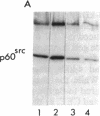
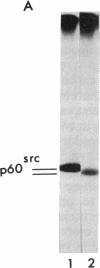
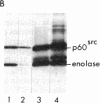
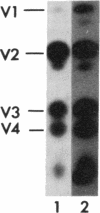
Abstract
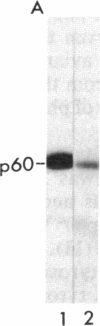
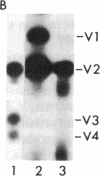
Avian and viral p60src proteins were expressed from a galactose-inducible promoter in the yeast Saccharomyces cerevisiae. Both the viral and cellular src proteins produced in yeast cells were myristoylated at their amino termini, as is the case for src proteins expressed in chicken embryo fibroblasts. The viral src protein produced in yeast autophosphorylated at tyrosine-416 in vivo and had approximately the same level of in vitro kinase activity as p60v-src expressed in Rous sarcoma virus-transformed cells. Unlike p60c-src expressed in chicken cells, which is phosphorylated on tyrosine in vivo almost exclusively at tyrosine-527, p60c-src expressed in yeast was phosphorylated 2.5-3 times more at tyrosine-416 than at tyrosine-527. The specific activity of the p60c-src produced in yeast was 2.5-5.0 times higher than that of p60c-src overexpressed from a retroviral vector in chicken cells, implicating the altered state of in vivo phosphorylation in modulation of the in vitro kinase activity. The expression of p60v-src substantially slowed down the growth of the yeast cells, suggesting that phosphorylation of yeast proteins essential for cell growth may have interfered with their proper functioning.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Mutations in the pho80 gene confer permeability to 5'-mononucleotides in Saccharomyces cerevisiae. Genetics. 1982 Nov;102(3):341–359. doi: 10.1093/genetics/102.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos R. M., Mazón M. J. Identification of phosphotyrosine in yeast proteins and of a protein tyrosine kinase associated with the plasma membrane. J Biol Chem. 1985 Jul 15;260(14):8240–8242. [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B., Jarausch J., Hartmann R., Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal Biochem. 1983 Mar;129(2):517–521. doi: 10.1016/0003-2697(83)90586-9. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hanafusa H. Viral and cellular src genes contribute to the structure of recovered avian sarcoma virus transforming protein. Cell. 1981 Apr;24(1):155–164. doi: 10.1016/0092-8674(81)90511-0. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Cross F. R., Harbison M., Hanafusa H. Transformation of chicken embryo fibroblasts and tumor induction by the middle T antigen of polyomavirus carried in an avian retroviral vector. Mol Cell Biol. 1986 May;6(5):1545–1551. doi: 10.1128/mcb.6.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K., Smart J. E. Homologous tyrosine phosphorylation sites in transformation-specific gene products of distinct avian sarcoma viruses. Nature. 1981 Jun 25;291(5817):675–677. doi: 10.1038/291675a0. [DOI] [PubMed] [Google Scholar]
- Ryder J. W., Gordon J. A. In vivo effect of sodium orthovanadate on pp60c-src kinase. Mol Cell Biol. 1987 Mar;7(3):1139–1147. doi: 10.1128/mcb.7.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieven G., Thorner J., Martin G. S. Protein-tyrosine kinase activity in Saccharomyces cerevisiae. Science. 1986 Jan 24;231(4736):390–393. doi: 10.1126/science.2417318. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Towler D., Glaser L. Protein fatty acid acylation: enzymatic synthesis of an N-myristoylglycyl peptide. Proc Natl Acad Sci U S A. 1986 May;83(9):2812–2816. doi: 10.1073/pnas.83.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp M. L., Piñon R., Meisenhelder J., Hunter T. Identification of phosphoproteins correlated with proliferation and cell cycle arrest in Saccharomyces cerevisiae: positive and negative regulation by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5973–5977. doi: 10.1073/pnas.83.16.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]