Abstract
Nicotine has been demonstrated to enhance the subsequent use of illicit drugs in animals and humans. We previously demonstrated in female, Holtzman rats that one low dose of nicotine will potentiate locomotor activity and dopamine (DA) efflux in response to a subsequent low dose of d-amphetamine (AMPH) given 1-4 hours later. In the present study, we show this also occurs in male rats and characterize the receptors required for the rapid sensitizing effect of nicotine on AMPH-stimulated locomotor behavior and AMPH-induced DA efflux. Pretreatment of male, Holtzman rats with a low dose (0.1 mg/kg, i.p.) of nicotine 2-4 hours before a challenge with AMPH (0.32 mg/kg, i.p.) enhanced locomotor behavior as compared to saline pretreatment. Dihydro-β-erythroidine (DHβE), a relatively selective antagonist at β2 subunit-containing (β2*) nicotinic acetylcholine receptors (nAChR), but not methyllycaconitine (MLA), a relatively selective antagonist at α7 nAChRs, blocked the sensitizing effect of nicotine on AMPH-stimulated locomotor activity. Pretreatment with varenicline, a partial agonist selective for β2* nAChRs, blocked the sensitizing effect of nicotine on AMPH-stimulated locomotor behavior. Nicotine pretreatment sensitized AMPH-induced DA overflow in slices from ventral (nucleus accumbens, NAc), but not dorsal striatum as compared to saline-pretreated rats. Nicotine sensitization of the DA overflow was blocked by DHβE. Pretreatment with the glutamate N-methyl-D-aspartate (NMDA) receptor antagonist (+)-MK801 (0.1 mg/kg, s.c.) 30 min before nicotine blocked sensitization of both locomotion and DA overflow in response to AMPH challenge. These results demonstrate that activation of the β2* nAChRs and NMDA receptors are required for the rapid sensitizing effect of nicotine on AMPH actions.
Keywords: Nucleus accumbens, stimulants, Holtzman rats, Locomotor activity, varenicline, dihydro-β-erythroidine
1. Introduction
Nicotine is an addictive drug and has been demonstrated to serve as a primary reinforcer in both humans and laboratory animals under some conditions (Henningfield and Goldberg, 1983; Le Foll and Goldberg, 2009). There is emerging evidence that nicotine can function as a reinforcer-enhancer, that is, it can enhance the incentive motivational and reinforcing properties of other stimuli (Caggiula et al., 2009; Raiff and Dallery, 2008). Nicotine use is often paired with use of other drugs, including stimulants, and repeated nicotine enhances subsequent use of other stimulant drugs (Henningfield et al., 1990; Wu and Anthony, 1999). Humans frequently use these drugs in close temporal proximity, and nicotine use is typically higher in individuals who abuse methamphetamine, d-amphetamine (AMPH), and cocaine (Budney et al., 1993; Burling et al., 1996; Teter et al., 2006). Nicotine exposure increased cocaine craving in crack cocaine-using smokers (Reid et al., 1998). In laboratory animals, repeated nicotine exposure sensitizes locomotor activity to a subsequent stimulation by AMPH (Birrell and Balfour, 1998; Celik et al., 2006; Collins et al., 2004), cocaine (Collins and Izenwasser, 2004), and methamphetamine (Suemaru et al., 1993), and facilitates cocaine self-administration (Horger et al., 1992; McQuown et al., 2007). Consistent with behavioral studies, AMPH-stimulated dopamine (DA) overflow in the nucleus accumbens (NAc) is enhanced in rats repeatedly exposed to nicotine (Birrell and Balfour, 1998). These data suggest that repeated nicotine produces persistent alterations in psychomotor and reward systems that are shared with other drugs of abuse such as AMPH and cocaine.
However, there are data suggesting that even one acute dose of nicotine increases reward salience and psychomotor activating effects of stimuli. Nicotine pretreatment via a transdermal patch increased positive subjective measures of cocaine in non-dependent smokers (Kouri et al., 2001). Administration of a one-time nicotine patch to non-smoking humans significantly increased response bias toward a more frequently rewarded task independent of effects on attention (Barr et al., 2008). In rats, acute nicotine selectively enhanced responding with conditioned reinforcement suggesting that acute nicotine could alter motivational behavior (Olausson et al., 2004). We previously reported that a single exposure to nicotine sensitizes locomotor responsiveness to other drugs of abuse, including AMPH, cocaine and methamphetamine. As compared to saline, a low dose of nicotine given to rats sensitized subsequent AMPH-induced locomotor behavior and AMPH-stimulated DA efflux in the striatum measured 1 to 4 hours later (Jutkiewicz et al., 2008), demonstrating a rapid sensitization following nicotine pretreatment. Conversely, an acute dose of AMPH rapidly enhanced locomotor behavior and DA efflux in response to nicotine (Jutkiewicz et al., 2008). Sensitization of both locomotor behavior and DA efflux in response to AMPH exemplifies neuroadaptations in the mesolimbic DA system; sensitization may underlie the incentive salience of drugs of abuse (Robinson and Berridge, 2000, 2008; Vezina, 2004) and enhance self-administration of psychostimulants (Vezina, 2004).
Activation of nicotinic acetylcholine receptors (nAChRs) on cell bodies in the mesolimbic system (ventral tegmental area, VTA) are crucial for the reinforcing and locomotor activating properties of nicotine (Vezina et al., 2007) and DA release in response to systemic nicotine (Nisell et al., 1997; Nisell et al., 1994). There are two predominant types of nAChRs in the brain: homomeric α7-containing, and heterotrimeric β2* nAChRs, both of which can enhance DA release upon activation by acetylcholine in the VTA. (* represents subunits other than those indicated that may be present in the heteropentameric receptors). The β2* nAChR subtypes have a higher affinity for nicotine than the α7 subtype, desensitize rapidly and are required for the DA-releasing, locomotor, and rewarding properties of nicotine (Corrigall et al., 1994; Grottick et al., 2000; Picciotto et al., 1998; Wonnacott et al., 2005).
In this study we further characterize the rapid sensitizing effect of nicotine on AMPH-induced locomotor behavior and AMPH-induced DA efflux in male and female Holtzman rats. We have extended this study to examine if β2* or α7 nAChRs are involved in the sensitizing effect of low-dose nicotine on AMPH-induced locomotor behavior. Further, we examine whether the sensitizing effect of nicotine on AMPH-stimulated DA efflux occurs primarily in the NAc, an area important for the initiation of drug reward, or in the dorsal striatum, an area important for anticipation and preoccupation stages of drug taking (Koob and Volkow, 2010), or both. We find that both β2* nAChRs and glutamate receptors are important for the sensitizing effect of acute nicotine.
2. Material and Methods
2.1. Animals
Male Holtzman rats (175-200 g) or female Holtzman rats (200-250 g) were purchased from Harlan Sprague Dawley (Indianapolis, IN, USA) and housed in groups of three for at least one week in a temperature- (21 °C) and humidity-controlled room with lights on at 7:00 AM and off at 7:00 PM. The rats could access food and water ad libitum. The experimental protocols were approved by the University of Michigan Committee on the Use and Care of Animals and followed the guidelines by the NIH Guide for the Use of Laboratory Animals.
2.2. Surgical implants and locomotor activity measurements
On surgery day, rats were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and a radiotransmitter (model ER-4000 E-mitter, Mini Mitter, Bend, OR, USA) was implanted into the peritoneal cavity of each rat. Rats were singly housed for one week to recover before measuring locomotor activity. One day before the locomotor activity measurements, home cages of individual rats were placed on top of radiotransmitter receivers (model ER-4000 Receiver, Mini Mitter) that record locomotor activity signals from the radiotransmitters transplanted in rats. The received signals were processed in real time by Vital View data acquisition system (Mini Mitter, Bend, OR). Locomotor activity is represented as gross locomotor counts per 5 min recording time. Prior to any treatments, rats were administered saline habituation injections and given 2 hours to allow locomotor activity to return to baseline before testing. Behavioral locomotor activity was collected 40 min before vehicle or drug pretreatment. Animals were pretreated with either nicotine (0.1 mg/kg, i.p.) or saline (1 ml/kg, i.p.) control. Two hours later, AMPH (0.32 mg/kg, i.p.) was injected in both nicotine- and saline-pretreated rats and locomotor activity was measured for 3 to 4 hours.
In experiments studying antagonism of the behavioral potentiating effect of nicotine, rats were injected with a drug or saline (1 ml/kg, i.p.) either 15 min or 30 min, as indicated, prior to nicotine (0.1 mg/kg, i.p.) or saline (1 ml/kg, i.p.) pretreatment. Drugs used were: dihydro-β-erythroidine (DHβE, 5.6 mg/kg, i.p.; a nAChR antagonist which acts primarily at non-α7-containing nAChRs and has high affinity for β2* subtypes), methyllycaconitine (MLA,10 mg/kg, i.p.; a nAChR antagonist selective for α7-containing receptors) or varenicline (0.1 mg/kg, i.p.), a nicotine partial agonist with relative specificity for the α4β2* nAChR subtypes at this dose (Rollema et al., 2007), and MK-801 (0.1 mg/kg, s.c.), a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist.
2.3. AMPH-stimulated DA efflux
Male Holtzman rats (250-350g) were pretreated with nicotine (0.1 mg/kg, i.p.) or saline (1 ml/kg, i.p.) as control. Two hours after nicotine pretreatment, the rats were sacrificed, and NAc and dorsal striatal slices were prepared as described (Jutkiewicz et al., 2008) and placed between two GF/B disk filters (Whatman, Maidstone, England) in a superfusion apparatus (Brandel SF-12, Gaithersburg, MD, USA). In order to obtain stable baseline DA release from the tissue, the slices were perfused for 75 min with Krebs-Ringer buffer (KRB) [24.9 mM NaHCO3, 1.2 mM KH2PO4, 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 10 mM glucose, 0.05 mM ascorbic acid, and 50 μM pargyline], saturated with 5 % CO2/95 % O2 gas mixture and adjusted to pH 7.4. KRB and tissue were maintained at 37 °C and the flow rate of superfusion was 0.4 ml/min. Overflow of endogenous DA in response to a 2-min bolus of 1 μM AMPH was collected into a vial containing (final concentrations) 50 mM HClO4, 25 μM sodium bisulfate, 25 μM ethylenediaminetetraacetic acid, and 10 nM 2-aminophenol (internal standard). The sample collection time was 2 min. The amount of DA released was measured using high performance liquid chromatography with electrochemical detection (HPLC-EC) (ESA biosciences, Chelmsford, MA, USA) and expressed as picomol per 2 min per mg wet weight tissue.
2.4. Drugs
All doses of nicotine are given as the base. (−)-Nicotine bitartrate, d-AMPH sulfate, and MLA were purchased from Sigma-Aldrich (St. Louis, MO, USA). (+)-MK801 maleate was purchased from Research Biochemicals Inc (Natick, MA, USA). DHβE was a kind gift from Dr. Edward Domino, University of Michigan. Varenicline was donated by Dr. Hans Rollema, Pfizer, Groton CT, USA.
2.5. Statistical Analysis
Time courses of locomotor stimulation and slice perfusion studies were analyzed by two-way ANOVA with Bonferroni post test using GraphPad Prism version 5.00 for Windows, GraphPad Software, (San Diego CA, USA, www.graphpad.com). Total locomotor activity counts or DA efflux following an AMPH challenge were compared by Student’s t-test, or one-way ANOVA with Bonferroni post test (GraphPad Prism Software).
3. Results
3.1. Acute nicotine sensitizes AMPH-stimulated locomotor activity in male Holtzman rats
Our previous studies demonstrating the potentiating action of nicotine pretreatment on AMPH-stimulated activities used female Holtzman rats. In order to demonstrate that there was not a sex-dependent effect, we tested the sensitizing effect in male Holtzman rats. In each experiment, following the 2 hours saline-injection-habituation phase, rats were injected with saline (1 ml/kg, i.p.) or nicotine (0.1 mg/kg, i.p.) in their home cages and behavior returned to baseline within 60-70 min. Two hours later, all rats were given an i.p. injection of 0.32 mg/kg AMPH. The nicotine pretreatment appeared to increase locomotor activity, but this effect was not statistically significant (Fig. 1A). However, the nicotine pretreatment as compared to saline pretreatment potentiated the response to a challenge of AMPH (0.32 mg/kg, i.p.) given two hours later. A two-way repeated measures ANOVA (pretreatment × time) indicates a significant main effect of nicotine pretreatment on AMPH-induced locomotor activity (130 – 210 min), [F(1, 153) = 8.422, p < 0.02]. Calculation of total locomotor activity over 80 min after the AMPH challenge revealed that AMPH-induced locomotor activity significantly differed between nicotine- and saline-pretreated rats (Fig. 1B). Nicotine-pretreated rats showed statistically greater total locomotor activity counts (364 ± 37, mean ± sem) than saline-pretreated rats (229 ± 28) after AMPH challenge in the home cage environment (p < 0.02, n = 6).
Fig. 1.
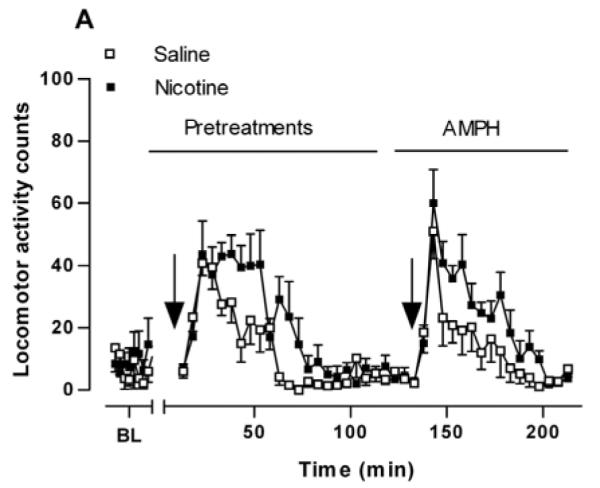
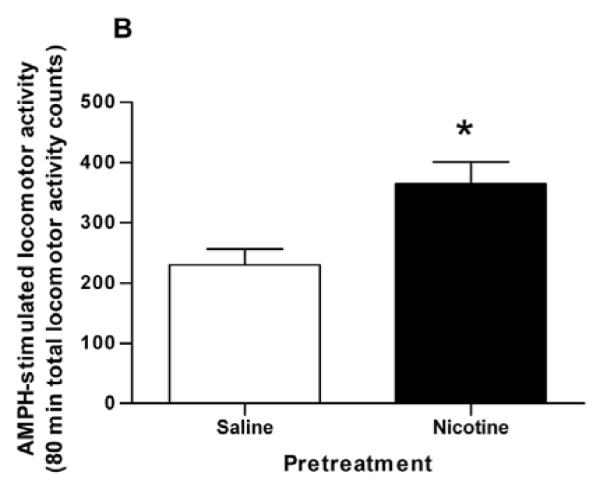
Locomotor response to AMPH in saline- and nicotine-pretreated rats. (A) Time course of locomotor activity after a single amphetamine (AMPH) injection following saline (Sal, □) or nicotine (Nic, ■) pretreatments. Sal (1 ml/kg, i.p.) or Nic (0.1 mg/kg, i.p.) was injected 2 hours before AMPH treatment (0.32 mg/kg, i.p.). Locomotor activity counts are expressed ± standard error of the mean (sem). A two-way repeated measures ANOVA (pretreatment × time) indicates a significant main effect of pretreatment on AMPH-stimulated locomotor activity, [F(1, 153) = 8.422, p < 0.02], n = 6. (B) Locomotor activity counts were summed for 80 min after AMPH challenge and are expressed ± sem. *p < 0.02 as compared to saline-pretreated rats (unpaired Student’s t-test).
3.2. The β2* nAChR, but not the α7 subtype, is important for the sensitizing effect of nicotine on AMPH-stimulated locomotor activity
In order to examine if β2* or α7-containing nAChRs were involved in the potentiative interaction between nicotine and AMPH, rats were injected with either saline, the β2*-subunit selective nAChR antagonist, DHβE (5.6 mg/kg, i.p.) or the α7-containing subtype selective nAChR antagonist, MLA (10 mg/kg, i.p.), 15 min prior to the nicotine or saline pretreatment. Two hours after the nicotine/saline pretreatment, rats were challenged with AMPH (0.32 mg/kg, i.p). For the sake of clarity, in Figures 2A and 2B only the behavioral response to the AMPH challenge is shown. A two-way repeated measures ANOVA (pretreatment × time) yields a significant effect of pretreatment on AMPH-induced locomotor activity [F(3, 368) = 3.63, p < 0.03]. Post hoc Bonferroni analysis of the data in Fig. 2A revealed a significant difference at 158 min Sal/Nic vs DHβE/Nic (p < 0.01) and for Sal/Nic, vs. DHβE/Sal (p < 0.05). These data suggest that the sensitizing effect of nicotine on AMPH locomotor activity was blocked by DHβE. On the contrary, the data in Fig. 2B suggest that MLA did not significantly prevent the sensitizing action of nicotine. The effect of treatment did not reach statistical significance when data were analyzed by a two-way repeated measures ANOVA (pretreatment × time), [F(3, 336) = 3.01, p = 0.053].
Fig. 2.
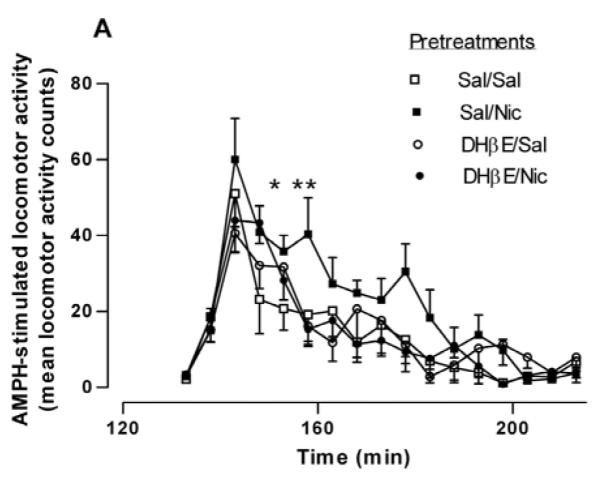
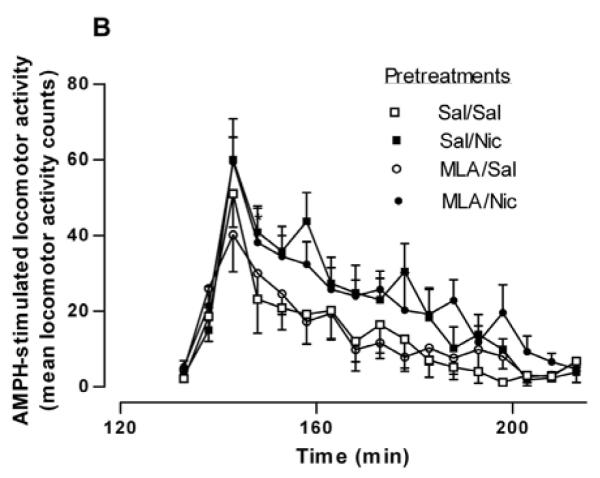
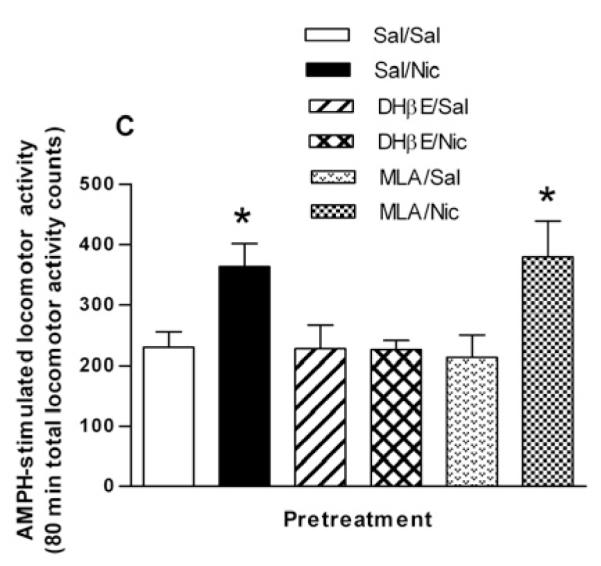
Effect of selective nAChR subtype inhibitors on the nicotine sensitization of AMPH-stimulated locomotor activity. (A) Rats were given either saline or the β-subunit selective drug DHβE (5.6 mg/kg, i.p.) 15 min before the pretreatment of saline or nicotine (0.1 mg/kg, i.p.) to create four groups: Sal/Sal, Sal/Nic, DHβE/Sal, and DHβE/Nic. Two hours later rats were challenged with AMPH (0.32 mg/kg, i.p.) and locomotor activity was recorded for 2 hours. Data are expressed as mean locomotor activity counts in response to AMPH ± sem. A two-way repeated measures ANOVA (preatment × time) indicates a significant main effect of pretreatment on AMPH-stimulated locomotor activity, [F(3, 368) = 3.63, p < 0.03]. Post hoc Bonferroni analysis of the data in Fig. 2A revealed a significant difference for Sal/Nic at 158 min (*p < 0.05 vs. DHβE/Sal ) and (**p < 0.01 vs. DHβE/Nic). (B) Rats were given either saline or the α7-subunit selective drug MLA (10 mg/kg, i.p.) 15 min before the pretreatment of saline or nicotine (0.1 mg/kg, i.p.) to create four groups: Sal/Sal, Sal/Nic, MLA/Sal, and MLA/Nic. Two hours later rats were challenged with AMPH (0.32 mg/kg, i.p.) and locomotor activity was recorded for 2 hours. Data are expressed as mean locomotor activity counts in response to AMPH ± sem. A two-way ANOVA (preatment × time) indicates statistically non significant effect, [F(3, 336) = 3.01, p = 0.053]. (C) Total locomotor activity counts ± sem summed over 80 min after AMPH challenge for data shown in (A) and (B). In one-way ANOVA, p = 0.014, n = 6-9. In post-hoc Bonferroni testing, *p < 0.05, for Sal/Nic compared to Sal/Sal and for MLA/Nic compared to MLA/Sal.
The total AMPH-induced locomotor activity counts over 80 min following DHβE or MLA pretreatments are represented in bar graph form in Fig. 2C. The pretreatments Sal/Sal (saline injection 15 min before second saline injection 2 hours before AMPH challenge),Sal/Nic, DHβE/Sal, DHβE/Nic, MLA/Sal and MLA/Nic produced total locomotor activity counts of 229 ± 26, 364 ± 37, 227 ± 39, and 226 ± 16, 216 ± 37 and 379 ± 60, respectively (F(3,23) = 4.838, p = 0.009, one-way ANOVA, n = 6-9). In Bonferroni post test, p < 0.05 for Sal/Nic compared to Sal/Sal and for MLA/Nic compared to MLA/Sal. These data demonstrate that blockade of nAChRs by DHβE but not MLA prevented the potentiative effect of nicotine pretreatment on AMPH-stimulated locomotor activity.
It was of interest to investigate the effect of the smoking cessation drug, varenicline (Chantix), on the nicotine sensitizing effect. Varenicline is a nAChR partial agonist with relative specificity for the α4β2* nAChRs at the dose used in these experiments, 0.1 mg/kg (Rollema et al., 2007). We first investigated whether varenicline and AMPH would interact when given simultaneously. Rats were injected with saline, varenicline (0.1 mg/kg, i.p.), AMPH (0.32 mg/kg, i.p.) or varenicline and AMPH together. These experiments were performed in female rats to compare the results most closely with previous nicotine results (Jutkiewicz et al., 2008)
Simultaneous administration of 0.1 mg/kg varenicline and 0.32 mg/kg AMPH produced greater activity levels than varenicline or AMPH alone (Fig. 3A) in one-way ANOVA (F(3,19) = 16.31, p < 0.001). In post hoc Bonferroni analysis, p < 0.05 for AMPH versus Sal and Var + AMPH; p < 0.001 for Var + AMPH versus Sal and Var. Varenicline and AMPH individually produced total activity counts over 90 min of 410 ± 54 and 538 ± 62, respectively; whereas simultaneous administration of varenicline and AMPH produced 809 ± 89. Considering a saline value of 228 ± 20, the activities produced by AMPH and varenicline together are very close to additive.
Fig. 3.
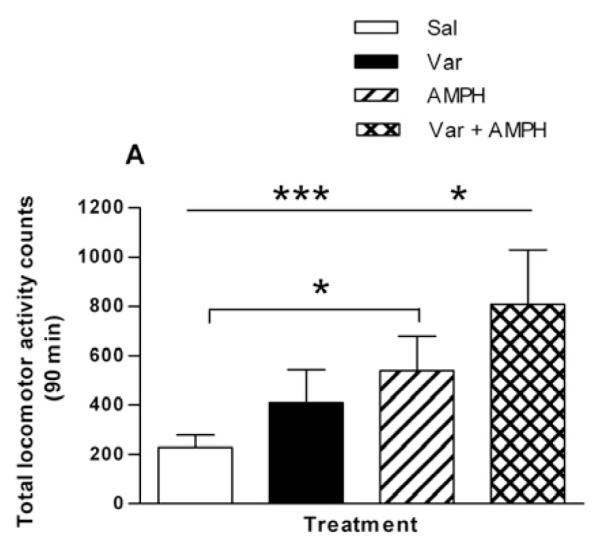
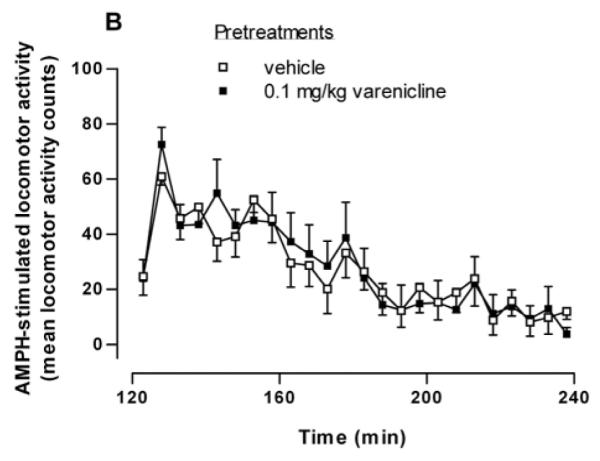
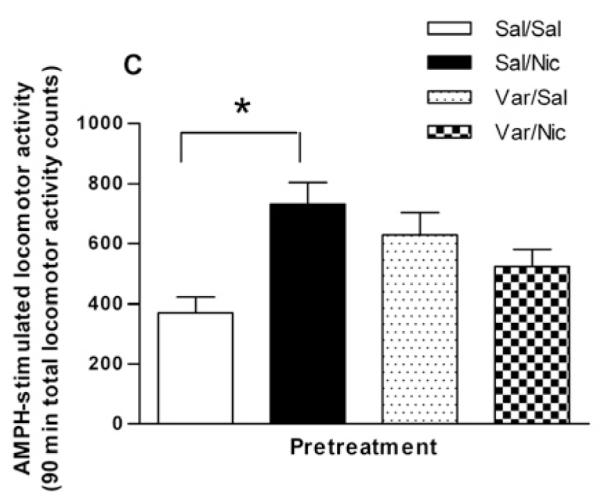
Effect of varenicline, an α4β2* nAChR subtype partial agonist, on nicotine sensitization. (A) Varenicline and AMPH have additive effects on locomotor activity when co-administered. Rats were given an injection of saline (Sal), varenicline (0.1 mg/kg, i.p., Var), AMPH (0.32 mg/kg, i.p., AMPH), or varenicline plus AMPH ( Var + AMPH). Locomotor activity counts were summed over for 90 min and are expressed as mean ± sem. In one-way ANOVA, p < 0.001, n = 5-6. In post hoc Bonferroni analysis, *p < 0.05 for AMPH versus Sal and Var + AMPH; ***p < 0.001 for Var + AMPH versus Sal and Var. (B) Rats were injected with saline or 0.1 mg/kg i.p. varenicline followed 2 hours later by 0.32 mg/kg i.p. AMPH. Locomotor activity counts were recorded and are expressed ± sem. A two-way repeated measures ANOVA indicates that there was no significant treatment effect [F(1, 230) = 0.01; p = 0.91] n = 6. (C) Varenicline blocks the sensitizing effect of nicotine. Rats were injected with either varenicline (0.1 mg/kg, i.p.) or saline 15 min before the pretreatment of saline or nicotine (0.1 mg/kg, i.p.). Four hours later rats were challenged with AMPH (0.32 mg/kg, i.p.) and locomotor activity was recorded for 90 min to create four groups: Sal/Sal, Sal/Nic, Var/Sal, and Var/Nic. Total locomotor activity counts are expressed ± sem. A one-way ANOVA indicates that p = 0.019, n = 6-9. In post hoc Bonferroni testing, *p < 0.05 for Sal/Sal versus Sal/Nic.
We next examined whether varenicline could substitute for nicotine in sensitizing the locomotor response to AMPH when the drugs were given sequentially. Rats were injected with saline or 0.1 mg/kg i.p. varenicline followed two hours later by 0.32 mg/kg i.p. AMPH. In contrast to nicotine (Fig. 1), varenicline did not sensitize AMPH-stimulated locomotor behavior. Locomotor counts over a 120 min period following the AMPH injection are shown in Fig. 3B. In a two-way repeated measures ANOVA (pretreatment × time), there was no significant effect of treatment [F(1, 230) = 0.01, p = 0.91, n = 6].
As a partial agonist, varenicline could block the sensitizing effect of nicotine. Therefore, varenicline was given using a similar experimental design as described for DHβE: the rat was given an i.p. injection of either saline or varenicline (Var, 0.1 mg/kg, i.p.) followed 15 min later by saline or nicotine (0.1 mg/kg, i.p.). Four hours later rats received a challenge dose of 0.32 mg/kg i.p. AMPH. The total locomotor counts for 90 min following the AMPH challenge are presented in the bar graph in Fig. 3C. The Sal/Sal, Sal/Nic, Var/Sal, and Var/Nic pretreatments produced total locomotor activity counts of 370 ± 51, 730 ± 73, 629 ± 73, and 524 ± 56, respectively (F(3,20) = 4.310, p = 0.017, one-way ANOVA, n = 5-9). Post-hoc Bonferroni testing revealed a statistically significant effect of the full agonist, nicotine, in eliciting sensitization of the locomotor activating effects of AMPH (p < 0.05). The partial agonist varenicline appeared partially effective in eliciting sensitization of AMPH-stimulated locomotor activity but the effect was not statistically significant. Varenicline, however, attenuated the ability of nicotine to elicit the sensitization as there was no significant difference between Var/Sal and Var/Nic groups.
3.3. Nicotine pretreatment enhances AMPH-stimulated DA efflux in the NAc
In our previous report on the sensitizing effects of nicotine (Jutkiewicz et al., 2008), we demonstrated that pretreatment with nicotine sensitized the AMPH-stimulated DA efflux in addition to the AMPH-stimulated locomotor behavior. The entire striatum, both dorsal and ventral areas, was used in that study. We now examined whether or not nicotine pretreatment would enhance AMPH-stimulated DA efflux selectively in the NAc or dorsal striatum. Experiments were conducted as described above except that the rats did not receive a challenge dose of AMPH. Instead, two hours following the nicotine (0.1 mg/kg, i.p.) or saline pretreatment, rats were sacrificed and DA efflux in response to AMPH was measured in perfused slices as described in Material and Methods (subsection 2.3.). DA efflux in slices from NAc or dorsal striatum in response to perfusion of AMPH (1 μM) are shown in Figures 4A and 4B, respectively. As compared to saline controls, pretreatment with nicotine significantly enhanced AMPH-induced DA efflux from slices of NAc (Fig. 4A) but not dorsal striatum (Fig. 4B). For the NAc, a two-way repeated measures ANOVA (pretreatment × fraction) indicates a significant effect of the interaction of pretreatment and time on AMPH induced efflux, [F(7, 70) = 2.22, p < 0.05]. In post-hoc Bonferroni testing, DA efflux in response to nicotine in fraction 7 differed from that of saline (p < 0.05).
Fig. 4.
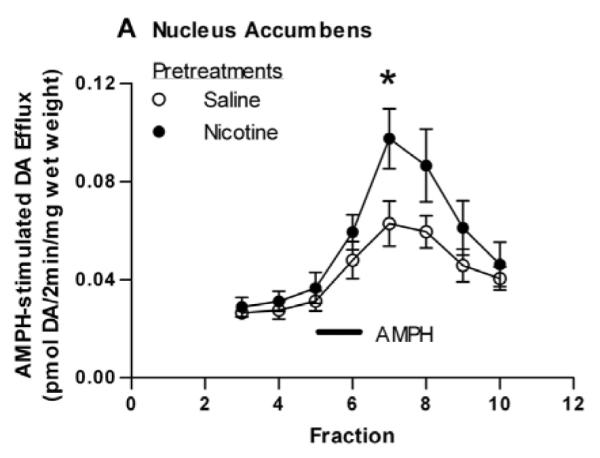
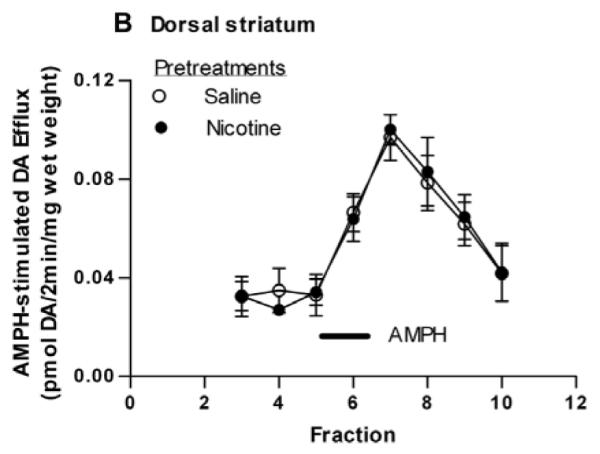
Effect of nicotine pretreatment on AMPH-stimulated DA efflux in the NAc and dorsal striatum. Rats were injected with saline (1 ml/kg, i.p.) or nicotine (0.1 mg/kg, i.p.) 2 hours before sacrifice. Rat striatum was separated into dorsal striatum and NAc and sliced. Slices were perfused with KRB (see subsection 2.2.) for 75 min and challenged with 1 μM AMPH for 2 min at fraction number 5. DA efflux was measured with HPLC-EC and are expressed ± sem. (A) NAc. A two-way repeated measures ANOVA (pretreatment × fraction) indicates a significant effect of pretreatment on AMPH induced efflux, [F(1, 80) = 11.31, p < 0.001]. *p < 0.05 with post-hoc Bonferroni test. n = 6. (B) Dorsal striatum. n = 5.
We determined if the nicotine-sensitizing effect on AMPH-stimulated DA efflux in the NAc was blocked by the nAChR antagonist, DHβE, similar to the locomotor results. Rats were pretreated with saline or DHβE (5.6 mg/kg, i.p.) 15 min before the saline or nicotine (0.1 mg/kg, i.p.) treatment. Two hours later rats were sacrificed and DA efflux in response to 1 μM AMPH was measured in slices of NAc. AMPH-stimulated DA efflux in the NAc slices is shown in Fig. 5A. A two-way repeated measures ANOVA (pretreatment × fraction) indicates a significant effect of pretreatment on AMPH induced efflux, [F(3, 84) = 4.125, p = 0.032] (n = 4). Total DA efflux in response to 1 μM AMPH was presented in Fig. 5B. One-way ANOVA of the data revealed statistical significance (F(3,11) = 6.45, p = 0.009). In a Bonferroni post test, the total amount of DA (in pmol/2min/mg wet weight) in the effluent following the Sal/Nic pretreatment is statistically greater than that following the Sal/Sal (p < 0.05) or DHβE/Nic (p < 0.01) pretreatments (n = 4). Overall, the behavioral and biochemical data with DHβE indicate a critical role of β2* nAChRs in the nicotine and AMPH interaction.
Fig. 5.
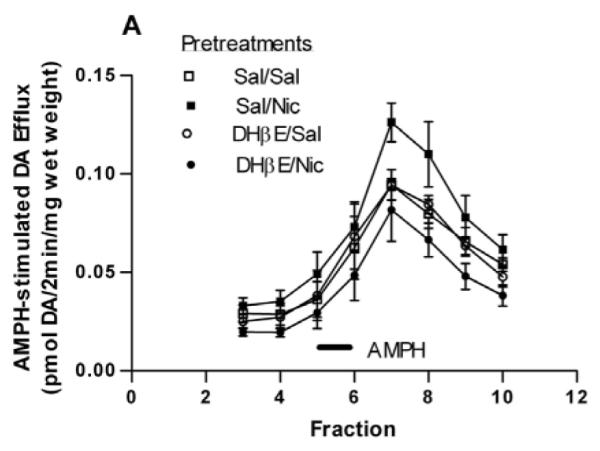
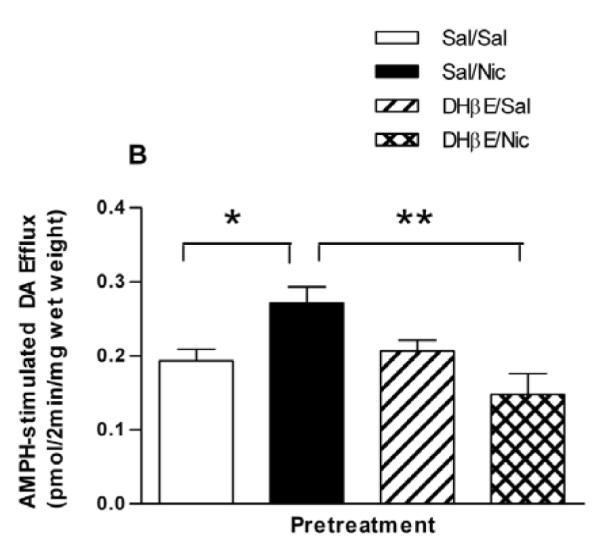
Effect of the β2* nAChRs antagonist, DHβE on AMPH-stimulated DA efflux. (A) Rats were pretreated with saline or DHβE (5.6 mg/kg, i.p.) 15 min prior to saline or nicotine pretreatment as described in the legend to Fig. 2A, to give 4 groups: Sal/Sal, Sal/Nic, DHβE/Sal, and DHβE/Nic. Rats were sacrificed 2 hours after the saline or nicotine injections. Sliced NAc was perfused with KRB for 75 min and challenged with 1 μM AMPH for 2 min at fraction number 5. DA efflux was measured with HPLC-EC and are expressed ± sem. A two-way repeated measures ANOVA (pretreatment × fraction) indicates a significant effect of pretreatment on AMPH induced efflux, [F(3, 84) = 4.125, p = 0.03] (n = 4). (B) Data from part (A) are presented as the total DA efflux elicited by AMPH (area under the curve) ± sem. One-way ANOVA of the data revealed statistical significance at p = 0.009. In post hoc Bonferroni testing, the Sal/Nic pretreatment group is statistically greater than the Sal/Sal (*p < 0.05) or DHβE/Nic (**p < 0.01) pretreatment groups.
3.4. The effect of the NMDA receptor antagonist, MK-801, on the nicotine sensitizing effect of AMPH
The NMDA receptor antagonist MK-801 blocks the induction of sensitization to several stimulants, including AMPH and nicotine (Jain et al., 2008; Sripada et al., 2001; Wolf, 1998). This suggests that NMDA receptor activation is a necessary step in the development of stimulant sensitization. Most of these studies have involved repeated co-administration of MK-801 or saline (control) with the stimulant. We investigated whether or not NMDA receptors were important for the sensitizing effect of a single injection of nicotine. Rats were treated with saline or MK-801 (0.1 mg/kg, s.c.) 30 min before saline or nicotine treatment. Two hours later, rats were injected with 0.32 mg/kg i.p. AMPH and locomotor activity was recorded for 2 hours. For the sake of clarity, in Figure 6A only the behavioral response to the AMPH challenge is shown. A two-way repeated measures ANOVA (pretreatment × time) yields a significant effect of pretreatment on AMPH-induced locomotor activity [F(3, 532) = 9.36, p < 0.0001]. The mean locomotor activity counts for each group over 90 min after AMPH challenge are graphed in Fig. 6B. The mean locomotor activity counts in the Sal/Sal, Sal/Nic, MK-801/Sal, and MK-801/Nic pretreatment groups are 275 ± 23, 407 ± 42, 207 ± 57, and 239 ± 30, respectively (n = 5-10). A one-way ANOVA indicates F(3,27) = 5.675 (p < 0.005) and Bonferroni post testing demonstrates that the Sal/Nic group was significantly greater than the Sal/Sal group at p < 0.05. In addition, the Sal/Nic group was significantly different from the MK-801/Sal and MK-801/Nic at p < 0.01. The rats that received MK-801 before the injection of saline or nicotine in the pretreatment stage did not demonstrate sensitized AMPH-stimulated locomotor activity, as there was no difference between the MK-801/Sal and MK-801/Nic groups in Bonferroni post-testing (t = 0.51). These results demonstrate that NMDA receptors are involved in the behavioral potentiation of nicotine on the action of AMPH.
Fig.6.
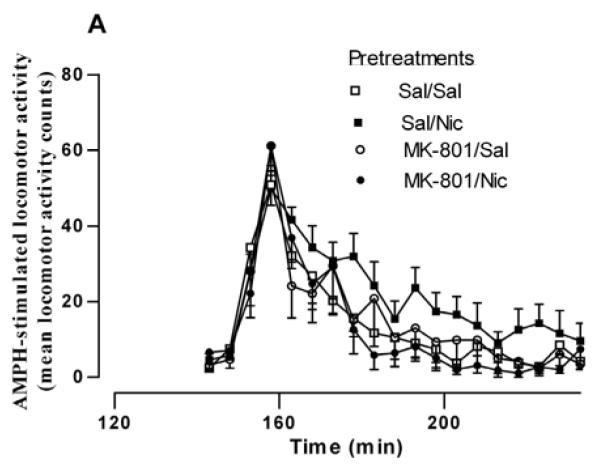
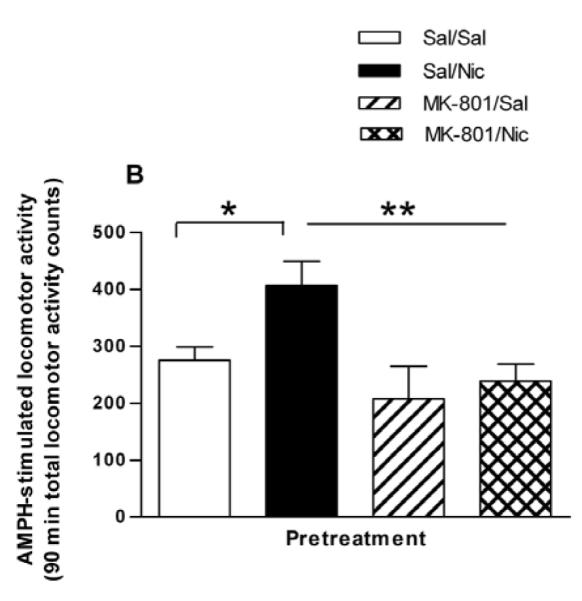
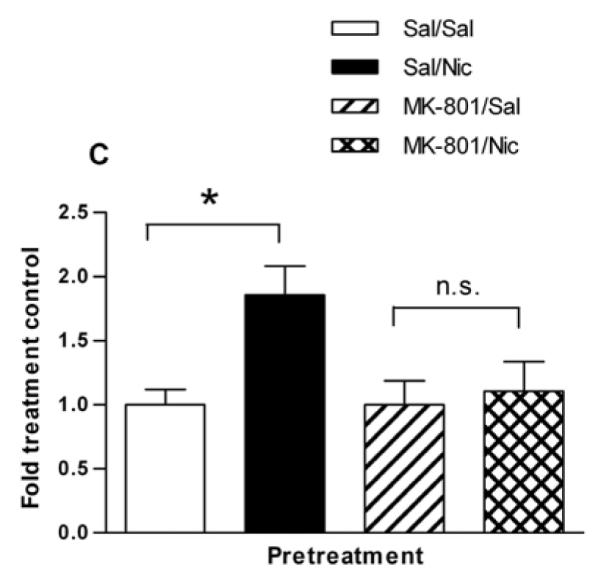
Effect of the NMDA receptor antagonist, MK-801 on the nicotine sensitization of (A) AMPH-stimulated locomotor behavior and (B) AMPH-stimulated DA efflux. (A) Rats were given either saline or the glutamate NMDA receptor antagonist MK-801 (0.1 mg/kg, s.c.) 30 min before the saline or nicotine (0.1 mg/kg, i.p.) to create four pretreatment groups: Sal/Sal, Sal/Nic, MK-801/Sal, and MK-801/Nic. Two hours later rats were challenged with AMPH (0.32 mg/kg, i.p.) and locomotor activity was recorded for 2 hours. A two-way repeated measures ANOVA (preatment × time) indicates a significant main effect of pretreatment on AMPH-stimulated locomotor activity, [F(3, 532) = 9.36, p < 0.0001]. (B) Total locomotor activity counts ± sem for 90 min are shown. A one-way ANOVA indicates p < 0.005, n = 5-10 and post-hoc Bonferroni testing revealed that the Sal/Nic group was significantly greater than the Sal/Sal group at p < 0.05 (t = 2.720) and different from the MK-801/Sal and MK-801/Nic groups at *p < 0.01(t = 3.457 and t = 3.240, respectively). (C) Rats were treated as described in Part (A), but were sacrificed 2 hours after the pretreatments. Sliced NAc was perfused with KRB for 75 min before challenge with 1 μM AMPH for 2 min at fraction number 5. DA efflux was measured with HPLC-EC. The DA efflux elicited by AMPH challenge was calculated as percent of control ± sem, where the Sal/Sal group and MK-801/Sal groups serve as controls. A one-way ANOVA demonstrated significance at p < 0.03, n = 5. Post hoc Bonferroni analysis revealed that the Sal/Nic group was statistically greater than the Sal/Sal group (p < 0.05, t = 2.88) but there was no difference between the MK-801-pretreated groups.
We examined if MK-801 pretreatment would similarly block the sensitizing effect of nicotine on AMPH-stimulated DA efflux. Rats were treated following the same pretreatment schedule as used in the behavioral study except that they did not receive an in vivo challenge with AMPH. The rats were sacrificed, and slices of NAc were prepared and placed in the perfusion apparatus. Following the KRB wash, 1 μM AMPH was perfused through the tissue for 2 min. The pretreatments Sal/Sal, Sal/Nic, MK-801/Sal, and MK-801/Nic produced accumulated DA efflux values of 0.15 ± 0.03, 0.27 ± 0.04, 0.21 ± 0.04, and 0.24 ± 0.05, respectively. A one-way ANOVA (p = 0.3) demonstrated no significant difference amongst the groups. However, examination of the data shows there was clearly no difference between the MK-801/Sal and the MK-801/Nic groups. To more clearly demonstrate this, the data are expressed as percent saline control; that is, Sal/Nic as a percentage of Sal/Sal, and MK-801/Nic as a percentage of its MK-801/Sal control (Fig. 6C). A one-way ANOVA of the results demonstrated significance at p < 0.03 (F(3,15) = 4.235, n = 5). Bonferroni post test analysis revealed that the Sal/Nic group was statistically greater than the Sal/Sal group (p < 0.05, t = 2.88) but there was no difference between the MK-801-pretreated groups (n.s., t = 0.37).
4. Discussion
We previously demonstrated that pretreatment of female, Holzman rats with nicotine 2-4 hours prior to an AMPH challenge sensitized the locomotor and DA releasing effects of AMPH (Jutkiewicz et al., 2008). Here, in addition to demonstrating that the nicotine sensitizing effect is not specific for females, we have identified receptor mechanisms that are important in the development of this effect. This study demonstrated the importance of β2* nAChRs to both the behavioral and biochemical indices, specifically the role of the NAc in the rapid effect, and the importance of NMDA receptor activation to the sensitizing effect of nicotine.
There are numerous studies demonstrating the effect of repeated nicotine on sensitization to other psychostimulants (Celik et al., 2006; Collins et al., 2004; Neugebauer et al., 2010), but there are relatively few studies examining the effect of acute nicotine. In many rodent strains nicotine has immediate depressant effects which would hide/mask subsequent effects of stimulants. The effects of nicotine, however, are dependent upon species, strain and dose of nicotine (Iyaniwura et al., 2001). The Holtzman strain of rat used in these studies may be less sensitive to the depressant effects of nicotine than other strains such as Sprague Dawley (Pehrson et al., 2008). At the low doses we used, nicotine did not significantly activate or inhibit locomotor activity. For example, a dose of 0.1 mg/kg s.c. nicotine immediately increased locomotor activity in Sprague-Dawley rats who were individually housed (Benwell and Balfour, 1992). The fact that additive effects are seen between nicotine and AMPH when administered together but sensitizing effects are demonstrated when the injections are spaced, suggests that a very rapid plasticity occurs in response to nicotine to alter the response to AMPH challenge. This plasticity endures long after nicotine, which has been cleared from the brain due to its half-life of 52 min. Because the sensitization lasts ≤ 8 hrs (Jutkiewicz et al., 2008) the response likely involves a slowly reversible signal transduction mechanism. It is unlikely to involve a nicotine metabolite, as discussed in Jutkiewicz et al. (2008). The acute nicotine sensitization effect involves receptors, pathways and neurochemical adaptations demonstrated to be critical for the ability of nicotine to increase DA in terminal areas. Nicotine preferentially stimulates mesolimbic VTA DA neurons (Mereu et al., 1987) as compared to DA neurons in the substantia nigra, and thus elicits greater DA release over basal output in the NAc than in the caudate putamen (Imperato et al., 1986). The VTA is the site of induction of sensitization to repeated nicotine (Vezina, McGehee et al. 2007), but the behavioral and neurochemical expressions of sensitization are manifested in the NAc (Balfour et al., 1998). The acute sensitization by nicotine also involved activation of this pathway as evidenced by the enhancement of DA efflux in response to AMPH challenge in the NAc but not the dorsal striatum. The enhancement in DA efflux from entire striatum in response to AMPH challenge was evident from two to four hours following nicotine pretreatment (Jutkiewicz et al., 2008). Birrell and Balfour (1998), however, did not find an enhancement of AMPH-stimulated DA efflux on day 5 following 5 consecutive days of treatment with 0.4 mg/kg s.c. nicotine. This could be due to the repeated nicotine treatment and a higher dose of nicotine than we used. It is also possible that time after repeated nicotine is necessary for a sensitized AMPH-stimulated DA efflux in response to be visualized, as is found following repeated AMPH treatment (Paulson and Robinson, 1995). In addition, subtle depressant effects of nicotine could be affecting AMPH action for some time after nicotine injection. Although the AMPH-induced behavior and AMPH-stimulated DA efflux were temporally correlated in the acute sensitizing effect of nicotine, the enhancement in AMPH-stimulated locomotor behavior may not be solely due to an enhancement in AMPH-stimulated DA efflux. Birrell and Balfour (1998) noted sensitized AMPH-stimulated locomotor behavior immediately following repeated nicotine, but not a sensitized response of AMPH-stimulated DA efflux. However, Fung and Lau (1992) reported that chronic nicotine, given subcutaneously in a minipump for 14 days, resulted in a sensitized release of [3H]DA from rat NAc slices in response to AMPH.
nAChRs in the mesolimbic pathway that contain the β2 subunit, particularly α4β2* and α6β2* subtypes, are important in mediating the effects of systemic nicotine on DA release, locomotor activity and reinforcement (Gotti et al., 2010). β2* nAChRs are localized directly on dopaminergic neurons and on GABAergic neurons in the VTA (Wonnacott et al., 2005). Our data suggest that the sensitizing effect of acute nicotine on both AMPH-stimulated locomotor behavior and AMPH-stimulated DA efflux requires activation of β2* nAChRs. DHβE alone did not influence the locomotor activity at any time after its injection as reported previously (Grottick et al., 2000). Thus we conclude that β2* nAChRs have a critical role in the sensitizing effect on nicotine on AMPH. On the contrary, the α7-selective nAChR antagonist, MLA, did not block the nicotine sensitizing effect on AMPH-stimulated locomotor behavior. Activation of α7 nAChRs in the VTA, which are located on glutamatergic terminals, induces a long-lasting potentiation of excitatory transmission by stimulating glutamatergic afferents (Mansvelder and McGehee, 2000) and are involved in burst firing of dopaminergic neurons (Schilstrom et al., 2003). Our data suggest that the plasticity controlled by α7 nAChRs plays a minimal role in the ability of acute nicotine to sensitize AMPH-stimulated locomotor activity. It has been proposed that activation of β2* nAChRs directly excites resting dopaminergic cells, while α7 nAChRs finely tune the excited state (Mameli-Engvall et al., 2006). Our data suggest that a direct excitatory action of nicotine on the VTA dopaminergic cells is required for the acute sensitizing effect.
The ability of varenicline to block the effect of nicotine accentuates the role of the β2* nAChRs in the acute nicotine sensitizing effect. Varenicline is a nAChR partial agonist selective for α4β2* subtype at the dose used in our study (0.1 mg/kg, i.p.) (Rollema et al., 2007). Varenicline was able to activate locomotor activity in the rats but its reduced efficacy at the β2* receptors likely prohibited a full substitution for nicotine in eliciting acute sensitization. There was a tendency for varenicline to mimic nicotine in the study in which varenicline was given four hours before the AMPH challenge, but there was no efficacy of varenicline when given two hours prior to the AMPH challenge. Because there was no statistical significance at either time point, the clearest conclusion is that varenicline does not have the efficacy to induce AMPH sensitization on its own, but will block the efficacy of the full agonist, nicotine. Although both varenicline and nicotine rapidly desensitize β2* nAChRs, it is unlikely that the sensitization effect is mediated by desensitized, inactive nAChRs. If that were true, then the antagonist, DHβE, would have been able to elicit the sensitization effect, but it did not.
Glutamatergic NMDA receptors promote subjective effects of smoking in humans (Jackson et al., 2009) and are essential for the reinforcing properties of nicotine in rats (Kenny et al., 2009). We found that administration of the non-competitive NMDA receptor antagonist, MK-801, before the nicotine or saline pretreatment blocked the nicotine sensitizing effect on both AMPH-stimulated locomotor behavior and on AMPH-stimulated DA efflux. These results concur with reports that pretreatment with NMDA receptor antagonists attenuates the sensitizing effects of repeated nicotine on nicotine-stimulated locomotor activity and blocks nicotine-stimulated DA overflow in the NAc (Shoaib et al., 1994; Shoaib et al., 1997).
It is currently thought that nicotine initially activates α4β2* nAChRs on DA neurons in the VTA to depolarize the neurons, which is subsequently reduced by elevated release of GABA due to activation of α4β2* nAChRs on GABAergic cells (Balfour, 2009). Following rapid desensitization of the α4β2* nAChRs, α7-containing nAChRs on glutamatergic terminals are activated resulting in the release of glutamate (Balfour, 2009; Mameli-Engvall et al., 2006). Activation of α7 nAChRs on glutamatergic terminals projecting from the prefrontal cortex into the VTA enhance glutamate release and activate NMDA receptors on DA neurons (Mansvelder and McGehee, 2002). Our data suggest that the acute nicotine sensitizing effect is due to the initial activation of α4β2* receptors by nicotine, in that nicotine initiated the sensitization, but the nAChR antagonists, DHβE or MLA could not. Our data also support a role for NMDA receptor activation in eliciting the sensitizing effect. It is puzzling, however, as to why MLA did not also block the sensitization if activation of α7 receptors is required for release of glutamate and subsequent activation of NMDA receptors. We gave MK-801 systemically, so it is uncertain as to whether NMDA receptors in the VTA were involved in the sensitization effect. Blockade of NMDA receptors in the amygdala, for example, reduced self-administration of nicotine demonstrating that NMDA receptors in areas other than the VTA affect functions of nicotine (Kenny et al., 2009).
In conclusion, we have demonstrated that acute nicotine can rapidly sensitize the locomotor and DA-releasing effects of AMPH in Holtzman rats. The effect is sex-independent and requires activation of β2* nAChRs and glutamate NMDA receptors. Because the potentiation is not visible when the drugs are added simultaneously, plasticity in downstream signal transduction systems is likely required for the sensitization. The involvement of the β2* nAChRs, which activates voltage-gated Ca2+ channels (Wonnacott et al., 2005), and the NMDA receptors, which are permeable to Ca2+, strongly suggest that Ca2+ plays an important role in the induction process. The ability of AMPH to increase smoking behavior in humans (Alsene et al., 2005; Cousins et al., 2001; Henningfield and Griffiths, 1981; Sigmon et al., 2003), and sensitize nicotine-stimulated behaviors and neurochemical activities in animals (Jutkiewicz et al., 2008; Santos et al., 2009), is well documented. Our results suggest that, under appropriate environmental conditions, acute nicotine also has sensitizing effects on behaviors elicited by other stimulants, notably AMPH, cocaine and methamphetamine. Although we measured locomotor behavior, locomotor sensitization is mediated by neuroadaptations in the mesolimbic DA system (Robinson and Berridge, 2008; Vezina, 2004). These neural systems overlap with those that mediate the reinforcing and incentive motivational properties of potentially addictive drugs (Robinson and Berridge, 2008; Vezina, 2004; Wise and Bozarth, 1987). Thus, drug treatments that produce psychomotor sensitization facilitate the subsequent acquisition of drug self-administration behavior and a conditioned place preference (Horger et al., 1990; Piazza et al., 1989; Vezina et al., 1999), and produce enhanced incentive motivation for drug (Deroche et al., 1999; Mendrek et al., 1998). Therefore it is possible that even acute nicotine could alter motivation for a paired stimulant.
Acknowledgements
We thank Dr. Edward F. Domino for the kind gift of DHβE, Dr. Rong Chen for helping the sacrifice of rats, Rheaclare Fraser for her help with the manuscript, and Conor Daining for animal surgery. This work was supported by the UM Tobacco Research Network, the MICHR Pilot and Collaborative Grant Program, and the United States Public Health Service grant T32 DA007268.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsene KM, Mahler SV, de Wit H. Effects of d-amphetamine and smoking abstinence on cue-induced cigarette craving. Exp Clin Psychopharmacol. 2005;13:209–218. doi: 10.1037/1064-1297.13.3.209. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb Exp Pharmacol. 2009:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell CE, Balfour DJ. The influence of nicotine pretreatment on mesoaccumbens dopamine overflow and locomotor responses to D-amphetamine. Psychopharmacology (Berl) 1998;140:142–149. doi: 10.1007/s002130050751. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Burling TA, Salvio MA, Seidner AL, Ramsey TG. Cigarette smoking in alcohol and cocaine abusers. J Subst Abuse. 1996;8:445–452. doi: 10.1016/s0899-3289(96)90005-x. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik E, Uzbay IT, Karakas S. Caffeine and amphetamine produce cross-sensitization to nicotine-induced locomotor activity in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:50–55. doi: 10.1016/j.pnpbp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl) 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Fung YK, Lau YS. Chronic effects of nicotine on mesolimbic dopaminergic system in rats. Pharmacol Biochem Behav. 1992;41:57–63. doi: 10.1016/0091-3057(92)90059-o. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Henningfield JE, Clayton R, Pollin W. Involvement of tobacco in alcoholism and illicit drug use. Br J Addict. 1990;85:279–291. doi: 10.1111/j.1360-0443.1990.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Griffiths RR. Cigarette smoking and subjective response: effects of d-amphetamine. Clin Pharmacol Ther. 1981;30:497–505. doi: 10.1038/clpt.1981.194. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Horger BA, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav. 1990;37:707–711. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Iyaniwura TT, Wright AE, Balfour DJ. Evidence that mesoaccumbens dopamine and locomotor responses to nicotine in the rat are influenced by pretreatment dose and strain. Psychopharmacology (Berl) 2001;158:73–79. doi: 10.1007/s002130100852. [DOI] [PubMed] [Google Scholar]
- Jackson A, Nesic J, Groombridge C, Clowry O, Rusted J, Duka T. Differential involvement of glutamatergic mechanisms in the cognitive and subjective effects of smoking. Neuropsychopharmacology. 2009;34:257–265. doi: 10.1038/npp.2008.50. [DOI] [PubMed] [Google Scholar]
- Jain R, Mukherjee K, Balhara YP. The role of NMDA receptor antagonists in nicotine tolerance, sensitization, and physical dependence: a preclinical review. Yonsei Med J. 2008;49:175–188. doi: 10.3349/ymj.2008.49.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Nicolazzo DM, Kim MN, Gnegy ME. Nicotine and amphetamine acutely cross-potentiate their behavioral and neurochemical responses in female Holtzman rats. Psychopharmacology (Berl) 2008;200:93–103. doi: 10.1007/s00213-008-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr., Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, Stull M, Lukas SE. Nicotine alters some of cocaine’s subjective effects in the absence of physiological or pharmacokinetic changes. Pharmacol Biochem Behav. 2001;69:209–217. doi: 10.1016/s0091-3057(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb Exp Pharmacol. 2009;335:367. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. 102-06-17 Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Mereu G, Yoon KW, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–399. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend. 2010;106:72–78. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Marcus M, Nomikos GG, Svensson TH. Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J Neural Transm. 1997;104:1–10. doi: 10.1007/BF01271290. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Philibin SD, Gross D, Robinson SE, Vann RE, Rosecrans JA, James JR. The effects of acute and repeated nicotine doses on spontaneous activity in male and female Sprague Dawley rats: analysis of brain area epibatidine binding and cotinine levels. Pharmacol Biochem Behav. 2008;89:424–431. doi: 10.1016/j.pbb.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology (Berl) 2008;201:305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha(4)beta(2) nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Santos GC, Marin MT, Cruz FC, Delucia R, Planeta CS. Amphetamine- and nicotine-induced cross-sensitization in adolescent rats persists until adulthood. Addict Biol. 2009;14:270–275. doi: 10.1111/j.1369-1600.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Rawal N, Mameli-Engvall M, Nomikos GG, Svensson TH. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int J Neuropsychopharmacol. 2003;6:1–11. doi: 10.1017/S1461145702003188. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Benwell ME, Akbar MT, Stolerman IP, Balfour DJ. Behavioural and neurochemical adaptations to nicotine in rats: influence of NMDA antagonists. Br J Pharmacol. 1994;111:1073–1080. doi: 10.1111/j.1476-5381.1994.tb14854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR, Pauly JR. Behavioural and biochemical adaptations to nicotine in rats: influence of MK801, an NMDA receptor antagonist. Psychopharmacology (Berl) 1997;134:121–130. doi: 10.1007/s002130050433. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of D-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology (Berl) 2003;167:393–402. doi: 10.1007/s00213-003-1416-z. [DOI] [PubMed] [Google Scholar]
- Sripada S, Gaytan O, Swann A, Dafny N. The role of MK-801 in sensitization to stimulants. Brain Res Brain Res Rev. 2001;35:97–114. doi: 10.1016/s0165-0173(00)00046-1. [DOI] [PubMed] [Google Scholar]
- Suemaru K, Gomita Y, Furuno K, Araki Y. Chronic nicotine treatment potentiates behavioral responses to dopaminergic drugs in rats. Pharmacol Biochem Behav. 1993;46:135–139. doi: 10.1016/0091-3057(93)90329-r. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Pierre PJ, Lorrain DS. The effect of previous exposure to amphetamine on drug-induced locomotion and self-administration of a low dose of the drug. Psychopharmacology (Berl) 1999;147:125–134. doi: 10.1007/s002130051152. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wu LT, Anthony JC. Tobacco smoking and other suspected antecedents of nonmedical psychostimulant use in the United States, 1995. Subst Use Misuse. 1999;34:1243–1259. doi: 10.3109/10826089909039407. [DOI] [PubMed] [Google Scholar]


