Abstract
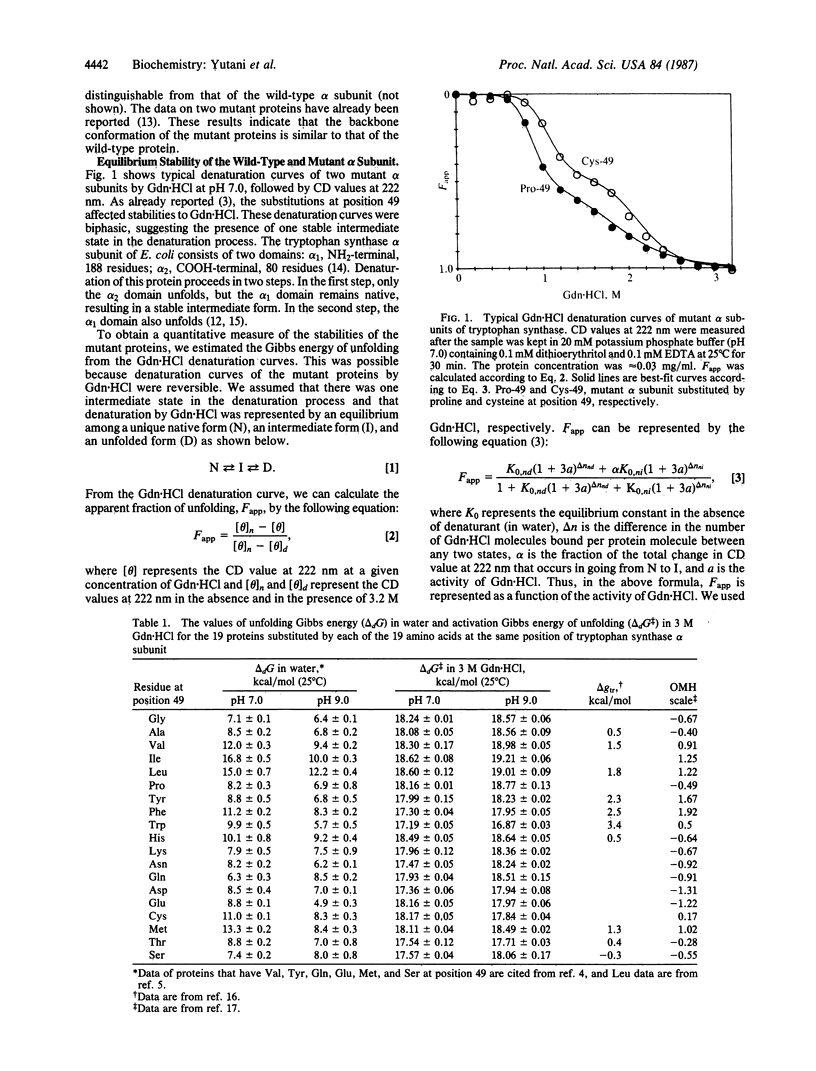
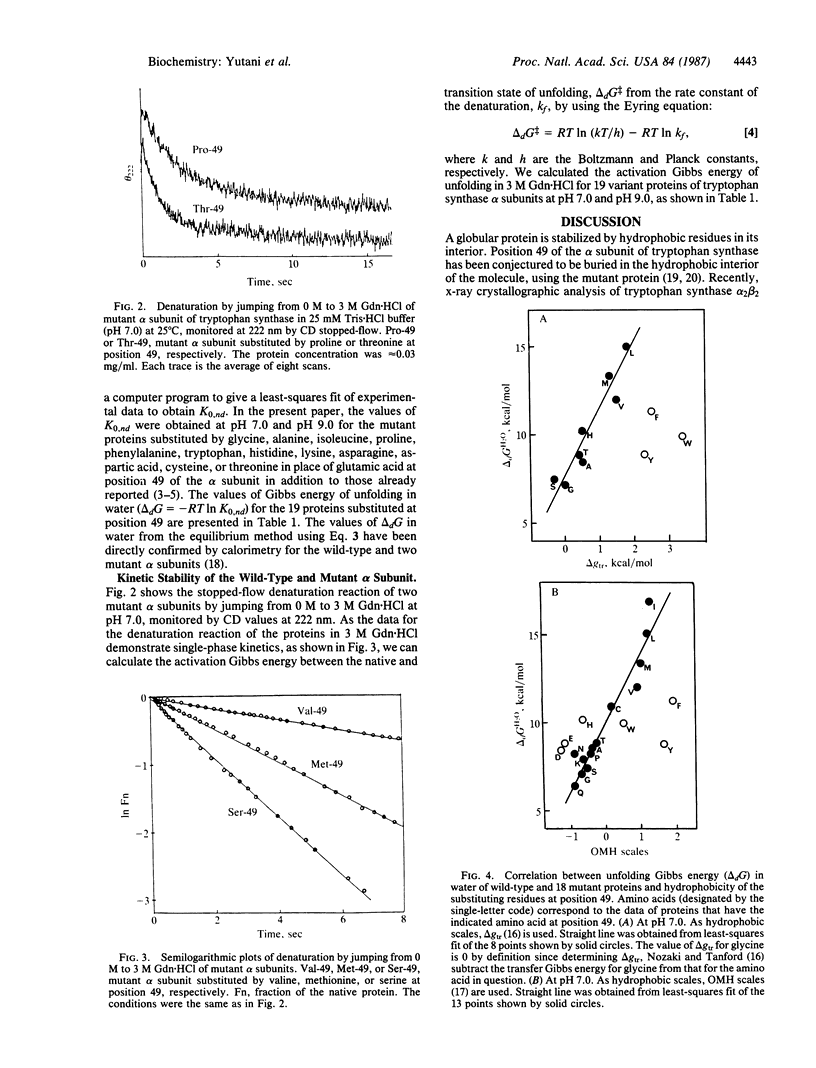
To elucidate the role of individual amino acid residues in stabilizing the conformation of a protein, we have constructed a series of variant alpha subunits of tryptophan synthase from Escherichia coli substituted by each of 20 amino acids at position 49, which is buried in the interior of the protein. The stabilities were quantitatively examined except for the mutant protein substituted by arginine, which was not obtained in enough quantity. The Gibbs energy of unfolding in water and the activation Gibbs energy of unfolding in 3 M guanidine hydrochloride for each protein were compared at pH 7.0 and pH 9.0. The Gibbs energy of unfolding in water at pH 7.0 varied from 0.72 to 1.92 times that of the wild-type protein by the substitutions, but the activation Gibbs energy of unfolding in 3 M guanidine hydrochloride varied only from 0.95 to 1.03 times that of the wild-type protein. Moreover, the stability of the protein substituted at this position, which is buried in the interior of the molecule, tended to increase linearly with increasing hydrophobicity of the substituted residue, unless the volume of the substituted residue was over a certain limit.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasty A. M., Hurle M. R., Manz J. T., Stackhouse T., Onuffer J. J., Matthews C. R. Effects of the phenylalanine-22----leucine, glutamic acid-49----methionine, glycine-234----aspartic acid, and glycine-234----lysine mutations on the folding and stability of the alpha subunit of tryptophan synthase from Escherichia coli. Biochemistry. 1986 May 20;25(10):2965–2974. doi: 10.1021/bi00358a035. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., McLachlan A. D. Solvation energy in protein folding and binding. Nature. 1986 Jan 16;319(6050):199–203. doi: 10.1038/319199a0. [DOI] [PubMed] [Google Scholar]
- Elwell M. L., Schellman J. A. Stability of phage T4 lysozymes. I. Native properties and thermal stability of wild type and two mutant lysozymes. Biochim Biophys Acta. 1977 Oct 26;494(2):367–383. doi: 10.1016/0005-2795(77)90166-0. [DOI] [PubMed] [Google Scholar]
- Higgins W., Fairwell T., Miles E. W. An active proteolytic derivative of the alpha subunit of tryptophan synthase. Identification of the site of cleavage and characterization of the fragments. Biochemistry. 1979 Oct 30;18(22):4827–4835. doi: 10.1021/bi00589a010. [DOI] [PubMed] [Google Scholar]
- Miles E. W., Yutani K., Ogasahara K. Guanidine hydrochloride induced unfolding of the alpha subunit of tryptophan synthase and of the two alpha proteolytic fragments: evidence for stepwise unfolding of the two alpha domains. Biochemistry. 1982 May 25;21(11):2586–2592. doi: 10.1021/bi00540a002. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Ogasahara K., Yutani K., Suzuki M., Sugino Y., Nakanishi M., Tsuboi M. State of Tyr49 in a mutant tryptophan synthase alpha-subunit substituted at position 49. J Biochem. 1980 Dec;88(6):1733–1738. doi: 10.1093/oxfordjournals.jbchem.a133148. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Geselowitz A. R., Lesser G. J., Lee R. H., Zehfus M. H. Hydrophobicity of amino acid residues in globular proteins. Science. 1985 Aug 30;229(4716):834–838. doi: 10.1126/science.4023714. [DOI] [PubMed] [Google Scholar]
- Schneider W. P., Nichols B. P., Yanofsky C. Procedure for production of hybrid genes and proteins and its use in assessing significance of amino acid differences in homologous tryptophan synthetase alpha polypeptides. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2169–2173. doi: 10.1073/pnas.78.4.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. M., Eisenberg D. Correlation of sequence hydrophobicities measures similarity in three-dimensional protein structure. J Mol Biol. 1983 Dec 25;171(4):479–488. doi: 10.1016/0022-2836(83)90041-4. [DOI] [PubMed] [Google Scholar]
- Ulmer K. M. Protein engineering. Science. 1983 Feb 11;219(4585):666–671. doi: 10.1126/science.6572017. [DOI] [PubMed] [Google Scholar]
- Yutani K., Khechinashvili N. N., Lapshina E. A., Privalov P. L., Sugino Y. Calorimetric study of tryptophan synthase alpha-subunit and two mutant proteins. Int J Pept Protein Res. 1982 Oct;20(4):331–336. doi: 10.1111/j.1399-3011.1982.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Aoki K., Kakuno T., Sugino Y. Effect of amino acid residues on conformational stability in eight mutant proteins variously substituted at a unique position of the tryptophan synthase alpha-subunit. J Biol Chem. 1984 Nov 25;259(22):14076–14081. [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Kimura A., Sugino Y. Effect of single amino acid substitutions at the same position on stability of a two-domain protein. J Mol Biol. 1982 Sep 15;160(2):387–390. doi: 10.1016/0022-2836(82)90184-x. [DOI] [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Sugino Y. Effect of amino acid substitutions on conformational stability of a protein. Adv Biophys. 1985;20:13–29. doi: 10.1016/0065-227x(85)90028-0. [DOI] [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Sugino Y., Matsushiro A. Effect of a single amino acid substitution on stability of conformation of a protein. Nature. 1977 May 19;267(5608):274–275. doi: 10.1038/267274a0. [DOI] [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Sugino Y. pH dependence of stability of the wild-type tryptophan synthase alpha-subunit and two mutant proteins (Glu49 replaced by Met or Gln). J Mol Biol. 1980 Dec 25;144(4):455–465. doi: 10.1016/0022-2836(80)90331-9. [DOI] [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Suzuki M., Sugino Y. Comparison of denaturation by guanidine hydrochloride of the wild type tryptophan synthase alpha-subunit of Escherichia coli and two mutant protein (Glu 49 replaced by Met or Gln). J Biochem. 1979 Apr;85(4):915–921. doi: 10.1093/oxfordjournals.jbchem.a132423. [DOI] [PubMed] [Google Scholar]
- Yutani K., Ogasahara K., Suzuki M., Sugino Y. Effect of a single amino acid substitution on the near-ultraviolet CD spectra of tryptophan synthase alpha-subunit. J Biochem. 1980 Jan;87(1):117–121. doi: 10.1093/oxfordjournals.jbchem.a132716. [DOI] [PubMed] [Google Scholar]


