Abstract
Various kinds of glycosaminoglycans (GAGs) and proteoglycans (PGs) have been known to be involved in structural and space-filling functions, as well as many physiological regulations in skin. To investigate ultraviolet (UV) radiation-mediated regulation of GAGs and PGs in cultured human dermal fibroblasts, transcriptional changes of many types of PGs and GAG chain-synthesizing enzymes at 18 hr after 75 mJ/cm2 of UV irradiation were examined using quantitative real-time polymerase chain reaction methods. Hyaluronic acid synthase (HAS)-1, -2, and -3 and hyaluronidase-2 mRNA expressions were significantly increased by UV irradiation. Expressions of lumican, fibromodulin, osteoglycin, syndecan-2, perlecan, agrin, versican, decorin, and biglycan were significantly decreased by UV irradiation, while syndecan-1 was increased. Expressions of GAG chain-synthesizing glycosyltransferases, xylosyltransferase-1, β1,3-glucuronyltransferase-1, β1,4-galactosyltransferase-2, -4, exostosin-1, chondroitin polymerizing factor, and chondroitin sulfate synthase-3 were significantly reduced, whereas those of β1,3-galactosyltransferase-6, β1,4-galactosyltransferase-3, -7, β-1,3-N-acetylglucosaminyltran sferase-2, and -7 were increased by UV irradiation. Heparanase-1 mRNA expression was increased, but that of heparanase-2 was reduced by UV irradiation. Time-course investigation of representative genes showed consistent results. In conclusion, UV irradiation may increase hyaluronic acid production through HAS induction, and decrease other GAG productions through downregulation of PG core proteins and GAG chain-synthesizing glycosyltransferases in cultured human dermal fibroblasts.
Keywords: Glycosaminoglycans, Proteoglycans, Glycosyltransferases, Ultraviolet Radiation, Dermal Fibroblasts
INTRODUCTION
Ultraviolet radiation has been known to induce premature skin aging during long human life time (1-4). In photoaged skin, dramatic changes of dermal components such as loss of collagen fiber and deposition of elastotic materials have been found (2, 5); however, changes of glycosaminoglycans (GAGs) during aging and their regulatory mechanisms have not been well-established.
GAGs are long polysaccharide chains consisting of specific disaccharide units, and have structural and physiological regulatory functions (6). Hyaluronic acid (HA) is the most abundant GAG in dermis, synthesized by HA synthase (HAS)-1, -2, and -3 in the plasma membrane of dermal fibroblasts, and secreted to the extracellular spaces as GAG form alone (7). HA turnover is tightly regulated by hyaluronidase-1, -2, and its receptor CD44 (8). Hyaluronidase-2, glycosylphosphatidylinositol-linked to the plasma membrane, cleaves HA, followed by CD44-mediated uptake of HA, and hyaluronidase-1 disintegrates it within lysosome (8).
Other GAGs, such as chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), and heparin (HP), are synthesized in endoplasmic reticulum (ER) and Golgi apparatus as glycosylation to the core proteins of their related proteoglycans (PGs) by numbers of common and GAG type-specific glycosyltransferases (9-11).
Lumican, fibromodulin, osteoglycin, and keratocan, which are members of KSPGs, and decorin, biglycan, and epiphycan, members of CS/DSPGs, belong to small leucine-rich PG family. Some of them have been reported to be involved in collagen fibril formation in cornea, tendon, and/or skin (12-17). Decorin and biglycan are reported to have critical roles in collagen and elastic fibril formation, and versican, a large CSPG, is also involved in elastic formation of microfibrils (17, 18). Epiphycan is known to play roles in maintenance of the joint integrity, but its expression is restricted to cartilage and testis (19). Perlecan, agrin, and collagen XVIII are ubiquitous basement membrane HSPGs, which play essential structural roles and contribute to local gradient formation of HS-binding cytokines, chemokines, and growth factors (20). Perlecan was reported to contribute to epidermal layer formation in the artificial skin model by inhibition of keratinocyte apoptosis (21). Syndecan-1 to -4 are plasma membrane-expressed HSPGs, and known to be involved in cell adhesion, migration, and signal transduction (22).
The initial common tetrasaccharide glycosylation for GAGs on serine residues of PG core proteins is started with a xylose addition in ER by xylosyltransferase-1 and -2, followed by adding two galactoses in cis/medial Golgi by β1,3-galactosyltransferase (B3GALT)-6 and β1,4-galactosyltransferase (B4GALT)-7, and the glucuronic acid is added in the trans Golgi by β1,3-glucuronyltransferase (B3GAT)-1, -2, and -3 (6). Thereafter, KS chain elongation is mediated by B4GALT1-4 and β-1,3-N-acetylglucosaminyltransferase (B3GNT)-1, -2, and -7 (23). CS chain elongation is mediated by chondroitin polymerizing factor (CHPF), CHPF2, CS synthase (CHSY)-1, -3, CS N-acetylgalactosaminyltransferase (CSGALNACT)-1, and -2, and DS chain is converted from CS chain by DS epimerase (10). HS and HP chain elongation is mainly mediated by exostosin-1, -2, and exostosin-like-1 to -3 are partly involved (6). Degradation of HS is mediated by heparanase-1, which has important roles in inflammatory response (20). Heparanase-2 was recently cloned, and its loss of function mutation was found in all urofacial syndrome patients, but its functionality is not still clear (24).
In this study, for estimating GAG and PG expressional changes and their involvement in skin photoaging process, single dose of UV-induced transcriptional changes of various PGs and GAG chain-synthesizing or degrading enzymes were investigated in primary human dermal fibroblasts.
MATERIALS AND METHODS
Cell culture and UV irradiation
Primary human dermal fibroblasts, which were isolated by outgrowth from foreskin of 7- to 30-yr old healthy donors, were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Gibco-BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS), 2 mM glutamine, penicillin (100 U/mL), and streptomycin (100 µg/mL). Cells were incubated at 37℃ in a humidified atmosphere containing 5% CO2, and grown up until > 95% confluence. Thereafter, cells were serum-starved with fresh medium with 0% FBS for 24 hr, washed twice with phosphate buffered saline (PBS), and then irradiated with 0 or 75 mJ/cm2 of UV under thin layer of PBS. Phillips TL 20W/12 RS florescent sun lamps with an emission spectrum between 275 and 380 nm (peak, 310-315 nm) were used as a UV source with a Kodacel filter (TA401/407; Kodak, Rochester, NY, USA) to block UV-C, which has wavelengths below 290 nm. UV strength was measured using a Waldmann UV meter (model 585100, Waldmann, Villingen-Schwenningen, Germany). After UV treatment, cells were incubated with fresh medium with 0% FBS, and further incubated for 6, 12, 18, or 24 hr.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted from UV-irradiated or sham-irradiated cultured dermal fibroblasts using the Trizol method (Invitrogen, Carlsbad, CA, USA), and 1 µg of total RNA was converted to cDNA using First Strand cDNA Synthesis Kit (MBI Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions. To quantitatively estimate the mRNA expression of target genes (Tables 1, 2), PCR was performed on a 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA) using the SYBR Premix Ex Taq™ (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions, using the primer pairs in the Tables 1, 2. The PCR conditions were 50℃ for 2 min, 95℃ for 2 min, followed by 40 cycles at 95℃ for 22 sec and 60℃ for 1 min. Data were analyzed using the comparative Ct method, normalized to 36B4, and presented as the fold changes in gene expression of UV-irradiated cells, relative to the control sham-irradiated cells. These experiments were carried out in duplicate or triplicate, and independently repeated at least three times.
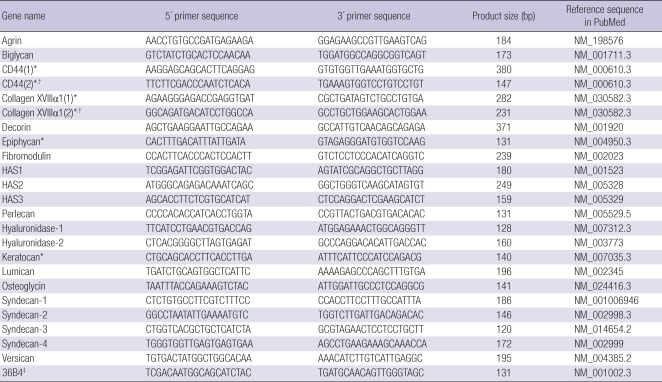
Table 1.
Real-time PCR primer sequences for HA-related genes and proteoglycans
*Not detected in human dermal fibroblasts with listed primer pairs; †Additional primer pair was applied for the undetected genes, but not detected; ‡36B4 was used as an endogenous control gene.
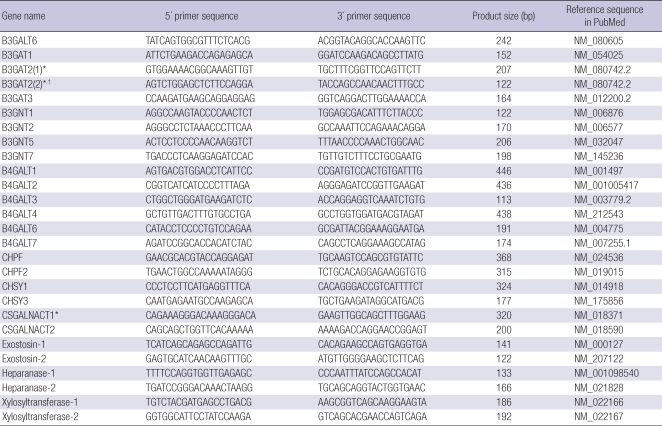
Table 2.
Real-time PCR primer sequences for GAG chain-synthesizing and degrading enzymes
*Not detected in human dermal fibroblasts with listed primer pairs; †Additional primer pair was applied for the undetected genes, but not detected.
Statistics
Statistical analyses between UV-irradiated and control shamirradiated samples in same time point were performed using Mann-Whitney U-test. P values below 0.05 were considered as statistically significant. Results were presented as mean fold changes ± standard deviation versus control sham-irradiated cells.
RESULTS
UV-induced mRNA expression of HA-related genes and various PGs in cultured primary human dermal fibroblasts
UV irradiation is a major cause of skin photoaging, and the induction of MMP-1 and reduction of procollagen by UV irradiation is the most well-known phenomena (1, 2). In our previous studies, statistically significant induction of MMP-1 in primary human dermal fibroblasts was observed at 75 mJ/cm2 or higher doses of UV irradiation (25), 75 mJ/cm2 of UV irradiation well caused both MMP-1 induction and type I procollagen reduction in human dermal fibroblast Hs27 (26). Therefore, we decided to use 75 mJ/cm2 of UV for our experiment.
In order to investigate the UV-induced transcriptional changes of HA-related genes and various PGs, cultured primary human dermal fibroblasts were irradiated with 75 mJ/cm2 of UV, and their mRNA expressions were determined by quantitative realtime PCR at 18 hr after UV irradiation (Fig. 1).
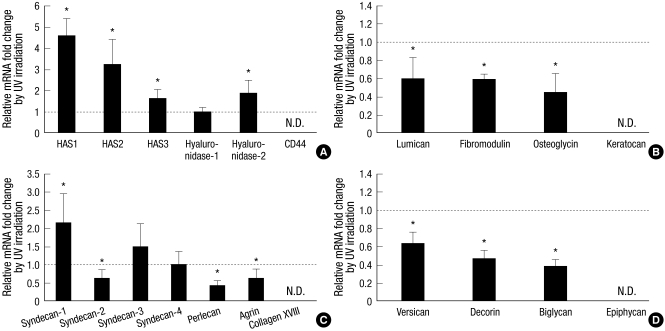
Fig. 1.
Fold changes of UV-induced mRNA expression of HA-related genes and various proteoglycans (PGs) in cultured human dermal fibroblasts. Human dermal fibroblasts were incubated for 18 hr after 75 mJ/cm2 of UV irradiation. Total RNA was isolated from UV-irradiated or sham-irradiated cells, converted to the cDNA, and applied to the quantitative real-time polymerase chain reaction experiments for each target genes. (A) Changes of HA-related genes. (B) Changes of KSPGs. (C) Changes of HSPGs. (D) Changes of CS/DSPGs. Values are mean fold changes ± standard deviation (SD) (n = 3 or 4). *P < 0.05 versus control sham-irradiated cells. N.D. means not detected in both control and UV-irradiated cells. HAS, hyaluronic acid synthase.
First, we examined UV-induced mRNA expression of HA-related genes, including HAS1-3, hyaluronidase-1, -2, and CD44 (Fig. 1A). The mRNA expressions of HAS1-3 and hyaluronidase-2 were significantly increased compared to sham-irradiated control at 18 hr after UV exposure, while that of hyaluronidase-1 was not changed significantly (Fig. 1A). CD44 mRNA expression was not detected with two different primer pairs (Fig. 1A, Table 1).
UV-induced mRNA expressions of members of KSPGs, HSPGs, and CS/DSPGs were also investigated (Fig. 1B-D). The mRNA expression levels of KSPGs such as lumican, fibromodulin, and osteoglycin were significantly downregulated at 18 hr by UV irradiation compared to sham-irradiated control, while keratocan was not detected (Fig. 1B). The mRNA expression levels of some HSPGs such as syndecan-2, perlecan, and agrin were significantly reduced, but that of syndecan-1 was significantly increased at 18 hr by UV irradiation (Fig. 1C). Those of syndecan-3 and -4 were not changed by UV irradiation, and collagen XVIII was not detected with two different primer pairs (Fig. 1C, Table 1). The mRNA expression levels of CS/DSPGs such as versican, decorin, and biglycan were also significantly decreased by UV irradiation, while epiphycan was not detected (Fig. 1D).
UV-induced mRNA expression of GAG chain-synthesizing glycosyltransferases in primary cultured human dermal fibroblasts
Since UV-mediated regulation of GAG production may be affected by regulation of not only core proteins but also GAG chain-synthesizing glycosyltransferases, we also investigated mRNA expressions of common and GAG type-specific glycosyltransferases in human dermal fibroblasts by quantitative real-time PCR at 18 h after UV irradiation (Fig. 2).
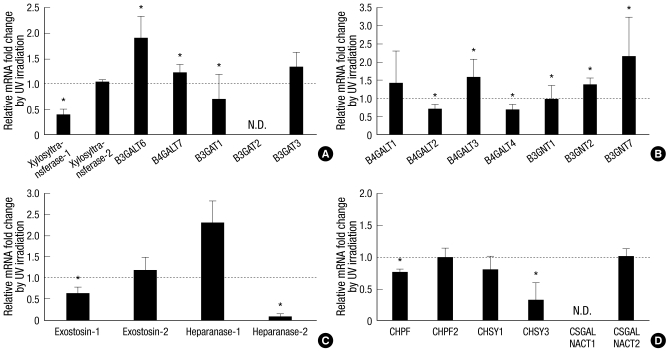
Fig. 2.
Fold changes of UV-induced mRNA expression of GAG chain-synthesizing glycosyltransferases in cultured human dermal fibroblasts. Human dermal fibroblasts were incubated for 18 hr after 75 mJ/cm2 of UV irradiation. Total RNA was isolated from UV-irradiated or sham-irradiated cells, converted to the cDNA, and applied to the quantitative real-time polymerase chain reaction experiments for each target genes. (A) Changes of GAG chain-synthesizing common glycosyltransferases. (B) Changes of KS chain-synthesizing glycosyltransferases. (C) Changes of HS chain-synthesizing glycosyltransferases and heparanases. (D) Changes of CS and DS chain-synthesizing glycosyltransferases. Values are mean fold changes ± SD (n = 3 or 4). *P < 0.05 versus control sham-irradiated cells. N.D. means not detected in both control and UV-irradiated cells. B3GALT, β1,3-galactosyltransferase; B4GALT, β1,4-galactosyltransferase; B3GAT, β1,3-glucuronyltransferase; B3GNT, β-1,3-N-acetylglucosaminyltransferase; CHPF, chondroitin polymerizing factor; CHSY, chondroitin sulfate synthase; CSGALNACT, chondroitin sulfate N-acetylgalactosaminyltransferase.
The glycosyltransferases responsible for the common tetrasaccharide were firstly examined (Fig. 2A). The mRNA expressions of xylosyltransferase-1 and B3GAT1 were significantly reduced by UV irradiation, and those of B3GALT6 and B4GALT7 were increased by UV irradiation (Fig. 2A). Those of xylosyltransferase-2 and B3GAT3 were not changed significantly, while that of B3GAT2 was not detected with two different primer pairs (Fig. 2A, Table 2).
KS chain-synthesizing glycosyltransferases were also investigated, and reduced mRNA expressions of B4GALT2 and B4GALT4 by UV irradiation were observed, while B4GALT3, B3GNT2, and B3GNT7 mRNA levels were increased (Fig. 2B). B4GALT1 and B3GNT1 mRNA expressions were not significantly changed by UV irradiation (Fig. 2B).
An HS chain-synthesizing glycosyltransferase, exostosin-1, was significantly downregulated by UV irradiation, but exostosin-2 did not changed compared to sham-irradiated control (Fig. 2C). An HS-degrading enzyme, heparanase-1, was significantly increased, but heparanase-2 was decreased by UV irradiation (Fig. 2C).
CS chain-synthesizing glycosyltransferases, including CHPF, CHPF2, CHSY1, CHSY3, CSGALNACT1, and CSGALNACT2, were investigated, and CHPF and CHSY3 were found to be significantly downreglated by UV irradiation (Fig. 2D). The mRNA expressions of CHPF2, CHSY1, and CSGALNACT2 were not significantly changed, while CSGALNACT1 was not detected (Fig. 2D).
Time-dependent mRNA expression of several PGs and GAG chain-synthesizing glycosyltransferases by UV irradiation in primary cultured human dermal fibroblasts
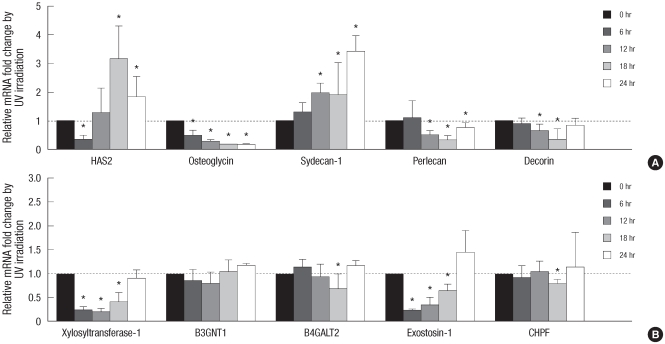
To further verify those changes, UV irradiation-induced time-dependent changes of several representative proteoglycans and GAG chain-synthesizing glycosyltransferases at 6, 12, 18, and 24 hr after irradiation were additionally investigated (Fig. 3). By UV irradiation, decorin, perlecan, osteoglycin, xylosyltransferase-1, and exostosin-1 showed decreased mRNA expression patterns in various times points, and syndecan-1 showed increased patterns (Fig. 3). HAS2 showed reduced mRNA expression at 6 hr, but increased mRNA expression at 18 and 24 hr after UV irradiation (Fig. 3). B4GALT2 and CHPF showed UV-mediated significant decrease of mRNA expression only at 18 hr, and B3GNT1 did not show any significant change (Fig. 3).
Fig. 3.
Time-dependent fold changes of UV-induced mRNA expression of HA-related genes, proteoglycans, and GAG chain-synthesizing glycosyltransferases in cultured human dermal fibroblasts. Human dermal fibroblasts were incubated for 6, 12, 18, or 24 hr after 75 mJ/cm2 of UV irradiation. Total RNA was isolated from UV-irradiated or sham-irradiated cells, converted to the cDNA, and applied to the quantitative real-time polymerase chain reaction experiments for each target genes. (A) Changes of HAS2, osteoglycin, syndecan-1, perlecan, and decorin. (B) Changes of xylosyltransferase-1, B3GNT1, B4GALT2, exostosin-1, and CHPF. Values are mean fold changes ± SD (n = 3 or 4). *P < 0.05 versus control sham-irradiated cells at each time point.
DISCUSSION
In this study, we tried to examine overall regulation of GAG production by UV irradiation in human dermal fibroblasts for the estimation of GAG changes and their contributions in skin photoaging process, and demonstrated that the mRNA levels of various PGs containing KS, HS, CS, or DS were downregulated by UV irradiation, while those of HAS family members were increased. These results suggest that UV irradiation may result in an increased HA production but reduced other GAG production in human dermis.
UV-induced increase of HAS1, 2, and 3 expression is similar with the previous report that mRNA expressions of HAS1 and HAS2 increased at 24 hr following UV irradiation, but not HAS3 (7). In that study, mRNA levels of hyaluronidase-2 and -3 were increased at 24 hr after UV irradiation but not hyaluronidase-1 (7), similar with our result that hyaluronidase-2 expression was increased by UV irradiation, but not hyaluronidase-1. However, other study showed that hyaluronidase-1 and -2 mRNA had no changes at 12 hr and 24 hr after UV-irradiation in cultured normal human keratinocytes (27). Time-course experiments of HAS2 showed significant increase of mRNA by UV irradiation at 18 and 24 hr, and significant decrease at 6 hr. This early decrease is also consistent with previous data (7), but its meaning remains unelucidated. CD44 mRNA expression was not detected in dermal fibroblasts in our experiment, although it can be detected in cultured normal human keratinocytes with same primers (data not shown), which should be further investigated.
Transcriptional levels of all detected PGs were downregulated by UV irradiation, except syndecan-1, -3, and -4. Time-course experiments of decorin, osteoglycin, perlecan, and syndecan-1 also revealed consistent patterns of UV effects. These downregulation of core protein expression may imply reduction of related GAG production. No evidence on UV irradiation-mediated PG expressions has been published, except decorin. Immunohistochemical stain and mRNA expression of decorin were reported to be decreased in UVA or UVB-irradiated human skin (28).
As similar, mRNA expressions of several GAG chain-synthesizing glycosyltransferases were also reduced by UV irradiation, including xylosyltransferase-1, B3GAT1 (GAG chain initializing enzymes), B4GALT2, B4GALT4 (KS-synthesizing enzymes), exostosin-1 (an HS-synthesizing enzyme), CHPF, and CHSY3 (CS-synthesizing enzymes), while those of B3GALT6, B4GALT7 (GAG chain initializing enzymes), B4GALT3, B3GNT2, and 7 (KS-synthesizing enzymes) were increased. Increase of heparanase-1 mRNA expression was also observed. In time-course experiments, xylosyltransferase-1 showed decreased mRNA expression from 6 to 24 hr, but B4GALT2 and CHPF showed decreased pattern only at 18 hr, implying their relatively less decrease than that of PGs. No evidence on UV irradiation-mediated GAG chain-synthesizing glycosyltransferase expressions has been published. These results imply that UV irradiation-induced transcriptional changes of GAG chain-synthesizing glycosyltransferases are complicated, but may result in reduced GAG production through significant downregulation of xylosyltransferase-1 and exostosin-1 expression, which are the first enzyme initializing GAG-chain synthesis and HS/HP chain synthesis enzyme, respectively (6).
Since detected KSPGs and CS/DSPGs are involved in collagen and/or elastic fiber network formation (12-18), their reduction may have weakening effects on dermal matrix structures with downregulation of procollagen I by UV.
Since perlecan has anti-apoptotic function by growth factor binding affinity in dermal-epidermal junction (21), downregulation of perlecan and agrin by UV irradiation may result in increased keratinocyte apoptosis in epidermis.
Syndecans are known to modulate inflammatory processes in the lung by regulating chemokines, and act as co-receptors for several growth factors and chemokines, mediating various intracellular signaling (20). Therefore, UV-induced changes of syndecan-1 and -2 expressions may participate in inflammatory regulation induced by UV. However, further evidence should be necessary.
In this study, we examined mRNA transcriptional changes of various PGs and glycosyltransferases by UV irradiation, which may imply increase of HA and decrease of other GAGs; however, there is limitation of our study that protein level changes of each target were not investigated, and it will be our next goal of further study. In our speculation, UV-induced increase of HA seems to be an unexpected beneficial result. Therefore, it is possible that, since GAGs are involved in maintenance of tissue water (6), decrease of GAGs may cause temporary unbalance of water homeostasis, and thereby increase of HA may be induced for its rescue.
In conclusion, UV irradiation may increase HA production through induction of HAS mRNA expressions, and decrease other GAG productions through downregulation of various PG core proteins and GAG chain-synthesizing glycosyltransferases in cultured human dermal fibroblasts.
Footnotes
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080283), by a grant (0420070470) from the SNUH Research Fund, by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-355-E00014), and by a research agreement with the Amore-Pacific Corporation, Seoul, Korea.
AUTHOR SUMMARY
Transcriptional Regulation of Proteoglycans and Glycosaminoglycan Chain-synthesizing Glycosyltransferases by UV Irradiation in Cultured Human Dermal Fibroblasts
Jeong-Eun Shin, Jang-Hee Oh, Yeon Kyung Kim, Ji-Yong Jung, and Jin Ho Chung
Glycosaminoglycans (GAGs) and proteoglycans (PGs) are involved in structural and space-filling functions, as well as many physiological regulations in skin. However, their changes during photoaging have not been well-elucidated. Therefore, ultraviolet (UV) irradiation-mediated transcriptional changes of many types of PGs and GAG chain-synthesizing enzymes were investigated in human dermal fibroblasts. After UV irradiation, hyaluronic acid synthase (HAS) 1-3 and hyaluronidase-2 mRNA expressions were increased, and expressions of lumican, fibromodulin, osteoglycin, syndecan-2, perlecan, agrin, versican, decorin, and biglycan were decreased, while syndecan-1 was increased. Expressions of GAG chain-synthesizing glycosyltransferases, xylosyltransferase-1, β1,3-glucuronyltransferase-1, β1,4-galactosyltransferase-2, -4, exostosin-1, chondroitin polymerizing factor, and chondroitin sulfate synthase-3 were reduced, whereas β1,3-galactosyltransferase-6, β1,4-galactosyltransferase-3, -7, β-1,3-N-acetylglucosa minyltransferase-2, and -7 expressions were increased. Heparanase-1 expression was increased, but heparanase-2 was reduced. Therefore, UV irradiation may increase HA production through HAS induction, and decrease other GAG productions through downregulation of PG core proteins and GAG chain-synthesizing glycosyltransferases in cultured human dermal fibroblasts.
References
- 1.Chung JH. Photoaging in Asians. Photodermatol Photoimmunol Photomed. 2003;19:109–121. doi: 10.1034/j.1600-0781.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 2.Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, Cho KH, Kim KH, Park KC, Eun HC. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001;117:1218–1224. doi: 10.1046/j.0022-202x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim EJ, Kim MK, Jin XJ, Oh JH, Kim JE, Chung JH. Skin aging and photoaging alter fatty acids composition, including 11,14,17-eicosatrienoic acid, in the epidermis of human skin. J Korean Med Sci. 2010;25:980–983. doi: 10.3346/jkms.2010.25.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin XJ, Kim EJ, Oh IK, Kim YK, Park CH, Chung JH. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J Korean Med Sci. 2010;25:930–937. doi: 10.3346/jkms.2010.25.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo JY, Lee SH, Youn CS, Choi HR, Rhie GE, Cho KH, Kim KH, Park KC, Eun HC, Chung JH. Ultraviolet radiation increases tropoelastin mRNA expression in the epidermis of human skin in vivo. J Invest Dermatol. 2001;116:915–919. doi: 10.1046/j.1523-1747.2001.01358.x. [DOI] [PubMed] [Google Scholar]
- 6.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: hostassociated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 7.Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 2007;127:687–697. doi: 10.1038/sj.jid.5700614. [DOI] [PubMed] [Google Scholar]
- 8.Carrino DA, Onnerfjord P, Sandy JD, Cs-Szabo G, Scott PG, Sorrell JM, Heinegård D, Caplan AI. Age-related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem. 2003;278:17566–17572. doi: 10.1074/jbc.M300124200. [DOI] [PubMed] [Google Scholar]
- 9.Trowbridge JM, Gallo RL. Dermatan sulfate: new functions from an old glycosaminoglycan. Glycobiology. 2002;12:117R–125R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 10.Pavão MS, Vilela-Silva AC, Mourão PA. Biosynthesis of chondroitin sulfate: from the early, precursor discoveries to nowadays, genetics approaches. Adv Pharmacol. 2006;53:117–140. doi: 10.1016/S1054-3589(05)53006-0. [DOI] [PubMed] [Google Scholar]
- 11.Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svensson L, Aszódi A, Reinholt FP, Fässler R, Heinegård D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 14.Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- 15.Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002;8:407–415. [PubMed] [Google Scholar]
- 16.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 17.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 18.Reinboth B, Hanssen E, Cleary EG, Gibson MA. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J Biol Chem. 2002;277:3950–3957. doi: 10.1074/jbc.M109540200. [DOI] [PubMed] [Google Scholar]
- 19.Nuka S, Zhou W, Henry SP, Gendron CM, Schultz JB, Shinomura T, Johnson J, Wang Y, Keene DR, Ramirez-Solis R, Behringer RR, Young MF, Hook M. Phenotypic characterization of epiphycan-deficient and epiphycan/biglycan double-deficient mice. Osteoarthritis Cartilage. 2010;18:88–96. doi: 10.1016/j.joca.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 21.Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage. 2006;14:1147–1154. doi: 10.1016/j.joca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 23.Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 24.Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, Ochoa B, Agundez JA, Voelckel MA, Fisher RB, Gu W, Xiong WC, Mei L, She JX, Wang CY. Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet. 2010;86:957–962. doi: 10.1016/j.ajhg.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MK, Shin JM, Eun HC, Chung JH. The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PLoS One. 2009;4:e4864. doi: 10.1371/journal.pone.0004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho S, Kim HH, Lee MJ, Lee S, Park CS, Nam SJ, Han JJ, Kim JW, Chung JH. Phosphatidylserine prevents UV-induced decrease of type I procollagen and increase of MMP-1 in dermal fibroblasts and human skin in vivo. J Lipid Res. 2008;49:1235–1245. doi: 10.1194/jlr.M700581-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Kakizaki I, Itano N, Kimata K, Hanada K, Kon A, Yamaguchi M, Takahashi T, Takagaki K. Up-regulation of hyaluronan synthase genes in cultured human epidermal keratinocytes by UVB irradiation. Arch Biochem Biophys. 2008;471:85–93. doi: 10.1016/j.abb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Gambichler T, Tomi NS, Skrygan M, Altmeyer P, Kreuter A. Significant decrease of decorin expression in human skin following short-term ultraviolet exposures. J Dermatol Sci. 2007;45:203–205. doi: 10.1016/j.jdermsci.2006.10.012. [DOI] [PubMed] [Google Scholar]