/− SEM and are from 3 independent experiments. *P<0.05 compared to the LPS-treated group. B. Confluent HK cells between 7–16 passages in a density of 5×105 cells were co-incubated with LPS +DMPO or LPS+Allo or LPS+DES, or LPS+APO for 24 and 48 h. To study the scavenging action of DMPO an excess concentration of DMPO (100mM) was added in the incubation mixture instead of the 25mM that was used for the purpose of spintrapping. At the end of each time point, cells were harvested and lysed. A direct anti-DMPO ELISA was performed using cell supernatants to detect DMPO-protein radical adducts. The results are shown as mean +/− SEM and are from 3 independent experiments. *P<0.05 compared to the LPS-treated group.
Caspase-3 activity and mode of cell death
The only study (3) that links FDC cell death to sepsis strongly suggests that caspase-mediated apoptosis leads to FDC depletion, resulting in immunosuppression. Alternatively, studies with HK cells indicate a clear role of TGF-β in opposing TNF-α-mediated apoptosis (33). To study the involvement of caspase-3-mediated apoptosis, we measured caspase-3 activity in FDCs isolated from the septic spleen and in HK cells. In the CD14/CD21+ve cells from LPS-treated mice, the caspase 3 activity shows an increase at 24 h which is restored to control levels by allopurinol, excess DMPO or desferrioxamine (Fig. 6A). At 48 h, caspase-3 activity has decreased in cells from the LPS-treated mice, while the activity in cells from mice co-administered LPS and either allopurinol, excess DMPO or desferrioxamine have control levels of activity.
Fig. 6.
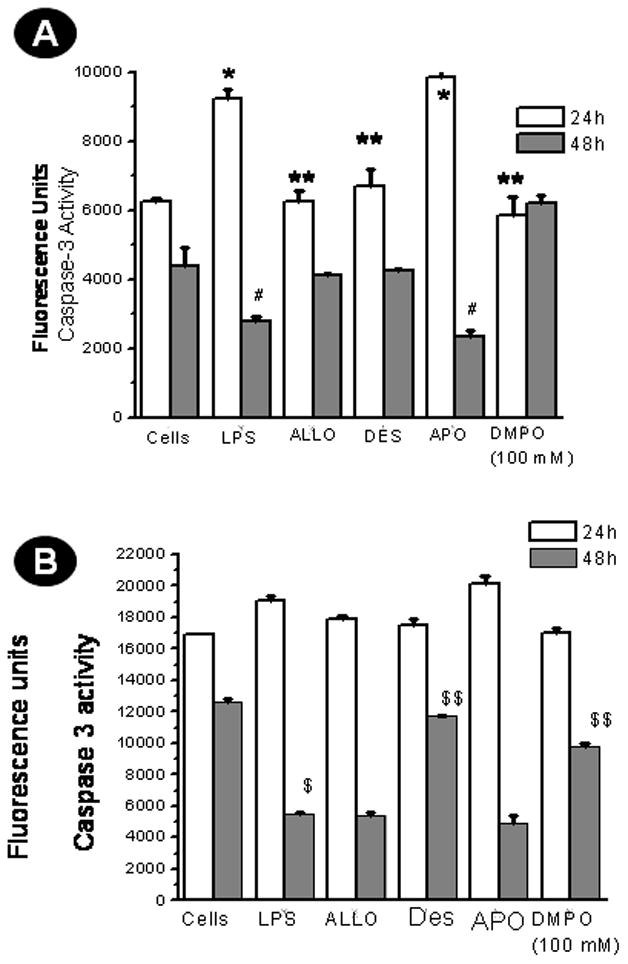
Protein radicals in FDCs modulate procaspase-3/caspase-3 activity. A. CD14/CD21+ve cells from sham-treated , LPS-treated, LPS+allopurinol (Allo)-treated, LPS+desferrioxamine (Des)-treated and LPS+Apocynin (APO)-treated mice were flow-sorted from spleen cell suspensions and cultured for 24 and 48 h using RPMI-1640 medium supplemented with 10% fetal bovine serum. For experiments to study the role of excess DMPO in scavenging protein radicals, sorted cells were incubated with 100 mM DMPO. Cell lysates collected at these time points were used to measure caspase-3 activity using a caspase-3 activity kit following the manufacturer’s protocol. The results are shown as mean +/− SEM and are from 3 independent experiments. * P<0.05 compared to the sham-treated group at 24 h. ** P<0.05 compared to the LPS-treated group at 24 h. # P<0.05 compared to the sham-treated group at 48 h. ## P<0.05 compared to the LPS-treated group at 48h. B. HK cells were untreated or co-incubated with LPS, LPS+allopurinol (Allo), LPS+Apocynin (APO) or LPS+desferrioxamine (Des) or for 24 and 48 h. The cell lysates were used for assaying caspase-3 activity. The results are shown as mean +/− SEM and are from 3 independent experiments. $ P<0.05 compared to the cells-only group at 48 h. $$ P<0.05 compared to the LPS-treated cells group at 48 h.
The caspase-3 activity in HK cells (Fig. 6B) responded somewhat differently than the CD14/CD21+ve spleen cells. At 24 h all treatments had equal activity, while at 48 h the LPS-treated cells showed a significant reduction in activity that was restored to control levels by desferrioxamine and excess DMPO but not by allopurinol. Apocynin had no effect either in cells derived from septic mice or HK cells (6A and 6B). 4-Aminotriazole had no effect either on CD14/CD21+ve or on HK cells at 24 h and 48 h (data not shown).
To study the pattern of cell death, we used confocal microscopy to visualize binding of Annexin V to the phosphatidyl serine moieties on the membrane. Results indicated that FDCs isolated from septic spleens (CD14/CD21+ve cells) had marked annexin-V binding on the cell membrane at 24 h, but the staining was diffuse in cells that were observed at 48 h (Fig. 7A). The DIC images at 24 h showed typical apoptotic characteristics like cell shrinkage and condensation of the nucleus, but were in sharp contrast to the 48 h view, which showed disintegration of the cell boundary, a characteristic of necrotic morphology.
Fig. 7.
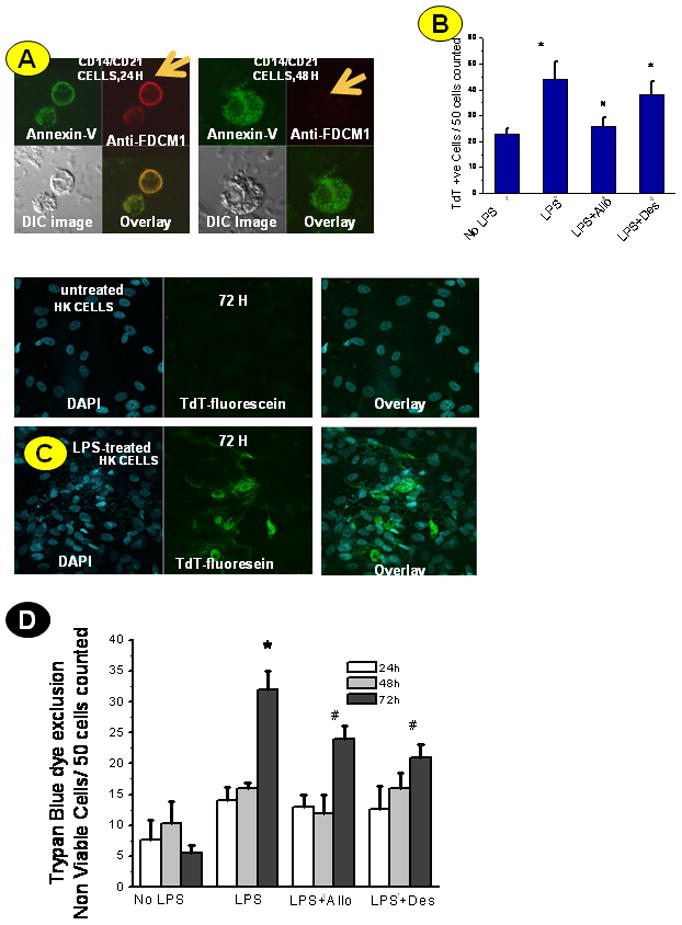
Cell death patterns in FDCs in early and late sepsis. A. CD14/CD21+ve cells from sham-treated (represented as “cells” in the figure) and LPS-treated mice were flow-sorted from spleen cell suspensions and cultured for 24 h and 48 h using RPMI-1640 medium supplemented with 10% fetal bovine serum. Cells were labeled with annexin –Alexa 488- conjugate and FDCM1 antibody (Alexa 567-conjugated secondary antibody) at the desired time points and analyzed by fluorescence microscopy. DIC image for each group was analyzed for the morphological features. B. HK cells were treated with LPS (10μg/ml), LPS+allopurinol (Allo) and LPS+desferrioxamine (Des) for 24, 48 and 72 h. At the desired time points, cells were fixed, permeabilized with cytonin and analyzed for TdT-fluorescein labeling using a fluorescence microscope. TdT+ve cells were counted in a 40× magnification field of view. Results are represented for 24 h cell counts. The results are shown as mean +/− SEM and are from 3 independent experiments.* P<0.05 compared to the cells only group. # P<0.05 compared to the LPS-treated group.
C. Photomicrograph of TdT-fluorescein labeling of HK cells at 72 hours showing extracellular and disintegrated chromatin structures (a phenomenon likely seen in necrosis) compared to untreated cells. D. Trypan blue dye exclusion test was carried out with HK cells at 24, 48 and 72 h for assessing the viability of cells in culture following treatment with LPS (10μg/ml), LPS+allopurinol (Allo) and LPS+desferrioxamine (Des) . The results are shown as mean +/− SEM and are from 3 independent experiments.* P<0.05 compared to the cells only group. # P<0.05 compared to the LPS-treated group.
Because the cells isolated from LPS-treated spleens were flow-sorted, which can cause stress-induced damage and perturb the results of the annexin-V analysis, we carried out TdT labeling experiments with HK cells (Fig. 7B). This labeling found that LPS-treated cells showed a significant increase in apoptotic nuclei at 24 h which was substantially returned to control levels by the xanthine oxidase inhibitor allopurinol apoptosis (P<0.05). Neither desferrioxamine nor 4-aminotriazole (data not shown), however, had a significant effect on apoptosis in these cells (Fig. 7B). Confocal analysis of HK cells at 72 h revealed condensed nuclei with the TdT-fluorescein label predominantly located outside the nuclei (Fig. 7C). Most of the cells observed had disintegrated cell membranes with the fluorescein localized in structures that did not resemble healthy cellular components, suggesting cellular necrosis. The cells that screened positive for TdT- fluorescein stain in the nucleus were significantly reduced at 48 h and 72 h when compared to 24 h in both cell types studied (data not shown).
Trypan Blue dye exclusion tests for assessing cell viability showed a time-dependent increase in nonviable cells in LPS-treated HK cells. Groups coincubated with allopurinol and desferrioxamine, respectively, had significantly fewer nonviable cells as compared to the LPS-treated group at the 72 h time point, suggesting that at 72 h superoxide, H2O2 (from the xanthine/xanthine oxidase system), and loosely bound iron in proteins or free iron had a significant role in the cell death process (P<0.05) (Fig. 7D).
Protein radical formation and post-translational protein oxidation are associated with FDC death and defects in B cell differentiation in the germinal center
The germinal center constitutes the dynamic microenvironment where antigen-activated B cells rapidly expand and differentiate, generating plasma cells and memory B cells (34). Therefore depletion of FDCs in the germinal center could derail the process of B cell differentiation, causing large scale apoptosis of B cells and ultimately amplifying the risk of secondary infections due to diminished immune response.
Activation of B cells leads to their differentiation into IgG-secreting plasma cells (35). To investigate whether FDC death impacts B cell differentiation in the germinal center, we used flow cytometry to analyze splenic cell populations from sham- and LPS-treated mice at 24, 48 and 72 h. CD 138, a cell surface marker for plasma cells, was used along with the B cell marker CD45R (43). When spleen cells from sham- and LPS-treated septic mice were isolated and restimulated, there was a significantly smaller number of CD45R/CD138+ve cells at 72 h compared to the sham-treated group (P<0.05) (Figs 8A and 8B). There was, however, no significant difference in the 24 h and 48 h time points (data not shown). These results suggest that the defect in differentiation of germinal center B cells might be due to the subtle depletion of FDCs in late sepsis (i.e., at time points >48 h) and are well correlated with the necrotic phase of cell death. Desferrioxamine administration to septic mice had no significant effect in CD45R/CD138ve cell number compared to the sham-treated group, indicating that chelation of iron might have led to reduced toxicity at 72 h, causing less FDC depletion and less cell death.
Fig. 8.
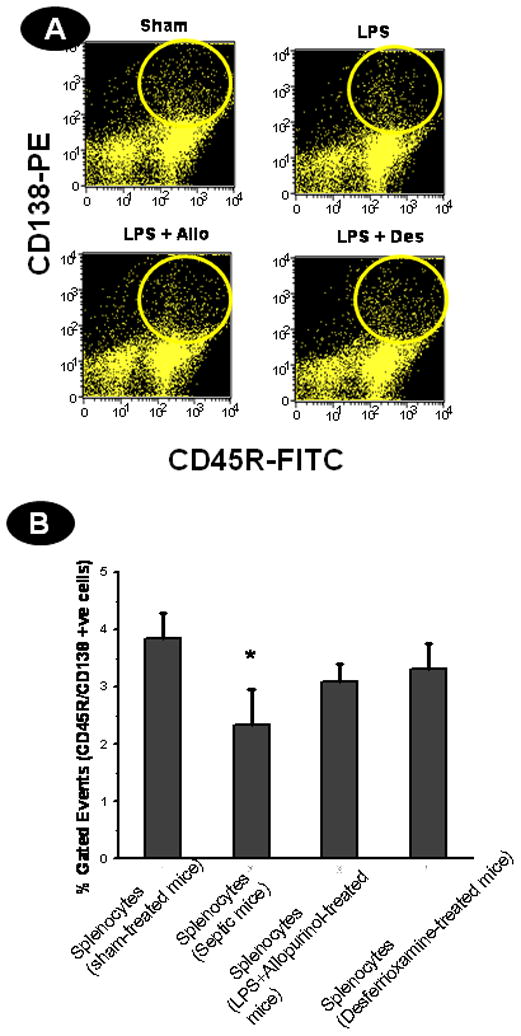
Oxidative stress in late sepsis modulates B cell differentiation following death of FDCs in septic mice. A. B cell differentiation into plasma cells is affected by oxidative stress induced FDC death. Spleen cells from sham-treated, LPS, LPS+allopurinol (Allo) and LPS+desferrioxamine (Des) were stimulated with 10 ng/ml LPS and incubated for 24, 48 and 72 h. At the end of each time point, cells were harvested and labeled with FITC-conjugated CD45R and PE-conjugated CD138 and analyzed by flow cytometry. Flow plots for the results at 72 h are shown. B. Column diagram of double positive cells at 72 h from sham, LPS, LPS+allopurinol (Allo) and LPS+desferrioxamine (Des) groups. The results are shown as mean +/− SEM and are from 3 independent experiments.* P<0.05 compared to the sham-treated group.
Discussion
Mortality and morbidity in sepsis is now known to be the result of severe immunosuppression, termed immunoparalysis. This condition is caused primarily by the profound depletion of lymphocytes, dendritic cells, interdigitating cells and follicular dendritic cells; enhanced dendritic cell survival attenuates LPS-induced immunosuppression (3,36,37,2,38). The rapid depletion of the immune effector cells is primarily due to apoptotic-like processes, and the molecular mechanisms involved are ascribed to both intrinsic and extrinsic pathways (2). Our studies for the first time identify the involvement of novel free radical-mediated, post-translational oxidation of proteins and DNA in cell death of FDCs both in septic mice and in an FDC-like cell line, HK. Injury to DNA can have severe pathophysiological consequences such as cell death and carcinogenic transformation (39).
In our previous studies, under conditions where severe inflammation and reactive oxygen species generation can influence mortality and morbidity in early sepsis, we identified the role of xanthine oxidase in radical formation and nitration of tyrosine residues of CPB1 resulting in an amplification of the inflammation cascade in spleen cells (14,17). In this study, we focused on FDCs in the spleen germinal center and on human tonsil-derived HK cells, which are a subtype of germinal center FDCs. We detected protein radical formation and tryptophan oxidation in FDCs in the septic spleen at 24 h (Fig. 1A and 2). LPS also stimulated the generation of protein and DNA radicals in HK cells (Fig. 1B, Fig. 2F, Fig. 3).
Sources of cellular superoxide and hydrogen peroxide can often be traced to either NADPH oxidase or xanthine oxidase (14,40), and we therefore assessed the role of these enzymes on radical formation. Spleens from LPS-treated NADPH oxidase knockout mice (both gp91 phox and p47 phox) accumulated DMPO radicals equal to those found in wild-type animals. Further administration of apocynin, an inhibitor of NADPH oxidase, did not affect the DMPO-nitrone adduct formation, suggesting that NADPH oxidase, did not contribute to radical formation in these cells (Figs. 4 and 5E). Xanthine oxidase, however, does play a role. Not only are amounts of xanthine oxidase increased (Fig. 5C and D), but co-treatments of LPS-treated mice and HK cells with the inhibitor allopurinol reduced accumulation of both DNA and protein radical adducts (Figs. 3, 4 and 5).
Transition metals are also recognized as important in the generation of H2O2 and as catalysts for free radical reactions (41,42). To study the role of iron-mediated production of hydroxyl radicals we used the iron-chelator desferrioxamine. In general, desferrioxamine reduction of radical adduct accumulation was similar to that of allopurinol inhibition. ELISA analysis and confocal microscopy show a reduction of both DNA and protein radical formation in HK cells and protein radical formation in FDCs from LPS-treated mice pretreated with desferrioxamine (Figs. 3, 4B and 5B), signifying a contributing role for iron-mediated radical production in our sepsis model.
Reactive oxygen species are known inducers of both apoptosis and necrosis under inflammatory conditions (29). Many agents that trigger apoptotic modes of cell death are stimulators of cellular oxidative metabolism, and thus ROS have been proposed as common mediators of cell death (7). Earlier studies have indicated that there is a significant increase in immunoreactivity of active caspase-3 in the germinal center FDCs, suggesting apoptosis as a major cause of their depletion in sepsis (3). Interestingly, our results with caspase-3 activity were time-dependent (Fig.6), showing an increase at 24 h and a marked decrease at 48 h in both cell types. Caspase-3 activity was restored to untreated levels by allopurinol and desferrioxamine in cells derived from the septic spleen and by desferrioxamine in HK cells (Fig. 6). The failure of allopurinol to restore caspase-3 activity in HK cells at 48 h might indicate an as yet unknown mechanism of ROS generation that is iron-dependent in a transformed cell line such as HK.
The observed caspase-3 activity was correlated with apoptotic features as seen by Annexin V staining in FDCs from septic spleens and by numbers of TdT-fluorescein positive nuclei of HK cells at 24 h (Fig.7), suggesting a time- and ROS-dependent modulation of cell death in septic FDCs. Various modes of caspase-3-independent cell death are known (43). It has been proposed that the necrosis-inducing effects are due to the sensitivity of the caspases to oxidative inactivation (44). In some cell types, TNF-α, a pleiotropic cytokine, elicits necrotic cell death either spontaneously or when caspases are blocked by inhibitors. Recent evidence of secondary necrosis of immune cells during inflammation has been ascribed to failure of phagocytosis (45).
Interestingly, our results show high numbers of tingible body macrophages (TIBMs) in the germinal center of septic mice at 24 h which decreased significantly over time (Fig. 2G). A higher magnification confocal image of one such TIBM showed that cell debris from FDCs had internal protein radical adducts at 24 h. This confirms that initially, following LPS treatment, there was apoptosis of FDCs. The result assumes significance since this is the first evidence of protein free radical adducts derived from a different cell in a TIBM which it essentially endocytosed (Fig. 2C). These events also confirm the role of TIBMs as major scavengers of FDCs in the germinal center. The decrease in the number of TIBMs in subsequent time periods also correlates well with a possible failure of the efficient removal of apoptotic cells, leading to alternate cell death. Our results thus suggest that sepsis along with a concomitant release of TNF-α and ROS might regulate the process of cell death from an initial apoptotic mode to a secondary necrotic mode at later time points. Thus, future studies are needed in order to establish a more direct role of caspase-3 inactivation by ROS generation and subsequent protein and DNA radical formation or downstream products of oxidative stress, e.g., hydroperoxides and aldehydes in modulating cell death patterns in the germinal center microenvironment.
Our studies in both FDCs isolated from the septic spleen and HK cells indicated clear evidence of apoptotic mechanisms at 24 h and necrosis post-LPS administration. It seems reasonable, therefore, to suggest that increased ROS generation coupled with protein and DNA radical adduct formation leads to rapid depletion of FDCs from the septic spleen. In summary, we report a novel mechanism of modulation of cell death patterns of FDCs in sepsis. Based on our evidence from this study, we propose a mechanism (Scheme 1, Fig. 9) whereby association of xanthine oxidase-derived superoxide anion radicals and hydrogen peroxide leads to the formation of protein and DNA radicals, causing modulation of caspase-3 activity, cell death patterns and decreased plasma cell numbers. The decreased plasma cell numbers might indicate that there is decreased differentiation of B cells into CD138+ve plasma cells (Fig.8). This might be one of the principal factors that contribute to the immunoparalysis seen in sepsis.
Fig. 9.
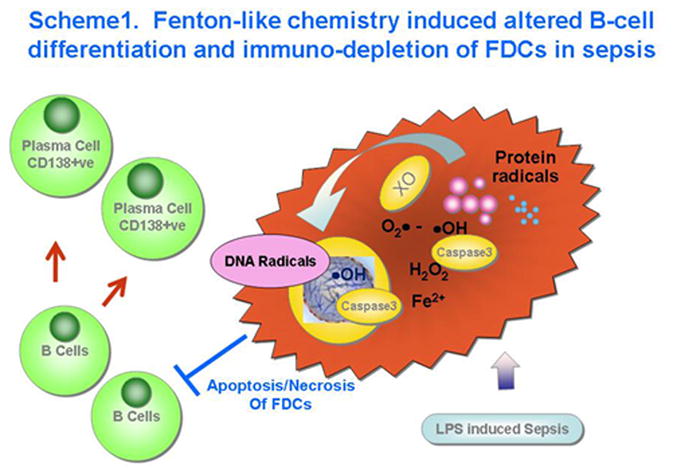
Scheme showing the proposed mechanism of oxidative stress-induced modulation of FDC depletion and altered B cell differentiation in the germinal center of spleen in late sepsis.
Supplementary Material
Time response of formation of NFK adducts in the germinal center of LPS-treated spleen. Sham-treated mice and LPS treated mice were sacrificed at 24, 36 and 48 h post LPS administration. Frozen spleen sections were probed for immunoreactivity to anti-NFK (blue) and anti-FDCM1 (red) antibodies in the germinal center. Co-localization of NFK adducts in FDCM1+ve cells can be seen in shades of purple (right lower quadrant of each time point).
Human tonsil-derived HK cells were probed for lysosomal accumulation of DMPO nitrone adducts following LPS and TNF-α treatment. Fixed cells in plastic glass bottomed dishes were probed for immunoreactivity to anti-DMPO (green) and anti-LAMP-2 (red) antibodies. Co-localization of DMPO-nitrone adducts in lysosomes (red) can be seen in shades of yellow (depicted with arrows in the right lower quadrant of each time point).
Acknowledgments
Grant Support: This work has been supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences (Z01 ES050139-13)
The authors sincerely acknowledge Tiwanda Marsh, Jeoffrey Hurlburt and Holly Rutledge for excellent technical assistance. We also thank Dr. Carl Bortner for help in analyzing flow cytometry data and Dr. Shyamal Peddada of Statistics Branch, NIEHS, for generous help in statistical analysis. We also sincerely thank Dr. Ann Motten and Mary Mason for help in the careful editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotchkiss RS, Coppersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 3.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 4.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:2367–2368. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H, Gibert C, Chastre J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Choi YS. Follicular dendritic cell-signaling molecules required for proliferation and differentiation of GC-B cells. Semin Immunol. 2002;14:259–266. doi: 10.1016/s1044-5323(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 7.Haddad JJ. Redox and oxidant-mediated regulation of apoptosis signaling pathways: immuno-pharmaco-redox conception of oxidative siege versus cell death commitment. Int Immunopharmacol. 2004;4:475–493. doi: 10.1016/j.intimp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity. Oxidant injury without apoptosis. J Biol Chem. 1996;271:15182–15186. doi: 10.1074/jbc.271.25.15182. [DOI] [PubMed] [Google Scholar]
- 9.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 10.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 11.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med. 2004;36:1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 12.Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de León A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadler K, Bonini MG, Dallas S, Duma D, Mason RP, Kadiiska MB. Direct evidence of iNOS-mediated in vivo free radical production and protein oxidation in acetone-induced ketosis. Am J Physiol Endocrinol Metab. 2008;295:E456–E462. doi: 10.1152/ajpendo.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee S, Ehrenshaft M, Bhattacharjee S, Deterding LG, Bonini MG, Corbett J, Kadiiska MB, Tomer KB, Mason RP. Immuno-spin trapping of a post-translational carboxypeptidase B1 radical formed by a dual role of xanthine oxidase and endothelial nitric oxide synthase in acute septic mice. Free Radic Biol Med. 2009;46:454–461. doi: 10.1016/j.freeradbiomed.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez DC, Gomez Mejiba SE, Mason RP. Immuno-spin trapping of DNA radicals. Nat Methods. 2006;3:123–127. doi: 10.1038/nmeth852. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S, Premachandran S, Shukla J, Poduval TB. Synergistic therapeutic potential of dexamethasone and L-arginine in lipopolysaccharide-induced septic shock. J Surg Res. 2007;140:99–108. doi: 10.1016/j.jss.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee S, Lardinois O, Bonini MG, Bhattacharjee S, Stadler K, Corbett J, Deterding LJ, Tomer KB, Kadiiska MB, Mason RP. Site-specific carboxypeptidase B1 tyrosine nitration and pathophysiological implications following its physical association with nitric oxide synthase-3 in experimental sepsis. J Immunol. 2009;183:4055–4066. doi: 10.4049/jimmunol.0900593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dikalova AE, Kadiiska MB, Mason RP. An in vivo ESR spin-trapping study: free radical generation in rats from formate intoxication--role of the Fenton reaction. Proc Natl Acad Sci USA. 2001;98:13549–13553. doi: 10.1073/pnas.251091098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura H, Liu S, Yamada S, Uchida K, Matsumoto K, Mukaida M, Yoshida K. Rapid increase in serum lipid peroxide 4-hydroxynonenal (HNE) through monocyte NADPH oxidase in early endo-toxemia. Free Radic Res. 2005;39:845–851. doi: 10.1080/10715760500161546. [DOI] [PubMed] [Google Scholar]
- 20.Liu YJ, Xu J, de Bouteiller O, Parham CL, Grouard G, Djossou O, de Saint-Vis B, Lebecque S, Banchereau J, Moore KW. Follicular dendritic cells specifically express the long CR2/CD21 isoform. J Exp Med. 1997;185:165–170. doi: 10.1084/jem.185.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukumar S, Szakal AK, Tew JG. Isolation of functionally active murine follicular dendritic cells. J Immunol Methods. 2006;313:81–95. doi: 10.1016/j.jim.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994;153:2951–2961. [PubMed] [Google Scholar]
- 23.El Shikh ME, El Sayed RM, Wu Y, Szakal AK, Tew JG. TLR4 on follicular dendritic cells: an activation pathway that promotes accessory activity. J Immunol. 2007;179:4444–4450. doi: 10.4049/jimmunol.179.7.4444. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez DC, Gomez-Mejiba SE, Mason RP. Immuno-spin trapping analyses of DNA radicals. Nat Protoc. 2007;2:512–522. doi: 10.1038/nprot.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrades ME, Ritter C, Dal-Pizzol F. The role of free radicals in sepsis development. Front Biosci (Elite Ed) 2009;1:277–287. doi: 10.2741/E27. [DOI] [PubMed] [Google Scholar]
- 26.Bayir H, Kagan VE. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis--there is nothing more practical than a good theory. Crit Care. 2008;12:206. doi: 10.1186/cc6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victor VM, Rocha M, Esplugues JV, De la Fuente M. Role of free radicals in sepsis: antioxidant therapy. CurrPharm Des. 2005;11:3141–3158. doi: 10.2174/1381612054864894. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D, Cuzzocrea S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med. 2002;33:1173–1185. doi: 10.1016/s0891-5849(02)00961-9. [DOI] [PubMed] [Google Scholar]
- 29.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphates. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Andrekopoulos C, Joseph J, Crow J, Kalyanaraman B. The carbonate radical anion-induced covalent aggregation of human copper, zinc superoxide dismutase, and alpha-synuclein: intermediacy of tryptophan- and tyrosine-derived oxidation products. Free Radic Biol Med. 2004;36:1355–1365. doi: 10.1016/j.freeradbiomed.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Ehrenshaft M, Silva SO, Perdivara I, Bilski P, Sik RH, Chignell CF, Tomer KB, Mason RP. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic Biol Med. 2009;46:1260–1266. doi: 10.1016/j.freeradbiomed.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SM, Kim S, Choi JS, Hur DY, Lee WJ, Choe J, Lee TH. TGF-β inhibits Fas-mediated apoptosis of a follicular dendritic cell line by down-regulating the expression of Fas and caspase-8: counteracting role of TGF-β on TNF sensitization of Fas-mediated apoptosis. J Immunol. 2005;174:6169–6175. doi: 10.4049/jimmunol.174.10.6169. [DOI] [PubMed] [Google Scholar]
- 34.Park CS, Choi YS. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology. 2005;114:2–10. doi: 10.1111/j.1365-2567.2004.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 36.Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, Hotchkiss RS, Clare-Salzler M, Moldawer LL. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J Immunol. 2004;173:3035–3043. doi: 10.4049/jimmunol.173.5.3035. [DOI] [PubMed] [Google Scholar]
- 37.Flohé SB, Agrawal H, Schmitz D, Gertz M, Flohé S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol. 2006;79:473–481. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 38.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Chapman MJ, Lesnik P. Enhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shock. J Immunol. 2008;180:6941–6946. doi: 10.4049/jimmunol.180.10.6941. [DOI] [PubMed] [Google Scholar]
- 39.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 40.Nakai K, Kadiiska MB, Jiang JJ, Stadler K, Mason RP. Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin. Proc Natl Acad Sci USA. 2006;103:4616–4621. doi: 10.1073/pnas.0510352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aust SD, Morehouse LA, Thomas CE. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1:3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- 42.Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987;1:358–364. [PubMed] [Google Scholar]
- 43.Vandenabeele P, Vanden Berghe T, Festjens N. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;2006:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
- 44.Hampton MB, Morgan PE, Davies MJ. Inactivation of cellular caspases by peptide- derived tryptophan and tyrosine peroxides. FEBS Lett. 2002;527:289–292. doi: 10.1016/s0014-5793(02)03240-4. [DOI] [PubMed] [Google Scholar]
- 45.Rydell-Törmänen K, Uller L, Erjefält JS. Direct evidence of secondary necrosis of neutrophils during intense lung inflammation. Eur Respir J. 2006;28:268–274. doi: 10.1183/09031936.06.00126905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time response of formation of NFK adducts in the germinal center of LPS-treated spleen. Sham-treated mice and LPS treated mice were sacrificed at 24, 36 and 48 h post LPS administration. Frozen spleen sections were probed for immunoreactivity to anti-NFK (blue) and anti-FDCM1 (red) antibodies in the germinal center. Co-localization of NFK adducts in FDCM1+ve cells can be seen in shades of purple (right lower quadrant of each time point).
Human tonsil-derived HK cells were probed for lysosomal accumulation of DMPO nitrone adducts following LPS and TNF-α treatment. Fixed cells in plastic glass bottomed dishes were probed for immunoreactivity to anti-DMPO (green) and anti-LAMP-2 (red) antibodies. Co-localization of DMPO-nitrone adducts in lysosomes (red) can be seen in shades of yellow (depicted with arrows in the right lower quadrant of each time point).