Summary
Background
There is ongoing debate regarding the initiation of psoriatic plaque as primarily arising from an anomaly in epidermal keratinocytes (KCs) or from abnormalities in immunocytes that secondarily activate otherwise normal KCs. In mice engineered to overexpress the angiopoietin receptor Tie2 in KCs, skin spontaneously develops the characteristic clinical, histological and immune cell phenotypes of psoriasis which can be reversed with either transgene repression or cyclosporin A administration, suggesting key roles for both KCs and T cells in mediating the skin disease in this murine model.
Objectives
To determine if antigen presenting cells and macrophages alone are sufficient to sustain psoriasiform inflammation in the KC-Tie2 murine model of psoriasis.
Methods
Clodronate liposomes were intradermally injected into involved dorsal skin of KC-Tie2 or control animals once a week for 6 weeks and acanthosis, angiogenesis, immune cell infiltration and cytokine production was quantitated using immunohistochemistry and interactive image analyses, enzyme linked immunosorbant assay (ELISAs) and quantitative real-time polymerase chain reaction (qRT-PCR).
Results
Clodronate liposome injection eliminated CD11c+, F4/80+, and CD11b+ cells in the skin and returned CD8+ T cell numbers to control mouse levels. Antigen presenting cell depletion in KC-Tie2 mouse skin resulted in resolution of the acanthotic skin phenotype, decreased dermal angiogenesis, and a return to control mouse levels for IL-1α, IL-6, IL-23 and TNFα expression and modest reductions in IFNγ and IL-17.
Conclusions
These findings suggest a critical role for APCs and myeloid cell derived IL-23 and TNFα and underscore the importance of Th1 and Th17 T cells in maintaining the psoriasiform skin phenotype in the KC-Tie2 mouse model.
Keywords: Clodronate, keratinocyte, macrophage, dendritic cell, cytokine, psoriasis
What's already known about this topic?
macrophage depletion in two different mouse models of psoriasis has previously been shown to improve the skin phenotype; however the mechanisms underlying this improvement remain unclear.
one group ruled out interferon gamma (IFNγ) as a potential mediator, but failed to identify the molecular mechanisms mediating their findings
the other group identified macrophage-derived Tumor Necrosis Factor alpha (TNFα) as a key player but did not examine other mechanisms.
What does this study add?
demonstrates that clodronate liposome injection results in a specific loss of CD11b, CD11c and F4/80+ cells (phagocytic dendritic cells and macrophages) in the KC-Tie2 psoriasis murine model
Confirms that a decrease in TNFα can reverse the phenotype but also demonstrates a key role for IL-23 but not IL-12 in maintaining psoriasiform dermatitis.
demonstrates that APC depletion leads to a return of CD8+ T cells to normal levels concomitant with decreased IFNγ and IL-17, highlighting the importance of lymphocytes as well as APCs in this murine model
further validates the KC-Tie2 mouse as a representative and useful mouse model of psoriasis.
Introduction
Psoriasis is dependent upon a series of coordinated cytokine signalling events between KCs, endothelial cells, cutaneous nerves, T cells, antigen presenting cells (APCs) including macrophages and DCs that once started, initiates a vicious pro-inflammatory cycle 1,2. However, a strict mitigating signal or a single antigenic target for psoriasis has yet to be identified. Rather, in patients with a genetic background predisposed to psoriasis, some unknown stimulus, perhaps antigenic in nature, such as an infection, initiates production of pro-inflammatory cytokines such as IL-12, IL-23 and TNFα by resident dendritic cells (DCs) and macrophages. IL-12 production in turn activates Th1 T cells that produce IFNγ. Together with IL-23, IL-6 and TGFβ these elevated cytokines lead to Th17 cell development, a subset of CD4+ and CD8+ T cells that produce IL-17, IL-21 and IL-22 3. IL-21 expression can then lead to the differentiation of more Th17 cells while IL-22 plays a role in KC proliferation and epidermal hyperplasia. IL-23 and IL-22 can also activate Stat3 phosphorylation within KCs, exacerbating pro-inflammatory cytokine production and subsequent KC proliferation 4-7. Once initiated, this cycle perpetuates sustained inflammatory responses in the dermal and epidermal tissue of psoriasis patients. Intervention at several points in this cycle results in clinical resolution, however durable remission and/or permanent clearance has not been achievable.
In human psoriasis 8-11 and in mouse models of psoriasis 12-21, macrophages are consistently found in involved areas of skin, even in the absence of T cells 20. These findings suggest that recruitment and activation of macrophages in the skin may represent a key pathogenic event in the development and maintenance of psoriatic skin. Recent work by others 19,20 has provided further preclinical evidence in mouse models to support a role for macrophages and macrophage-derived TNFα 19 in the development and maintenance of psoriasiform disease. Biological therapeutics currently being used as clinical treatments target macrophage-derived cytokines, including TNFα and IL12/23p40 22-26. We have previously described a psoriasis mouse model in which Tie2 is overexpressed in KCs, resulting in a psoriasiform skin phenotype with an abundance of infiltrating macrophages, DCs and T cells, and increases in many pro-inflammatory cytokines including TNFα, IFNγ, IL-1, IL12, IL-23, and IL-17. This model also demonstrated resolution of the phenotype following CsA treatment, consistent with a role for T cells in disease maintenance27. We now report that clodronate liposome depletion of F4/80+ macrophages, CD11b+ myeloid cells, and CD11c+ dendritic cells from the skin of KC-Tie2 animals resulted in resolution of acanthosis, a return of CD8+ T cells to control mouse levels, significant reductions in TNFα, IL-23, IL-1α, IL-6 and modest decreases in IL-17 and IFNγ. Moreover, systemic inhibition of TNFα signalling also led to the reversal of the psoriasiform skin phenotype. These findings suggest a critical role for myeloid cells and myeloid cell derived TNFα and IL-23 in maintaining psoriasis and further underscore the importance of lymphocytes, IFNγ and IL-17 in disease pathogenesis.
Materials and Methods
Animals, macrophage depletion and TNFα inhibition experiments
The KC-specific (K5-tTA) driver line and the TetosTek/Tie2 responder line have been described previously 27-29. Matings were performed between the K5tTA line and the TetosTek/Tie2 line and offspring were genotyped by polymerase chain reaction (PCR) using DNA extracted from ear biopsies. DNA was prepared and PCR performed using primers as previously described 27. We have previously demonstrated that animals inheriting a single copy of each gene (KC-Tie2 mice) have ∼50-fold increase in Tie2 mRNA and spontaneously develop a psoriasisform skin phenotype 27. Littermates inheriting one or no transgenes do not express either transgene and therefore served as experimental controls.
Phagocytic cells were depleted in vivo by using dichloromethylene diphosphonate (clodronate) encapsulated in liposomes obtained through www.clodronateliposomes.org. Mechanistically, liposomes serve as an intracellular delivery vehicle for clodronate, and are readily engulfed by phagocytic cells, following which the liposomal phospholipid bilayer is disrupted via phospholipases resulting in the release of the clodronate into the cell. Following intracellular clodronate accumulation, the cell becomes irreversibly damaged and apoptosis occurs30. This approach for macrophage depletion has been used successfully before by others20,30-32. At least two weeks prior to beginning these experiments, pre-treatment punch biopsies were taken from individual KC-Tie2 mice and control littermates in order to obtain baseline measurements of acanthosis prior to treatment. Administration of clodronate- or PBS-encapsulated liposomes was accomplished by briefly exposing mice to isofluorane followed immediately by intradermal injection of 50μl of liposome into one of 4 injection locations on the dorsal surface of the mouse, for a total of 200μl per animal, consistent with approaches previously described 19. Animals were injected once a week for 6 weeks. Efficacy of clodronate liposome depletion was confirmed by staining frozen skin sections using antibodies specific for F4/80 (clone BM8; eBioscience, San Diego, CA), CD11c (Clone HL3; BD Biosciences, San Jose, CA), and CD11b (Clone M1/70, BD Biosciences). The efficacy of cutaneous cell depletion using clodronate-encapsulated liposomes has been comprehensively reported in the literature, including in skin of other mouse models of psoriasis 19,20,33.
TNFα inhibition was completed using a rat/mouse chimeric monoclonal IgG2a,κ antibody specific for murine TNFα (CNTO5048) generously provided by Dr. David Shealy (Centocor). Mice were injected with either TNFα antibody or IgG2a,κ at 5mg/kg IP, once a week for a four week period.
All animal protocols were approved by the Case Western Reserve University institutional animal care and use committee (IACUC) and conformed to the American Association for Accreditation of Laboratory Animal Care guidelines.
Tissue collection and histological and morphometric analyses
Adult mice were euthanized; their hair shaved and skin from the back where liposomes were injected and adjacent tissue was processed for either thin frozen or paraffin sectioning. For paraffin sectioning, skin was placed in 10% buffered formalin (Surgipath Medical Industries, Richmond, IL), overnight at 4°C prior to dehydration and embedding (Sakura Finetech, Torrance, CA). For frozen sectioning, skin was either embedded in Tissue Freezing Medium (TFM; Triangle Biomedical Sciences, Durham, NC), and then flash frozen in liquid nitrogen or fixed in the non-cross-linking fixative Histochoice (Amresco, Solon, OH) for 4 hours at 4°C and then transferred to 5% sucrose for 1 hour at 4°C and placed in 20% sucrose overnight at 4°C prior to embedding.
H&E staining was completed on 5μm thick paraffin sections using standard protocols. Immunohistochemistry was performed on TFM embedded frozen skin sectioned at 8μm using antibodies specific for CD4, CD8 (BD Biosciences, San Jose, CA), CD11b, CD11c, F4/80 and on histochoice-fixed MECA-32 stained tissues (MECA-32, Developmental Studies Hybridoma Bank, Iowa City, Iowa) or matched isotype controls. Antibodies and isotype controls were detected using either rabbit anti-rat IgG biotinylated or rabbit anti-hamster IgG biotinylated (Southern Biotech, Birmingham, AL). Biotinylated antibodies were then amplified with Avidin/Biotinylated Enzyme Complex (ABC, Vector) and then visualized using the enzyme substrate DAB (Vector). Slides were counterstained with hematoxylin. Epidermal thickness and blood vessel analyses, and immune cell counts were quantified using approaches similar to those described previously 27.
RNA and protein isolation and analysis
Dorsal skin from regions in which liposomes were injected and adjacent regions was homogenized using a Mikro-Dismembrator S (Sartorius Stedim Biotech, Edgewood, NY) and RNA and protein isolated and quantified as described previously 27. IFNγ, IL17, IL-12p70 (R&D Systems, Minneapolis, MN), IL12p40 (BD Biosciences) and IL-23p19/40 (eBiosciences, San Diego, CA) expression was quantitated by ELISA according to the individual manufacturer's instructions.
RNA was reversed transcribed using MMLV reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Quantitative real-time RT-PCR was performed using Taqman technology from Applied Biosystems on an ABI Prism 7700 Sequence Detector. Probes and primers for IL-1α, IL-6, TNFα, S100A8/9 and 18S were obtained from Applied Biosystems. Expression levels were normalized to 18S.
Statistical analysis
All data are represented as mean ± standard error of the mean (SEM). Between group comparisons were analyzed using either a Student's T test or a Mann Whitney U test and statistical significance was defined as p<0.05.
Results
Injection of clodronate liposomes eliminates CD11c+, F4/80+ and CD11b+ cells from skin
In an effort to identify the contribution of phagocytic antigen presenting cells (APCs) to the maintenance of the psoriasisform skin phenotype, KC-Tie2 transgenic mouse skin was intradermally injected with either PBS or clodronate encapsulated liposomes once a week for a 6 week period. Efficacy of the liposome-mediated depletion was confirmed using skin tissues stained and analysed for the presence of antigen presenting cells (APCs) CD11c+, F4/80+ and CD11b+ cells (Fig. 1a-i). KC-Tie2 mice treated with PBS encapsulated liposomes had significantly more myeloid derived cells than control mice treated with clodronate encapsulated liposomes (n=5 KC-Tie2 + PBS liposomes; n=9 controls + Clodronate liposomes; p≤0.01; Fig. 1j), consistent with previous work 27. KC-Tie2 animals receiving clodronate liposomes (n=12) had significantly reduced levels of not only CD11b+ and F4/80+ macrophages, but also CD11c+ cells compared to KC-Tie2 mice treated with PBS liposomes (p≤0.01; Fig. 1j) and these levels did not differ from control animals. No differences in cutaneous CD11c+ or F4/80+ cell numbers were observed between untreated KC-Tie2 mice and KC-Tie2 mice treated with PBS encapsulated liposomes or between untreated control mice and control mice treated with clodronate encapsulated liposomes (Supplemental Figure 1a).
Figure 1. Clodronate liposome administration results in antigen cell depletion.
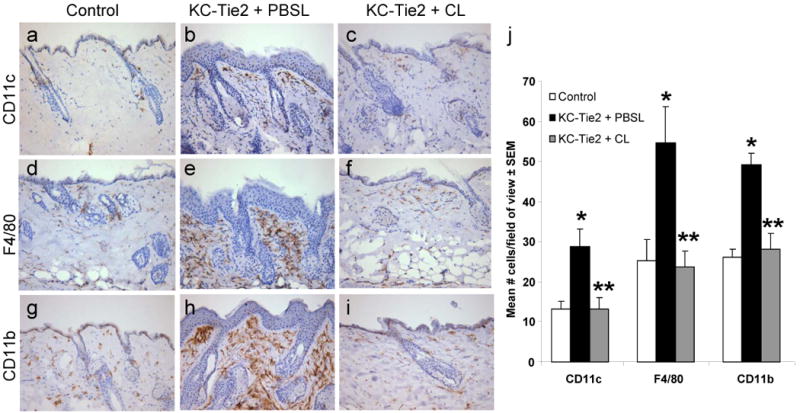
Immunohistochemical staining detecting CD11c+ (a-c) F4/80+ (d-f), and CD11b+ (g-i) cells in skin sections from KC-Tie2 or control animals treated with either clodronate or PBS filled liposomes for 6 weeks. Quantitation using interactive image analyses approaches (j) reveals increased numbers of antigen presenting cells in KC-Tie2 + PBS liposomes (PBSL) compared to control mice, and that these numbers return to control mouse levels following clodronate liposome (CL) administration. * p≤0.05 compared to controls; ** p≤0.05 compared to KC-Tie2 + PBSL.
Elimination of antigen presenting cells significantly improves acanthosis and dermal angiogenesis and returns CD8+ T cell numbers back to control levels
H&E histological analyses confirmed our previous report 27 of a ∼3 fold increase in epidermal thickness in KC-Tie2 animals treated with PBS liposomes compared with littermate controls treated with clodronate liposomes (Fig. 2a-b, e). This increase disappeared in KC-Tie2 mice treated with clodronate liposomes (Fig. 2c). Longitudinal comparison within the same KC-Tie2 mouse across the treatment period revealed a statistically significant decrease in epidermal thickness (paired student T-test, p=0.032; n=12; Fig. 2d) following clodronate liposome administration. Further analyses revealed that 7/12 animals treated with clodronate liposomes had a significant reduction in epidermal acanthosis; 3/12 treated animals failed to respond; and 2/12 did not exhibit elevated levels of acanthosis at the initiation of liposome treatment, consistent with previous findings that ∼10% of KC-Tie2 animals do not develop acanthosis 27 (Fig. 2d). Evaluation of acanthosis levels in KC-Tie2 responders (n=7) compared with KC-Tie2 mice treated with PBS liposomes (n=3) revealed a 40% decrease in acanthosis (p=0.0002) but levels remained elevated compared to control animals treated with clodronate encapsulated liposomes (p=0.023; n=9; Fig. 2a-c, e). No differences were observed between untreated KC-Tie2 mice and KC-Tie2 mice treated with PBS encapsulated liposomes or between untreated control mice and control mice treated with clodronate encapsulated liposomes (Supplemental Figure 1b).
Figure 2. Acanthosis improves in KC-Tie2 mice following clodronate liposome administration.
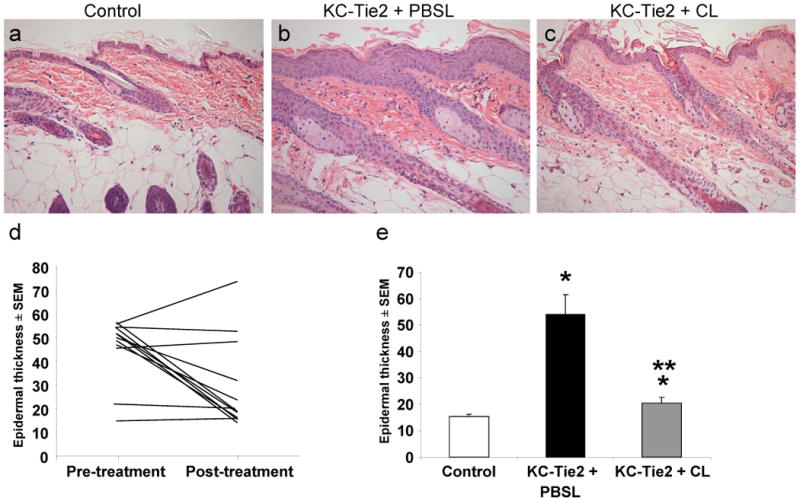
(a-c) H&E stained skin following either PBSL or CL liposome treatment. (d) Longitudinal measurement of back skin epidermal thickness in individual KC-Tie2 mouse skin before and after treatment with clodronate liposomes. (e) Average epidermal thickness measures following 6 weeks of treatment with either PBSL or CL demonstrates increased acanthosis in KC-Tie2 + PBSL skin compared to control skin; KC-Tie2 + CL epidermis is significantly less thick than KC-Tie2 + PBSL, but still thicker than controls. * p≤0.05 compared to controls; ** p≤0.05 compared to KC-Tie2 + PBSL.
Further analyses of the skin phenotype across the different treatment groups also revealed that KC-Tie2 animals treated with PBS liposomes (n=3) had more blood vessels compared to control mice treated with clodronate encapsulated liposomes (n=9; p=0.024) and that following exposure to clodronate liposomes and APC depletion, the number of dermal blood vessels (identified using with the pan endothelial cell marker, MECA), returned to control mouse levels indicative of reductions in angiogenesis (Fig. 3). No differences were observed between untreated KC-Tie2 mice and KC-Tie2 mice treated with PBS encapsulated liposomes or between untreated control mice and control mice treated with clodronate encapsulated liposomes (Supplemental Figure 1c).
Figure 3. Dermal angiogenesis returns to control levels in KC-Tie2 + CL animals.
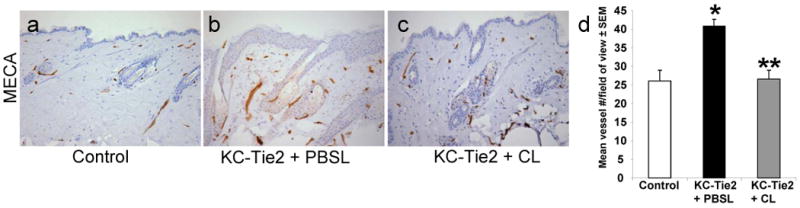
(a-c) Immunohistochemical staining of skin following PBSL or CL treatment using the pan endothelial cell marker, mouse endothelial cell antigen (MECA). (d) KC-Tie2 + CL mouse skin has significantly more MECA+ endothelial cells than control animals, and this number decreases to control mouse levels in KC-Tie2 + CL mice. * p≤0.05 compared to controls; ** p≤0.05 compared to KC-Tie2 + PBSL.
Examination of the other immune cell types in the tissues revealed significant increases in CD4+ and CD8+ T cells in KC-Tie2 animals treated with PBS-encapsulated liposomes (n=3) compared to control animals treated with clodronate encapsulated liposomes (n=9; p≤0.001). Significant decreases in CD4+ T cell numbers were observed in KC-Tie2 animals responsive to treatment with clodronate encapsulated liposomes (n=7; p=0.019), although these numbers remained significantly higher than control mice (p=0.041; Fig. 4a-c, g). In contrast, CD8+ T cell numbers returned to control mouse levels in KC-Tie2 mice treated with clodronate encapsulated liposomes (n=7; p=0.10 vs control mice; p=0.018 vs KC-Tie2 + Clodronate liposomes; Fig. 4d-g). As before, no differences were observed between untreated KC-Tie2 mice and KC-Tie2 mice treated with PBS encapsulated liposomes or between untreated control mice and control mice treated with clodronate encapsulated liposomes (Supplemental Figure 1d).
Figure 4. T cell numbers decrease significantly following clodronate liposome treatment.
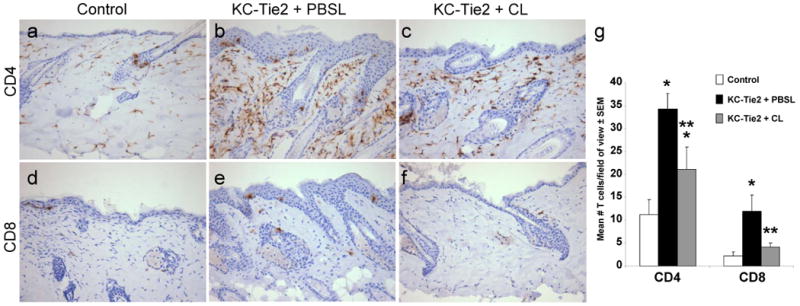
Immunohistochemical staining detecting CD4+ (a-c) and CD8+ (d-f) T cells in skin sections from KC-Tie2 or control animals treated with either clodronate or PBS filled liposomes. (g) Quantitative analyses demonstrates KC-Tie2 + PBSL skin has significantly more T cells than control animals, and that this number decreases significantly in KC-Tie2 + CL mouse skin. * p≤0.05 compared to controls** p≤0.05 compared to KC-Tie2 + PBSL.
Proinflammatory IL-1, IL-6, TNFα and IL-23 are significantly decreased following clodronate liposome administration
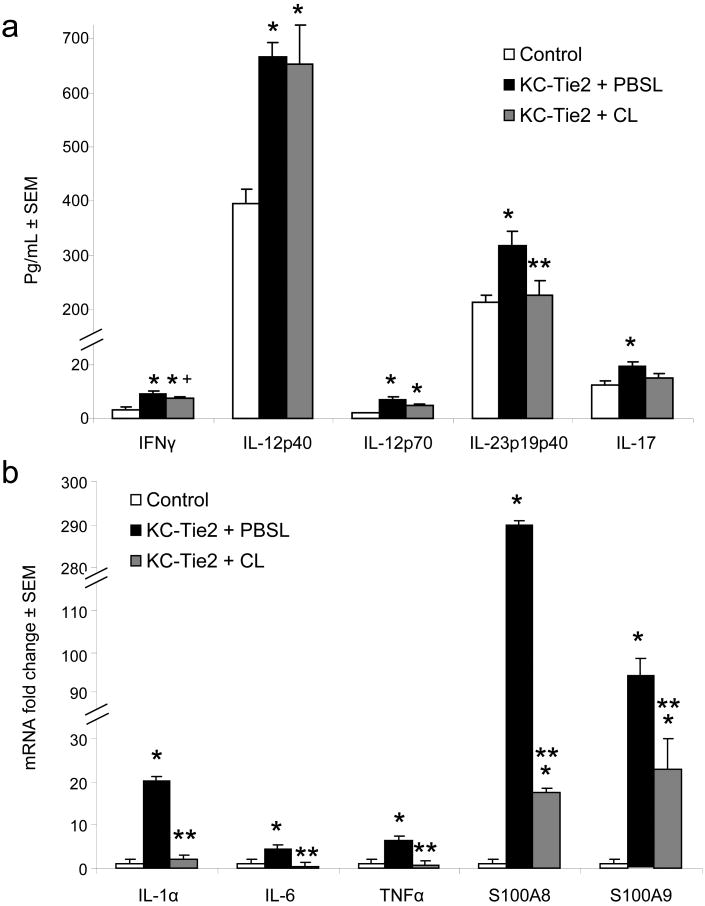
A large number of APC derived cytokines have been proposed to contribute to the pathogenesis of psoriasis 34,35. We used ELISA and qRT-PCR approaches to quantify changes in APC- and T cell-derived cytokines between clodronate and PBS liposome treated KC-Tie2 skin. Significant increases in APC, Th1 and Th17 T cell-derived cytokines were confirmed between control animals (n=5) and KC-Tie2 mice treated with PBS liposomes (n=3), including increases in IFNγ, IL-12, IL-23 and IL-17 protein (Fig. 5a), and IL-1α, IL-6, TNFα and S100A8/A9 mRNA (Fig. 5b). KC-Tie2 mice treated with clodronate liposomes (n=5) had significant decreases in cutaneous IL-23 protein (p=0.036) and modest reductions in IFNγ expression (p=0.08; Fig. 5a) compared to KC-Tie2 animals treated with PBS encapsulated liposomes. IL-17 expression also decreased to levels between control animals and KC-Tie2 mice treated with PBS encapsulated liposomes (Fig. 5a). At the mRNA level, IL-1α, IL-6 and TNFα expression returned to control mouse levels (p<0.05 vs KC-Tie2 + Clodronate liposomes); S100A8/S100A9 were significantly decreased, however levels remained elevated compared to control mice (p<0.05).
Figure 5. Protein and RNA expression analysis of cytokines and innate defence molecules derived from antigen presenting cells (APC) and T cells.
ELISA analyses (a) of T cell derived cytokines IFNγ, IL-17 and APC derived cytokines IL12 and IL23 demonstrates significant decreases in IL-23 and modest decreases in IFNγ and IL-17 expression following clodronate liposome exposure. qRT-PCR analyses (b) of pro-inflammatory cytokines IL-1α, IL-6 and TNFα and the calcium binding proteins S100A8 and S100A9 demonstrates significant decreases in expression of each molecule following clodronate liposome exposure. * p≤0.05 compared to controls; ** p≤0.05 compared to KC-Tie2 + PBSL; + p=0.08 compared to KC-Tie2 + PBSL.
Functional inhibition of TNFα eliminates cutaneous inflammatory cell infiltrate and improves acanthosis
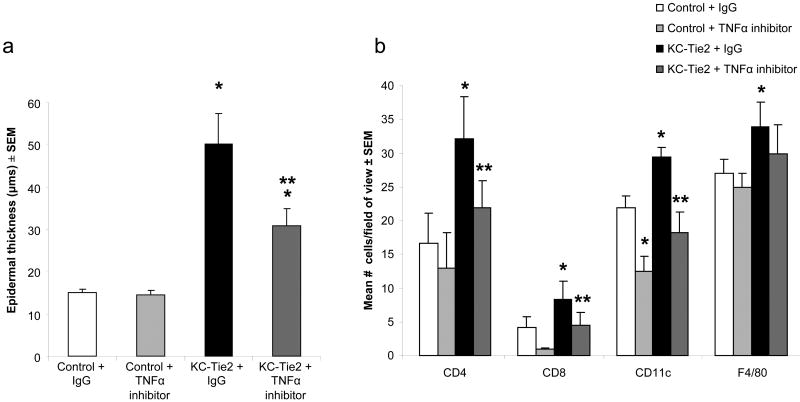
To further validate the contribution and significance of TNFα in the KC-Tie2 psoriasiform murine model, KC-Tie2 transgenic mice were injected IP with TNFα blocking antibodies once a week for a 4 week period. As before, KC-Tie2 mice administered IgG (n=6) alone showed a ∼3-fold increase in epidermal thickness (p=0.0007 vs Control + IgG) and significant increases in inflammatory infiltrate in the skin including increases in CD4+ (p=0.0025 vs Control + IgG), CD8+ (p=0.013 vs Control + IgG), CD11c+ (p=0.0093 vs Control + IgG) and F4/80+ (p= 0.05 vs Control + IgG) cells compared to control mice treated with either IgG or TNFα antibodies (n=6 each; Fig. 6). TNFα inhibition (n=5) lead to a significant reduction in acanthosis (p=0.05), however the thickness of the epidermis remained significantly thicker than control mice (p=0.0024, Fig.6a); whether control mouse levels would be obtained with a longer treatment approach is likely, but remains unknown. In contrast, targeting TNFα in KC-Tie2 mice lead to a return of T cell and APC inflammatory infiltrate back to control mouse levels (Fig. 6b).
Figure 6. Systemic administration of antibodies targeting murine TNFα leads to significant improvement in the psoriasiform skin phenotype in KC-Tie2 mice.
Average epidermal thickness measures performed on H&E stained sections (a) following 4 weeks of treatment with either TNFα antibodies or IgG control demonstrates increased acanthosis in KC-Tie2 + IgG skin compared to control skin treated with either IgG or TNFα antibodies. Epidermal thickness in KC-Tie2 mice treated with TNFα antibodies is significantly decreased compared to KC-Tie2 + IgG animals, but remains thicker than control animals. TNFα antibody treatment results in a return of T cell and APC numbers back to control mouse levels. * p≤0.05 compared to control + IgG; ** p≤0.05 compared to KC-Tie2 + IgG.
Discussion
We have previously reported the responsiveness of the KC-Tie2 mouse to T cell targeted therapies, such as CsA 27 but also identified a large population of infiltrating macrophages in psoriatic lesions on the skin of the mouse. Here we report that administration of clodronate liposomes into the skin of KC-Tie2 animals results in the elimination of F4/80+ macrophages, CD11b+ myeloid cells, and CD11c+ dendritic cells. Elimination of these APCs occurred concomitant with the resolution of acanthosis, decreases in T cell numbers and significant decreases in TNFα, IL-23, IL-1α, IL-6 and S1008/9 and modest reductions in IFNγ and IL-17. We further demonstrated that systemic TNFα inhibition also resulted in resolution of the skin disease phenotype. These findings suggest a critical role for myeloid cells and myeloid cell derived cytokines in maintaining psoriasis and continues to highlight the importance of lymphocytes in disease pathogenesis. Reversal of pathogenesis by TNFα inhibition also highlights the importance of TNFα in the KC-Tie2 murine model of psoriasis.
To date, there are only two other reports of similar findings albeit with identifiable areas of divergence following macrophage depletion in other murine models of psoriasis 19,20. Wang et al, utilizing a T cell dependent mouse model (CD18hypo on a PL/J background), demonstrated in adult mice an improvement in gross external phenotype following clodronate administration, macrophage depletion and reduced expression of TNFα, however moderate levels of acanthosis were still apparent after treating animals for 6 weeks, as were sustained levels of T cells, neutrophils, mast cells and mature Langerhans cells 19. Pasparakis et al, used a KC-specific inhibitor of NFκB (IκB) kinase 2 (IKK2) mouse model which they previously had shown developed a psoriasiform like skin disease that was T cell independent and began treating postnatal day (P)4 neonates with either clodronate or PBS liposomes for 3 days 36. This resulted in a significant improvement in acanthosis by normalizing epidermal differentiation, depleting macrophages in addition to decreasing the number of skin infiltrating CD3+ T cells and GR-1+ granulocytes. This group ruled out significant contributions of granulocytes as well as IFNγ as critical for the sustainment of the phenotype in their mouse model 20. Both groups observed elevated levels of phagocytic cells in their murine models that exhibited skin lesions and determined that depletion of these cells lead to a significant improvement in various aspects of the skin disease; and in the case of the CD18hypo model, reduced levels of TNFα.
Our current findings support the importance of myeloid-derived phagocytic cells and their derived cytokines, including TNFα and IL-23, as key molecules in disease maintenance in the KC-Tie2 murine model of psoriasis. These results are consistent with the current efficacy and usage of both TNFα inhibitors, Infliximab (Remicade)37-39, Etanercept (Enbrel)23,40,41, Adalimumab(Humira) 42 and most recently the usage of an IL-12/23p40 inhibitor, ustekinumab (Stelara) in human psoriasis 25,26,43. Although significant changes in IL-23 were observed, we did not detect changes in IL-12p70 or IL12/23p40; which suggests that the p19 subunit of IL-23 may be the critical component to sustaining the inflammation within the skin. This confirms a prior finding from a clinical trial showing that following 2 weeks of ustekinumab treatment a significant decrease in IL-23p19 and IL12/23p40 mRNA concomitant with an increase in IL12p35 25 was observed. Our observations of a return to control mouse levels for CD8+ T cells and modest reductions in both IL-17, IFNγ and IL-6, despite an ongoing presence of CD4+ T cells, further underscores the role of lymphocytes in this murine model and suggests that targeting APCs decreases the cytotoxic CD8+ T cell population and leads to sufficient decreases in T cell-derived cytokines to further aid in the rebalancing of the skin to non-psoriatic phenotype. These results also reinforce the idea that treatment strategies focused on the elimination of inhibition of APCs may be efficacious in clinical psoriasis as this approach circumvents a specific T cell inhibition and still results in a decrease of T cell derived inflammatory cytokines. Our previous report in which we demonstrate improved skin response following CsA administration 44 resulted in a subset of outbred mice that failed to respond, much like what has been reported in the clinical environment. Here again, we report three animals of 12 that also failed to respond to APC depletion targeted therapy. Whether these animals would respond following the addition of CsA in a combination therapy approach has not been tested, but if successful would support the idea of specific subsets of animals having differential responses to targeted therapy.
These results confirm the importance of activated lymphocytes in psoriasis pathogenesis and provide further support for the critical role for APCs in disease progression. Myeloid cell derived IL-23 in addition to TNFα appears critical for maintaining the psoriasiform skin phenotype in the KC-Tie2 mouse model. The combined results suggest that the KC-Tie2 psoriasiform murine model is ideal as a preclinical tool for evaluating the efficacy of therapeutic strategies that directly target APCs in psoriasis.
Supplementary Material
Acknowledgments
This work was funded by grants to NLW and TSM from the National Institutes of Health (P30AR39750 and P50AR05508) and the Murdough Family Center for Psoriasis; and to NLW from the American Skin Association, the Dermatology Foundation and the National Psoriasis Foundation; JAW was supported by training grant T32 GM-08056-23.
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Bata-Csorgo Z, Hammerberg C, Voorhees JJ, et al. Intralesional T-lymphocyte activation as a mediator of psoriatic epidermal hyperplasia. J Invest Dermatol. 1995;105:89S–94S. doi: 10.1111/1523-1747.ep12316121. [DOI] [PubMed] [Google Scholar]
- 2.Strange P, Cooper KD, Hansen ER, et al. T-lymphocyte clones initiated from lesional psoriatic skin release growth factors that induce keratinocyte proliferation. J Invest Dermatol. 1993;101:695–700. doi: 10.1111/1523-1747.ep12371678. [DOI] [PubMed] [Google Scholar]
- 3.Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 4.Kryczek I, Bruce AT, Gudjonsson JE, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–41. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 6.Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol. 2008;128:1064–7. doi: 10.1038/jid.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461–7. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowes MA, Chamian F, Abello MV, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci U S A. 2005;102:19057–62. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu KJ, Cheng H, Mao XH, et al. Increased endocytic activity in monocyte-derived dendritic cells in patients with psoriasis vulgaris. Indian J Med Res. 2006;123:43–50. [PubMed] [Google Scholar]
- 10.Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–30. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Lee E, Lowes MA, et al. Prominent production of IL-20 by CD68+/CD11c+ myeloid-derived cells in psoriasis: Gene regulation and cellular effects. J Invest Dermatol. 2006;126:1590–9. doi: 10.1038/sj.jid.5700310. [DOI] [PubMed] [Google Scholar]
- 12.Xia YP, Li B, Hylton D, et al. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–8. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Turksen K, Yu QC, et al. Cachexia and graft-vs.-host-disease-type skin changes in keratin promoter-driven TNF alpha transgenic mice. Genes Dev. 1992;6:1444–56. doi: 10.1101/gad.6.8.1444. [DOI] [PubMed] [Google Scholar]
- 14.Cook PW, Pittelkow MR, Piepkorn M. Overexpression of amphiregulin in the epidermis of transgenic mice induces a psoriasis-like cutaneous phenotype. J Invest Dermatol. 1999;113:860. doi: 10.1046/j.1523-1747.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- 15.Carroll JM, Crompton T, Seery JP, et al. Transgenic mice expressing IFN-gamma in the epidermis have eczema, hair hypopigmentation, and hair loss. J Invest Dermatol. 1997;108:412–22. doi: 10.1111/1523-1747.ep12289702. [DOI] [PubMed] [Google Scholar]
- 16.Groves RW, Mizutani H, Kieffer JD, et al. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proc Natl Acad Sci U S A. 1995;92:11874–8. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groves RW, Rauschmayr T, Nakamura K, et al. Inflammatory and hyperproliferative skin disease in mice that express elevated levels of the IL-1 receptor (type I) on epidermal keratinocytes. Evidence that IL-1-inducible secondary cytokines produced by keratinocytes in vivo can cause skin disease. J Clin Invest. 1996;98:336–44. doi: 10.1172/JCI118797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zenz R, Eferl R, Kenner L, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–75. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Peters T, Kess D, et al. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J Clin Invest. 2006;116:2105–14. doi: 10.1172/JCI27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratis A, Pasparakis M, Rupec RA, et al. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J Clin Invest. 2006;116:2094–104. doi: 10.1172/JCI27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li AG, Wang D, Feng XH, et al. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004;23:1770–81. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb AB, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139:1627–32. doi: 10.1001/archderm.139.12.1627. discussion 32. [DOI] [PubMed] [Google Scholar]
- 23.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–22. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 24.Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–92. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 25.Toichi E, Torres G, McCormick TS, et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177:4917–26. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 26.Kauffman CL, Aria N, Toichi E, et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J Invest Dermatol. 2004;123:1037–44. doi: 10.1111/j.0022-202X.2004.23448.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolfram JA, Diaconu D, Hatala DA, et al. Keratinocyte but not endothelial cell specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–58. doi: 10.2353/ajpath.2009.080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones N, Voskas D, Master Z, et al. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2:438–45. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond I, Owolabi T, Marco M, et al. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–94. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 30.van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. Journal of Immunological Methods. 1996;193:93–9. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 31.Calin MV, Manduteanu I, Dragomir E, et al. Effect of depletion of monocytes/macrophages on early aortic valve lesion in experimental hyperlipidemia. Cell Tissue Res. 2009;336:237–48. doi: 10.1007/s00441-009-0765-2. [DOI] [PubMed] [Google Scholar]
- 32.Ylitalo R, Syvala H, Tuohimaa P, et al. Suppression of immunoreactive macrophages in atheromatous lesions of rabbits by clodronate. Pharmacol Toxicol. 2002;90:139–43. doi: 10.1034/j.1600-0773.2002.900305.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Peters T, Sindrilaru A, et al. Key role of macrophages in the pathogenesis of CD18 hypomorphic murine model of psoriasis. J Invest Dermatol. 2009;129:1100–14. doi: 10.1038/jid.2009.43. [DOI] [PubMed] [Google Scholar]
- 34.Nickoloff BJ, Xin H, Nestle FO, et al. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568–73. doi: 10.1016/j.clindermatol.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med. 2007;13:242–4. doi: 10.1038/nm0307-242. [DOI] [PubMed] [Google Scholar]
- 36.Pasparakis M, Courtois G, Hafner M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–6. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 37.Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150–7. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–36. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–42. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis & Rheumatism. 2004;50:2264–72. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 41.Carlin CS, Feldman SR, Krueger JG, et al. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol. 2004;50:859–66. doi: 10.1016/j.jaad.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Beuthien W, Mellinghoff HU, von Kempis J. Skin reaction to adalimumab. Arthritis & Rheumatism. 2004;50:1690–2. doi: 10.1002/art.20155. [DOI] [PubMed] [Google Scholar]
- 43.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) The Lancet. 2008;371:1675–84. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 44.Wolfram J, Diaconu D, Tessier P, et al. Antigen presenting cell depletion in the KC-Tie2 mouse model of psoriasis attenuates acanthosis, angiogenesis and leads to decreases in IL-23 and S100A8/9 but not IL-12. Journal of Investigative Dermatology. 2009;129:S122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.