Abstract
AIM: To analyze the upregulated CD133 expression in tumorigenesis of primary colon cancer cells.
METHODS: Upregulated CD133 expression in tumorigenesis of colorectal cancer cell lines (Lovo, Colo205, Caco-2, HCT116 and SW620) was analyzed by flow cytometry. Human colon cancer tissue samples were stained with anti-human CD133. SW620 cells were sorted according to the CD133 expression level measured by fluorescence-activated cell sorting. Spheroids of colorectal cancer cells were cultured with the hanging drop. Expression of CD133 and Lgr5 in spheroids of colorectal cancer cells and monolayer culture was detected by RT-qPCR. Spheroids of colorectal cancer cells were analyzed using anti-human CD133 with immunohistochemical staining.
RESULTS: CD133 antigen was expressed in colorectal cancer cell lines (Lovo, Colo205, Caco-2, HCT116 and SW620) as well as in primary and metastatic human colon cancer tissues. However, the CD133 was differently expressed in these cell lines and tissues. The expression levels of CD133 and Lgr5 were significantly higher in spheroids of parental, CD133hi and CD133- cells than in their monolayer culture at the mRNA level (P < 0.05). Immunohistochemical staining of spheroids of CD133- cells showed that CD133 was highly expressed in colorectal cancer cell lines.
CONCLUSION: Upregulated CD133 expression plays a role in tumorigenesis colorectal cancer cells, which may promote the expression of other critical genes that can drive tumorigenesis.
Keywords: CD133, Colon cancer cells, Tumorigenesis, Cancer stem cells
INTRODUCTION
CD133, also known as prominin-1, a transmembrane pentaspan protein, is originally described as a surface antigen specific for human hematopoietic stem and progenitor cells[1,2]. Later, CD133 is recognized as a stem cell marker for other normal tissues of brain[3], kidney[4], prostate[5], liver[6], pancreas[7], and skin[8]. It has been increasingly reported that CD133 is a marker of putative cancer stem cells (CSC) in brain tumor[9,10], prostate cancer[11], colon cancer[12-14], lung cancer[15], hepatocellular carcinoma[16], melanoma[17], ovarian cancer[18], and pancreatic cancer[19]. Accordingly, CD133 has been referred to as “the molecule of the moment”[20].
It has been recently shown that CD133 expression is broadly distributed in primary colon cancer cells including cancer stem cells, both CD133+ and CD133- metastatic colon cancer cells initiate tumors[21-23]. However, whether CD133 expression plays a role in tumorigenesis of colorectal cancer cells is unknown.
In the present study, upregulated CD133 expression in several colorectal cancer cell lines as well as in human primary and metastatic colon cancer tissue samples was analyzed. SW620 cell line was sorted using CD133 antigen. Spheroids of parental, CD133- and CD133hi cells were cultured with the hanging drop. Expressions of CD133 and Lgr5 were detected in spheroids of colorectal cancer cells. CD133 was widely expressed in human colorectal cancer cell lines as well as in primary and metastatic colon cancer tissues and upregulated CD133 expression was detected in spheroids of colorectal cancer cells, indicating that upregulated CD133 expression may promote the expression of other critical genes that can drive tumorigenesis.
MATERIALS AND METHODS
Cell lines and cell culture and tissue samples
Human colorectal cancer cell lines (Lovo, Colo205, Caco-2, HCT116 and SW620) were cultured in RPMI1640 medium containing 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, 10 μmol/L thioglycerol, 12.5 U insulin, 0.5 mg hydrocostisone, and 30mg penicillin G/0.05 g streptomycin. Colorectal cancer cells were cultured at 37°C in a humidified atmosphere containing 10% CO2. CD133 expression was detected in formalin-fixed, paraffin-embedded primary and metastatic colorectal cancer tissue samples from Affiliated Sixth People’s Hospital of Shanghai Jiaotong University. The study was approved by the Ethics Committee of Affiliated Sixth People’s Hospital of Shanghai Jiaotong University.
Fluorescence-activated cell sorting
Single-cell suspensions were stained with antibodies against human CD133 (AC133, 1:40) and human CD133/1 and CD133/2(1:10, APC conjugated, Miltenyi Biotech, Germany). Dead cells, cell debris, doublets and aggregates were excluded by forward and side scattering and pulse-width gating. Colorectal cancer ells (1 × 105) were stained in an eppendorf tube. Primary antibody was incubated for 45 min on ice and second antibody (anti-mouse Alexa488, 1:400) was incubated for 30 min on ice in the dark. Flow cytometry analysis was carried out on a fluorescence-activated cell sorting (FACS) caliber (BD). Colorectal cancer ells (1 × 106) were prepared for sorting, stained with human CD133/1 (1:10, APC conjugated, Miltenyi Biotech) and 1 μg/mL propidium iodide (PI) to exclude dead cells during sorting. The cells were sorted using FACSAria (BD). Matched isotype antibodies were applied in parallel as controls.
Colon spheroids were culture with hanging drop
SW620 colorectal cancer cells and their sorted CD133- and CD133hi cells were prepared as a single cell suspension. The cells were counted and diluted in RPMI1640 containing 20% FBS and antibiotics to a concentration of 500 cells per 20 μL/drop in a sterile basin. The lid was lifted, inverted and placed on top of the dish containing 10 mL PBS. An 8-channel pipette was used to make rows of 20 μL drops on the up-turned inner surface of the tissue culture dish lid. The drops were incubated at 37°C in an atmosphere containing 10% CO2 for 10 d.
Immunohistochemistry
Frozen sections of the spheroids of colorectal cancer cells were fixed in acetone at -20°C for 10 min and rehydrated in PBS. Endogenous peroxidase was inactivated by immersing the sections in 0.3% hydrogen peroxide for 20 min. The primary antibody for frozen sections of the spheroids of colorectal cancer cells and paraffin-embedded sections of colorectal cancer tissue samples was a mouse anti-human monoclonal CD133/2 (1:40, Miltenyi Biotech, Germany) and a rabbit anti-human polyclonal CD133 (1:100, Abcam, England), respectively. The sections were incubated overnight at 4°C in a humidified chamber, then with biotinylated secondary antibody (VECTASTAIN ABC kit, Vector Laboratories) for 30 min at room temperature. Each section was incubated with the VECTASTAIN ABC reagent for 30 min at room temperature. The sections were developed using the DAB (Vector Laboratories) as the substrate and then counterstained with hematoxylin. The negative control was performed by incubating samples with PBS.
Quantification of CD133 expression by quantitative polymerase chain reaction
Total RNA was isolated from cultured colorectal cancer cells and their spheroids using the RNeasy extraction kit (GE Healthcare) and reverse transcribed using high-capacity cDNA reverse transcription kit (Applied Biosystems) according to their manufacturer’s instructions, respectively. Relative quantitative polymerase chain reaction (PCR) was performed on a 7300 fast real-time PCR system (Applied Biosystems) using SYBR green PCR master mix (Applied Biosystems). The human-specific intron spanning primer pairs for CD133 were provided by QIAGEN (Catalog number: QT00075586). The sequences of primer pairs used for GAPDH and Lgr5 are CAATGACCCCTTCATTGACC (forward) and TGATGACAAGCTTCCCGTTC (reverse), and CTTCCAACCTCAGCGTCTTC (forward) and TTTCCCGCAAGACGTAACTC (reverse), respectively. PCR was performed for 1 cycle at 50°C for 2 min and 1 cycle at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Specificity of PCR products was tested according to the dissociation curves. Relative values of transcripts were calculated using the equation: 2-ΔΔCt, where ΔCt is equal to the difference in threshold cycles for target and reference.
Statistical analysis
Results were expressed as mean ± SD for three repeated individual experiments in each group. Statistical analyses were conducted using the SPSS software (version 10.0). Correlation between sample groups and molecular variables was assayed with paired t test. P < 0.05 was considered statistically significant.
RESULTS
CD133 expression in colon cancer cell lines and human colon cancer tissues
CD133 antigen was expressed in all colorectal cancer cell lines with a difference of 30%-95% (Figure 1A). CD133 in human colorectal cancer tissue samples was stained with polyclonal antibody. CD133 expression was detected in 18 of the 20 primary cancer tissue samples, exclusively on the membrane of the vast majority of colorectal cancer gland cells (Figure 1B), and in 9 of the 10 metastatic colorectal cancer tissue samples with positive staining in cytoplasm of cancer cells (Figure 1C).
Figure 1.
Fluorescence-activated cell sorting showing CD133 expression in different colorectal cancer cell lines (A), CD133 staining of human primary colorectal cancer tissue (B) and metastatic colorectal cancer tissue (C) (Original magnification × 100). Brown indicates positive staining.
CD133 expression in spheroids of sorted colorectal cancer cell subpopulations
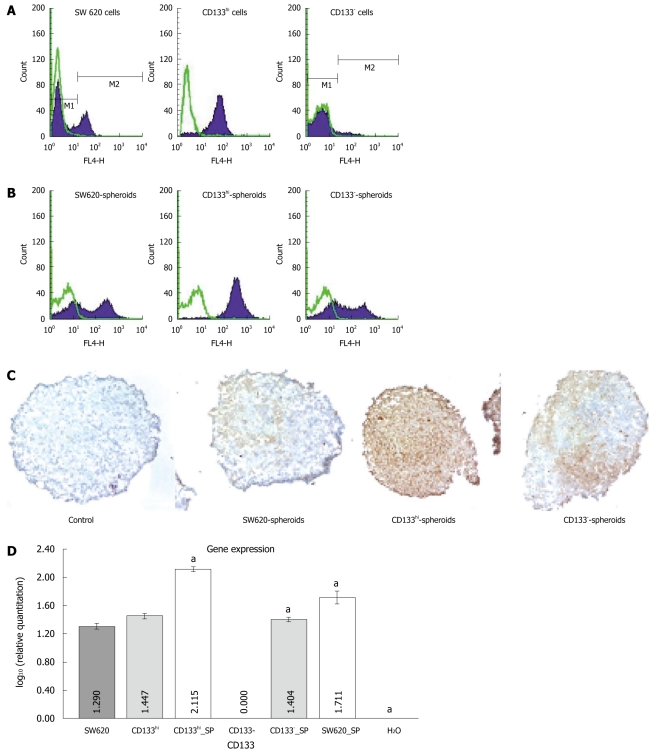
To minimize the contamination between the sorted CD133+ and CD133- cells, a high CD133 expression cell subpopulation (CD133hi) and a CD133-cell subpopulation sorted from the SW620 cells could be persistently passed. CD133 antigen was stably expressed in the monolayer culture (Figure 2A). To mimic the tumorigenesis of colorectal cancer cells in vivo, spheroids of the sorted cells were cultured with hanging drop. The parental, CD133hi and CD133- cells could grow into spheroids. CD133 expression was upregulated in spheroids of CD133- cells. Although the CD133 expression rate was not changed, the mean fluorescence intensity (MFI) was significantly increased in spheroids of CD133hi cells, and the CD133 expression rate and MFI were significantly increased in spheroids of parental cells detected by FACS assay (Figure 2B). Immunohistochemical staining of CD133 antigen was observed in spheroids of CD133- cells (Figure 2C). The CD133 gene expression level was significantly higher in spheroids of SW620, CD133hi and CD133- cells than in their monolayer culture at the mRNA level (4.224 ± 0.063 vs 2.680 ± 0.117, 3.653 ± 0.061 vs 1.325 ± 0.044, 8.746 ± 0.029 vs 3.761 ± 0.065, P < 0.05) (Figure 2D).
Figure 2.
Fluorescence-activated cell sorting showing CD133 expression in SW620, CD133- and CD133hi cells (A) and in their spheroids (B), CD133 staining in spheroids of SW620, CD133- and CD133hi cells (original magnification × 100, brown indicates positive staining) (C), and reverse transcription-polymerase chain reaction showing CD133 expression in SW620, CD133- and CD133hi cells and their spheroids. aP < 0.05 vs monolayer cells. SP: Spheroid.
Lgr5 expression in spheroids of sorted colorectal cancer cell subpopulations
Lgr5 expression was analyzed by RT-qPCR in order to observe the role of the expression of other colon stem cell genes in tumorigenesis of colorectal cancer cells. The results showed that the Lgr5 expression level was significantly higher in spheroids of parental, CD133hi and CD133- cells than in their monolayer cells (5.942 ± 0.091 vs 4.003 ± 0.039, 6.611 ± 0.214 vs 3.645 ± 0.046, 5.910 ± 0.035 vs 3.903 ± 0.083, P < 0.05) (Figure 3).
Figure 3.
Quantitative reverse transcription-polymerase chain reaction showing Lgr5 expression in SW620, CD133- and CD133hi cells and their spheroids. aP < 0.05 vs monolayer cells. SP: Spheroid.
DISCUSSION
Whether CD133 antigen can be used as a marker of colorectal cancer stem cells is still controversial. The focus is that CD133 expression is not restricted to just a small number of colorectal cancer cells. In this study, the CD133 expression was upregulated in colorectal cancer cell lines and primary or metastatic colorectal cancer tissue samples, showing that CD133 antigen can be expressed in colorectal cancer cell lines with a difference of 30%-95%. CD133 expression was detected in 18 of the 20 primary colorectal cancer tissue samples, exclusively on the membrane of a large number of colorectal cancer gland cells, and in 9 of the 10 metastatic colorectal cancer tissue samples with a positive staining in cytoplasm of colorectal cancer cells, which is consistent with the reported findings[21-23]. The different CD133 expression levels in colorectal caner cell lines may be related to the different glycosylation to the mask specific epitopes of CD133 antigen in colorectal cancer cell differentiation[24]. Therefore, our data indicate that CD133 is commonly expressed in colorectal cancer cells.
To investigate whether the upregulated CD133 expression plays a role in tumorigenesis of colorectal cancer cells, SW620 cell line containing two cell subpopulations (CD133hi, CD133-) was selected and sorted using CD133 antigen, the spheroids of parental, CD133hi and CD133- cells were cultured with the hanging drop in vitro, which is based on the natural disposition of cells to aggregate without the need for polymer scaffolds such as matrigel, polyglycolic acid or microporous supports to achieve homogeneous multicellular tumor spheroids[25]. The spheroids represent a popular in vitro 3D tissue structure that mimics in vivo tumor tissue organization and microenvironment[26,27]. In the present study, CD133hi and CD133- cells could be cultured into their spheroids, CD133 expression was upregulated in spheroids of CD133- cells. Although the CD133 expression was not changed, the mean fluorescence intensity (MFI) was significantly increased in spheroids of CD133hi cells as detected by FACS assay. Immunohistochemical staining of CD133 antigen was observed in spheroids of CD133- cells, indicating that CD133 antigen expression is upregulated in spheroids of CD133- and CD133hi cells. Further analysis revealed that the CD133 gene expression level was significantly higher in spheroids of SW620, CD133hi and CD133- cells than in their monolayer culture at the mRNA level, suggesting that the upregulated expression of CD133 including protein and gene plays a role in tumorigenesis of colorectal cancer cells.
Since the upregulated CD133 expression plays a role in tumorigenesis of colorectal cancer cells, whether CD133 protein supports the growth of colorectal cancer is a subject that should be actively studied. As CD133 by itself may lack of a functional role in initiation of tumors and metastasis of human colorectal cancer[28,29], it has an impact on the survival of colorectal cancer patients[22,29]. It has been recently demonstrated that prominin 1 (also called CD133)-marked mouse intestinal stem cells are susceptible to neoplastic transformation[30], possibly due to the fact that upregulated CD133 expression may promote the expression of other critical genes that can drive tumorigenesis of colorectal cancer cells. In this study, the expression level of Lgr5 (leucine-rich-repeat-containing G-protein-coupled receptor 5), also known as Gpr49, a colon stem cell marker gene[31], was significantly higher in spheroids of parental, CD133hi and CD133- cells than in their monolayer cells.
In conclusion, the upregulated CD133 expression plays a role in tumorigenesis of colorectal cancer cells, which may be related to the expression of other critical genes that can drive tumorigenesis of colorectal cancer cells. Further study is needed to confirm the present results in vivo.
COMMENTS
Background
It has been recently shown that CD133 expression is broadly distributed in primary colorectal cancer cells, and not restricted to cancer stem cells. Whether the upregulated CD133 expression plays a role in tumorigenesis of colorectal cancer cells is unknown.
Research frontiers
It has been increasingly reported that CD133 is a marker of putative cancer stem cells (CSC) in some cancers. However, it has been recently shown that CD133 expression is broadly distributed in primary colon cancer cells and not restricted to cancer stem cells, and both CD133+ and CD133-metastatic colorectal cancer cells initiate tumors. Whether the upregulated CD133 expression plays a role in tumorigenesis of colorectal cancer cells is unknown. In this study, the upregulated CD133 expression was found to play a role in tumorigenesis of colorectal cancer cells.
Innovations and breakthroughs
Recent reports have shown that whether CD133 antigen can be used as a marker of colorectal cancer stem cells is controversial. This is the first study to report the role of upregulated CD133 expression in tumorigenesis of colorectal cancer cells. Furthermore, our in vitro studies suggested that the upregulated CD133 expression may promote the expression of other critical genes that can drive tumorigenesis of colorectal cancer cells.
Applications
Whether the upregulated CD133 expression plays a role in tumorigenesis of colorectal cancer cells was studied, the results may help to solve the controversy on CD133 antigen as a marker of colorectal cancer stem cells.
Terminology
CD133, also known as prominin-1, a transmembrane pentaspan protein, is originally described as a surface antigen specific for human hematopoietic stem and progenitor cells. Lgr5 (leucine-rich-repeat-containing G-protein-coupled receptor 5), also known as Gpr49, is a colon stem cell marker gene.
Peer review
The authors detected the expression of CD133 in a panel of colorectal cancer cell lines and human colorectal cancer tissue samples. The expression of CD133 and Lgr5 in spheroids of the sorted colorectal cancer cell subpopulations suggests that the upregulated expression plays a role in tumorigenesis of colorectal cancer cells, which may promote the expression of other critical genes that can drive tumorigenesis. The results are interesting.
Acknowledgments
The authors thank Francesca Walker and Hui-Hua Zhang (Ludwig Institute, Melbourne) for their assistance to cell experiments and FACS studies, and You-Fang Zhang (Ludwig Institute, Melbourne) for his support in immunohistochemical studies.
Footnotes
Peer reviewer: Ioannis Kanellos, Professor, 4th Surgical Department, Aristotle University of Thessaloniki, Antheon 1, Panorama, Thessaloniki 55236, Greece
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
References
- 1.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 2.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 3.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 5.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 6.Kordes C, Sawitza I, Müller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, Häussinger D. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci USA. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 9.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 10.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 14.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 16.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 19.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 21.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–1289. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima M, Ishii G, Atsumi N, Fujii S, Saito N, Ochiai A. Immunohistochemical detection of CD133 expression in colorectal cancer: a clinicopathological study. Cancer Sci. 2008;99:1578–1583. doi: 10.1111/j.1349-7006.2008.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G, et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010;70:719–729. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 25.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 26.Weiswald LB, Richon S, Validire P, Briffod M, Lai-Kuen R, Cordelières FP, Bertrand F, Dargere D, Massonnet G, Marangoni E, et al. Newly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressiveness. Br J Cancer. 2009;101:473–482. doi: 10.1038/sj.bjc.6605173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 28.Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 29.Horst D, Scheel SK, Liebmann S, Neumann J, Maatz S, Kirchner T, Jung A. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol. 2009;219:427–434. doi: 10.1002/path.2597. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]