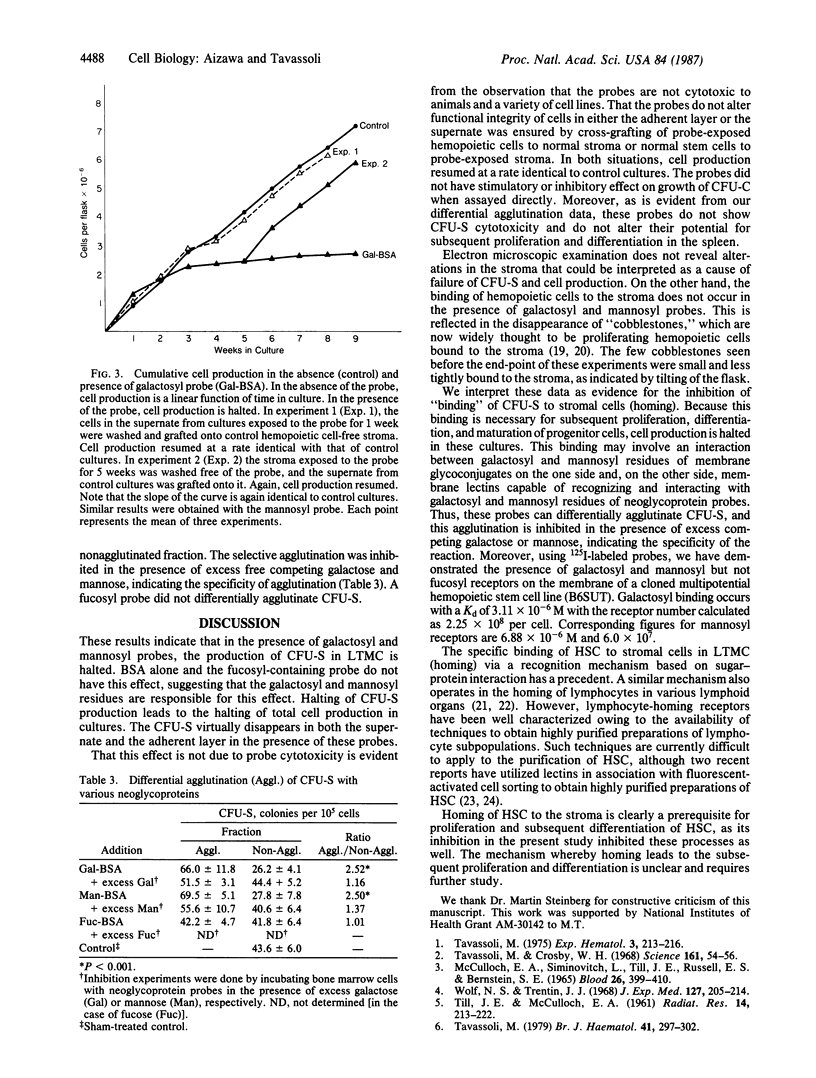
Abstract
We synthesized a number of neoglycoprotein probes by covalently linking three biologically relevant sugars (mannose, galactose, and fucose) to a protein molecule so as to retain the pyranose (ring) form of sugars necessary for their interaction with lectins. In the presence of galactosyl and mannosyl but not fucosyl probes, the production of CFU-S [colony-forming unit(s) in spleen] and total cells was halted in murine long-term marrow cultures. Cobblestone areas disappeared in these cultures, indicating the inhibition of binding of hemopoietic cells to the stroma. Electron microscopy revealed no alterations of the stroma, and the probes did not have direct cytotoxic or inhibitory effects on the growth of CFU-S or CFU-C [colony-forming unit(s) in culture]. Stroma grown for 5 weeks in the presence of the probes could subsequently support the growth of hemopoietic progenitor cells when the probes were removed from the medium. Conversely, the proliferative capacity of CFU-S in the supernate, grown in the presence of the probes, was retained upon grafting to control stroma. Galactosyl and mannosyl but not fucosyl probes differentially agglutinated CFU-S in whole-marrow-cell suspensions, suggesting the presence of membrane lectins with specificity for these sugars on the surface of CFU-S. We conclude that the binding of CFU-S to marrow stroma (homing) is mediated by a recognition system with galactosyl and mannosyl specificities.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. D., Dexter T. M. Cellular interrelationships during in vitro granulopoiesis. Differentiation. 1976 Oct 7;6(3):191–194. doi: 10.1111/j.1432-0436.1976.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Bauman J. G., Wagemaker G., Visser J. W. A fractionation procedure of mouse bone marrow cells yielding exclusively pluripotent stem cells and committed progenitors. J Cell Physiol. 1986 Jul;128(1):133–142. doi: 10.1002/jcp.1041280120. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Gallatin M., St John T. P., Siegelman M., Reichert R., Butcher E. C., Weissman I. L. Lymphocyte homing receptors. Cell. 1986 Mar 14;44(5):673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Ichikawa Y. Control of macrophage and granulocyte colony formation by two different factors. Exp Cell Res. 1977 Nov;110(1):79–85. doi: 10.1016/0014-4827(77)90272-5. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969 Aug 16;223(5207):710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Tavassoli M. Synthetic neoglycoproteins: a class of regents for detection of sugar-recognizing substances. J Histochem Cytochem. 1984 Oct;32(10):1091–1098. doi: 10.1177/32.10.6434628. [DOI] [PubMed] [Google Scholar]
- McCulloch E. A., Siminovitch L., Till J. E., Russell E. S., Bernstein S. E. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl-Sld. Blood. 1965 Oct;26(4):399–410. [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Staber F. G., Johnson G. R., Metcalf D., Battye F. L. Differential expression of lectin receptors during hemopoietic differentiation: enrichment for granulocyte-macrophage progenitor cells. J Cell Physiol. 1980 May;103(2):217–237. doi: 10.1002/jcp.1041030207. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Itzicovitch L., Meshorer A., Sharon N. Hemopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2933–2936. doi: 10.1073/pnas.75.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. Bone-marrow transplantation: man and mouse. Nature. 1979 Aug 30;280(5725):720–721. doi: 10.1038/280720a0. [DOI] [PubMed] [Google Scholar]
- Samlowski W. E., Daynes R. A. Bone marrow engraftment efficiency is enhanced by competitive inhibition of the hepatic asialoglycoprotein receptor. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2508–2512. doi: 10.1073/pnas.82.8.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M., Tenforde T. S., Rosen S. D. Phosphomannosyl receptors may participate in the adhesive interaction between lymphocytes and high endothelial venules. J Cell Biol. 1984 Oct;99(4 Pt 1):1535–1540. doi: 10.1083/jcb.99.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Tavassoli M., Crosby W. H. Transplantation of marrow to extramedullary sites. Science. 1968 Jul 5;161(3836):54–56. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- Tavassoli M. Radiosensitivity of stromal cells responsible for in vitro maintenance of hemopoietic stem cells in continuous, long-term marrow culture. Exp Hematol. 1982 May;10(5):435–443. [PubMed] [Google Scholar]
- Tavassoli M. Studies on hemopoietic microenvironments. Report of a workshop held in La Jolla,, California, August 8-9, 1974. Exp Hematol. 1975 Aug;3(4):213–226. [PubMed] [Google Scholar]
- Tavassoli M., Takahashi K. Morphological studies on long-term culture of marrow cells: characterization of the adherent stromal cells and their interactions in maintaining the proliferation of hemopoietic stem cells. Am J Anat. 1982 Jun;164(2):91–111. doi: 10.1002/aja.1001640202. [DOI] [PubMed] [Google Scholar]
- Tavassoli M. The marrow-blood barrier. Br J Haematol. 1979 Mar;41(3):297–302. doi: 10.1111/j.1365-2141.1979.tb05862.x. [DOI] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N. S., Trentin J. J. Hemopoietic colony studies. V. Effect of hemopoietic organ stroma on differentiation of pluripotent stem cells. J Exp Med. 1968 Jan 1;127(1):205–214. doi: 10.1084/jem.127.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]