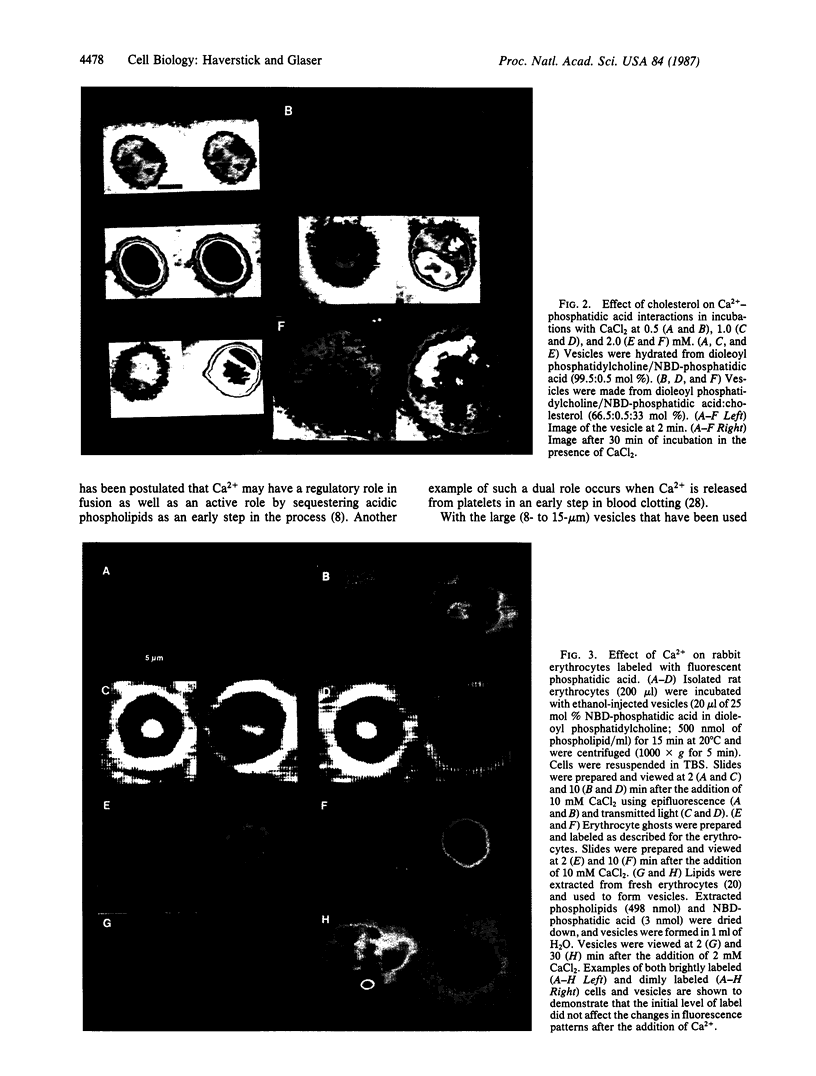
Abstract
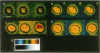
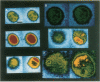
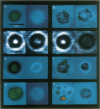
Large vesicles (5-15 microns) were formed by hydrating a dried lipid film containing phospholipids labeled with a fluorophore in one fatty acid chain. By using a fluorescence microscope attached to a low-light-intensity charge-coupled-device camera and digital-image processor, the vesicles were easily viewed and initially showed uniform fluorescence intensity across the surface. The fluorescence pattern of vesicles made with a fluorophore attached to phosphatidylcholine or phosphatidylethanolamine was unaffected by the presence of divalent cations such as Ca2+, Mg2+, Mn2+, Zn2+, or Cd2+. The fluorescence pattern of vesicles containing a fluorophore attached to the acidic phospholipids phosphatidylserine or phosphatidic acid showed distinct differences when treated with Ca2+ or Cd2+, although they were unaffected by Mg2+, Mn2+, or Zn2+. Treatment with 2.0 mM Ca2+ or Cd2+ resulted in the movement of the fluorophore to a single large patch on the surface of the vesicle. When vesicles were formed in the presence of 33 mol % cholesterol, patching was seen at a slightly lower Ca2+ concentration (1.0 mM). The possibility of interactions between Ca2+ and acidic phospholipids in plasma membranes was investigated by labeling erythrocytes and erythrocyte ghosts with fluorescent phosphatidic acid. When Ca2+ was added, multiple (five or six) small patches were seen per individual cell. The same pattern was observed when vesicles formed from whole lipid extracts of erythrocytes were labeled with fluorescent phosphatidic acid and then treated with Ca2+. This shows that the size and distribution of the Ca2+-induced domains depend on phospholipid composition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Darszon A., Vandenberg C. A., Schönfeld M., Ellisman M. H., Spitzer N. C., Montal M. Reassembly of protein-lipid complexes into large bilayer vesicles: perspectives for membrane reconstitution. Proc Natl Acad Sci U S A. 1980 Jan;77(1):239–243. doi: 10.1073/pnas.77.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Feigenson G. W. Fluorescence quenching in model membranes. Biophys J. 1982 Jan;37(1):165–165. doi: 10.1016/S0006-3495(82)84652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson G. W. Polypeptide clearing in model membranes: an analysis of the partition of gramicidin a' between cadmium ion induced gel and liquid-crystalline phases in vesicles of phosphatidic acid and phosphatidylcholine. Biochemistry. 1983 Jun 21;22(13):3106–3112. doi: 10.1021/bi00282a013. [DOI] [PubMed] [Google Scholar]
- Hoekstra D. Role of lipid phase separations and membrane hydration in phospholipid vesicle fusion. Biochemistry. 1982 Jun 8;21(12):2833–2840. doi: 10.1021/bi00541a004. [DOI] [PubMed] [Google Scholar]
- Iot T., Ohnish S., Ishinaga M., Kito M. Synthesis of a new phosphatidylserine spin-label and calcium-induced lateral phase separation in phosphatidylserine-phosphatidylcholine membranes. Biochemistry. 1975 Jul 15;14(14):3064–3069. doi: 10.1021/bi00685a004. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Esker M. W., Pathmamanoharan C., Wiersema P. H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry. 1977 Aug 23;16(17):3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- Lee B., McKenna K., Bramhall J. Kinetic studies of human erythrocyte membrane resealing. Biochim Biophys Acta. 1985 Apr 26;815(1):128–134. doi: 10.1016/0005-2736(85)90482-1. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Tamm L. K., Weis R. M. Periodic structures in lipid monolayer phase transitions. Proc Natl Acad Sci U S A. 1984 May;81(10):3249–3253. doi: 10.1073/pnas.81.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. J., Gibson C. C., Smith P. D., Greif P. C., Stirk C. W., Bradley D., Haynes D. H., Blumenthal R. Rapid kinetics of Ca2+-induced fusion of phosphatidylserine/phosphatidylethanolamine vesicles. The effect of bilayer curvature on leakage. J Biol Chem. 1985 Apr 10;260(7):4122–4127. [PubMed] [Google Scholar]
- Newton C., Pangborn W., Nir S., Papahadjopoulos D. Specificity of Ca2+ and Mg2+ binding to phosphatidylserine vesicles and resultant phase changes of bilayer membrane structure. Biochim Biophys Acta. 1978 Jan 19;506(2):281–287. doi: 10.1016/0005-2736(78)90398-x. [DOI] [PubMed] [Google Scholar]
- Nichols J. W., Pagano R. E. Kinetics of soluble lipid monomer diffusion between vesicles. Biochemistry. 1981 May 12;20(10):2783–2789. doi: 10.1021/bi00513a012. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J., Martin O. C. Intracellular translocation and metabolism of a fluorescent phosphatidic acid analogue in cultured fibroblasts. J Biol Chem. 1983 Feb 10;258(3):2034–2040. [PubMed] [Google Scholar]
- Pagano R. E., Longmuir K. J., Martin O. C., Struck D. K. Metabolism and intracellular localization of a fluorescently labeled intermediate in lipid biosynthesis within cultured fibroblasts. J Cell Biol. 1981 Dec;91(3 Pt 1):872–877. doi: 10.1083/jcb.91.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977 Mar 17;465(3):579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- Peters R., Beck K. Translational diffusion in phospholipid monolayers measured by fluorescence microphotolysis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7183–7187. doi: 10.1073/pnas.80.23.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., Papahadjopoulos D. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 1979 Mar 6;18(5):780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Parsegian V. A. Mimicry and mechanism in phospholipid models of membrane fusion. Annu Rev Physiol. 1986;48:201–212. doi: 10.1146/annurev.ph.48.030186.001221. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Pagano R. E. Capping of a phospholipid analog in the plasma membrane of lymphocytes. Cell. 1981 Jan;23(1):105–112. doi: 10.1016/0092-8674(81)90275-0. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Schroit A. J. Calcium/phosphate-induced immobilization of fluorescent phosphatidylserine in synthetic bilayer membranes: inhibition of lipid transfer between vesicles. Biochemistry. 1986 Apr 22;25(8):2141–2148. doi: 10.1021/bi00356a044. [DOI] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]