Abstract
AIM: To retrospectively analyze the computed tomography (CT) and magnetic resonance imaging (MRI) appearances of primary clear cell carcinoma of the liver (PCCCL) and compare the imaging appearances of PCCCL and common type hepatocellular carcinoma (CHCC) to determine whether any differences exist between the two groups.
METHODS: Twenty cases with pathologically proven PCCCL and 127 cases with CHCC in the Second Affiliated Hospital of Sun Yat-sen University were included in this study. CT or MRI images from these patients were retrospectively analyzed. The following imaging findings were reviewed: the presence of liver cirrhosis, tumor size, the enhancement pattern on dynamic contrast scanning, the presence of pseudo capsules, tumor rupture, portal vein thrombosis and lymph node metastasis.
RESULTS: Both PCCCL and CHCC were prone to occur in patients with liver cirrhosis, the association rate of liver cirrhosis was 80.0% and 78.7%, respectively (P > 0.05). The mean sizes of PCCCL and CHCC tumors were (7.28 ± 4.25) cm and (6.96 ± 3.98) cm, respectively. Small HCCs were found in 25.0% (5/20) of PCCCL and 19.7% (25/127) of CHCC cases. No significant differences in mean size and ratio of small HCCs were found between the two groups (P = 0.658 and 0.803, respectively). Compared with CHCC patients, PCCCL patients were more prone to form pseudo capsules (49.6% vs 75.0%, P = 0.034). Tumor rupture, typical HCC enhancement patterns and portal vein tumor thrombosis were detected in 15.0% (3/20), 72.2% (13/18) and 20.0% (4/20) of patients with PCCCL and 3.1% (4/127), 83.6% (97/116) and 17.3% (22/127) of patients with CHCC, respectively. There were no significant differences between the two groups (all P > 0.05). No patients with PCCCL and 2.4% (3/127) of patients with CHCC showed signs of lymph node metastasis (P > 0.05).
CONCLUSION: The imaging characteristics of PCCCL are similar to those of CHCC and could be useful for differentiating these from other liver tumors (such as hemangioma and hepatic metastases). PCCCLs are more prone than CHCCs to form pseudo capsules.
Keywords: Clear cell carcinoma, Hepatocellular carcinoma, Pathology, Magnetic resonance imaging, Computed Tomography, X-ray
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver. It can be classified according to its histological architecture or cytological features. HCC includes various cytological types; the less common ones are clear cell type, spindle cell type, giant cell type, small cell type and squamous cell type[1,2]. Primary clear cell carcinoma of the liver (PCCCL) is rare, with a frequency varying between 2.2% and 6.7% among HCCs reported in the published literatures[3,4]. Due to the accumulation of glycogens and/or fats, the PCCCL cell cytoplasm is clear to hematoxylin-eosin staining. PCCCL may pose a diagnostic dilemma even with histological evaluation because the morphology of PCCCL cells is similar to that of extrahepatic clear cell tumors, such as clear cell cancers of the kidneys, adrenal glands, ovaries, thyroid, endometrium, uterine cervix, and vagina[5,6]. PCCCLs should be differentiated from metastatic clear cell cancer because their treatment strategies and prognoses are quite different. The prognosis of PCCCL is generally considered better than that of the common type of HCC (CHCC)[3,7,8].
Computed tomography (CT) and magnetic resonance imaging (MRI) are important examinations for the detection and characterization of liver tumors[9,10]. To our knowledge, the imaging features of PCCCL have rarely been reported in the English literature[11]. The purpose of this study was to describe the CT and MRI findings of PCCCL and compare them to CHCC to determine whether any differences exist between the two groups.
MATERIALS AND METHODS
Patients
Between January 2005 and August 2009, a total of 570 patients with primary HCC underwent hepatectomy at the Second Affiliated Hospital of Sun Yat-sen University. Twenty (3.5%) of these patients had pathologically confirmed PCCCL. The participants of this study included 20 patients with PCCCL and 127 patients with CHCC (randomly selected from the other 550 cases of primary HCC). No patient had received preoperative treatment, such as interventional therapy or chemotherapy.
Of the 20 patients with PCCCL, 14 had right upper abdominal pain, two complained of fatigue and four were asymptomatic. All patients with PCCCL were positive for HBsAg, and two were positive for anti-hepatitis C virus-IgG. The serum concentration of α-fetoprotein (AFP) was 5.8-68 787.0 μg/L for PCCCL patients, with a median of 149.9 μg/L. Of the 20 patients with PCCCL, 17 were AFP-positive (> 25 μg/L).
Pathologic examinations were retrospectively reviewed by an experienced pathologist. According to diagnostic criteria generally accepted by pathologists in China, PCCCL was diagnosed when clear cells accounted for more than 50% of the tumor[1,3,4,12].
Imaging protocols
CT or MRI examinations were performed no more than 5 days before hepatectomy. Thirteen patients with PCCCL and 73 patients with CHCC underwent dynamic CT examination using a spiral CT scanner (HiSpeed NX/I; GE Medical Systems, Milwaukee, WI) or a multi-detector CT scanner (Sensation 64; Siemens Medical Solutions, Erlangen, Germany). The scan parameters were as follows: 5-7 mm slice thickness reconstructions, 120-kV, 220-400 mA current, 25 cm field of view, and 256 × 256 matrix. Scans began at the dome of the diaphragm and proceeded in a caudal direction. After pre-contrast CT scans, the patients underwent dynamic contrast-enhanced scans. A bolus injection of 80-100 mL of non-ionic contrast medium (Iopamidol, Bracco, Milano, Italy) with a concentration of 350 mg I/mL was given via the antecubital vein at a rate of 3.5 mL/s. Images of the hepatic arterial phase (HAP), portal venous phase (PVP) and equilibrium phase (EP) were obtained at 25 s, 70 s and 120 s, respectively, after the injection of contrast agent.
Seven patients with PCCCL and 54 patients with CHCC underwent MRI studies with a 1.5-T MR unit (Gyroscan Intera, Philips Medical System, Best, the Netherlands). Unenhanced MR images included T1-weighted images with a water-selective excitation technique (FFE, TR 218ms, TE 4.9 ms, flip angle of 80, one acquisition) and turbo spin-echo T2-weighted images with fat saturation (TR 1600 ms, TE 70 ms, TSE Factor 24, three acquisitions). Five patients with PCCCL and 43 patients with CHCC underwent dynamic contrast-enhanced MR scans using a high-resolution turbo spin-echo sequence (TR 5.3 ms, TE 1.4 ms, flip angle of 40, 3.0-mm slice thickness, no gap, one acquisition) via a power injector; contrast agent was administrated at a rate of 2.5 mL/sec. HAP, PVP and EP scans were obtained at 20, 60, and 110 s, respectively. The other 13 patients (2 with PCCCL and 11 with CHCC) received manual injections of gadopentetate dimeglumine (Magnevist, Bayer Schering, Berlin, Germany) at a dose of 0.1 mmol/kg; post-contrast T1-weighted images were obtained at PVP (60-80 s after injection) with the same scanning parameters as the pre-contrast T1W scan. Regardless of the technique employed, axial and coronal images were acquired with 5.0-mm slice thickness.
Image interpretation
The CT and MRI images were retrospectively analyzed by two radiologists who have 10 and 15 years of experience in diagnosing abdominal diseases. Neither radiologist was aware of the patients’ clinicopathological data. Reviews were performed jointly and by consensus. The presence of liver cirrhosis, tumor size, the enhancement pattern on dynamic contrast scanning, the presence of pseudocapsule, tumor rupture, portal vein thrombus, and lymph node metastasis were recorded. A typical HCC enhancement pattern was defined as early enhancement at HAP and rapid contrast medium washout at PVP or EP with hypo-attenuation/intense signal or iso-attenuation/intense signal[9,10].
Statistical analysis
Differences in mean age and tumor size were assessed with an independent-samples t test. Differences in the frequencies of liver cirrhosis, tumor capsule formation, tumor rupture, typical enhancement pattern, portal vein tumor thrombus and lymph node metastases between the two groups were compared using the Chi-squared test or Fischer’s exact test. A P value of 0.05 or less was considered significant. Statistical analysis was performed using the SPSS 13.0 software package (SPSS Inc., Chicago, IL, USA).
RESULTS
The male-to-female ratio was 4.0:1 in the PCCCL group and 6.1:1 in the CHCC group. The mean age was 52.00 ± 10.09 years (range, 29-66 years) in the PCCCL group and 51.82 ± 13.20 years (range, 19-83 years) in the CHCC group. There were no statistical differences between the two groups regarding sex or age (P = 0.733 and P = 0.953, respectively).
Table 1 summarizes the imaging features observed in patients with PCCCL and patients with CHCC. Both PCCCL and CHCC were prone to occur in patients with liver cirrhosis, with a rate of 80.0% and 78.7%, respectively. The mean sizes of PCCCLs and CHCCs were 7.28 ± 4.25 cm (range, 2.0-15.9 cm), and 6.96 ± 3.98 cm (range, 1.0-17.0 cm), respectively. Small HCCs with diameters ≤ 3.0 cm were found in 25.0% (5/20) of PCCCL cases and 19.7% (25/127) of CHCC cases. No statistically significant differences in mean size or ratio of small HCC were found between the two groups (P = 0.658 and 0.803, respectively). Compared with CHCCs, PCCCLs were more prone to form pseudo capsules, with a rate of 49.6% and 75.0%, respectively (P = 0.034). Pseudo capsules showed hypo-attenuation/intensity haloes on pre-contrast scans and rim enhancement after contrast administration (Figures 1 and 2).
Table 1.
Characteristics of clear cell hepatocellular carcinoma in the liver
| Parameters | PCCCL (n = 20) | CHCC (n = 127) | P value |
| Sex | 0.733 | ||
| Male | 16 | 109 | |
| Female | 4 | 18 | |
| Liver cirrhosis | 1.000 | ||
| Positive | 16 | 100 | |
| Negative | 4 | 27 | |
| Tumor diameter (cm) | 0.803 | ||
| ≤ 3.0 | 5 | 25 | |
| > 3.0 | 15 | 102 | |
| Capsule formation | 0.034 | ||
| Positive | 15 | 63 | |
| Negative | 5 | 64 | |
| Rupture | 0.053 | ||
| Positive | 3 | 4 | |
| Negative | 17 | 123 | |
| Typical enhancement pattern | 0.399 | ||
| Positive | 13 | 97 | |
| Negative | 5 | 19 | |
| Portal vein tumor thrombus | 1.000 | ||
| Positive | 4 | 22 | |
| Negative | 16 | 105 | |
| Lymph node metastases | 1.000 | ||
| Positive | 0 | 3 | |
| Negative | 20 | 124 |
PCCCL: Primary clear cell carcinoma of the liver; CHCC: Common type of hepatocellular carcinoma.
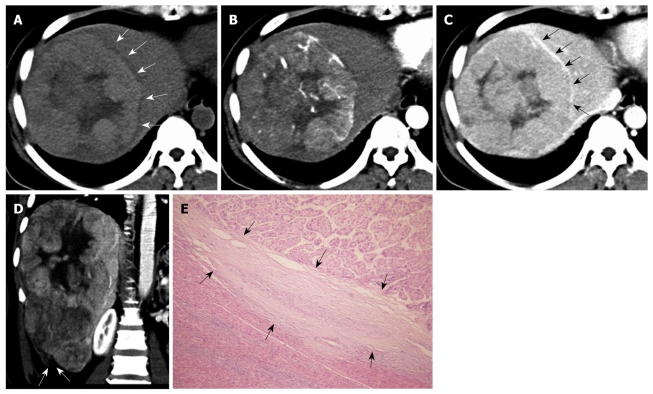
Figure 1.
Primary clear cell carcinoma of the liver in a 47-year-old woman. A: On pre-contrast computed tomography scan, the mass shows slight hyper-attenuation with a hypo-attenuation halo (arrows); B: At hepatic arterial phase, the mass shows early enhancement; C: At the equilibrium phase, the mass presents hypo-attenuation with rim enhancement (arrows); D: At portal venous phase, the reconstructed coronal image shows the mass with a discontinuous liver capsule (arrows) at Segment VI, indicating tumor rupture, which was surgically confirmed; E: Pathologically, the mass shows a pseudocapsule (arrows) (HE, × 100).
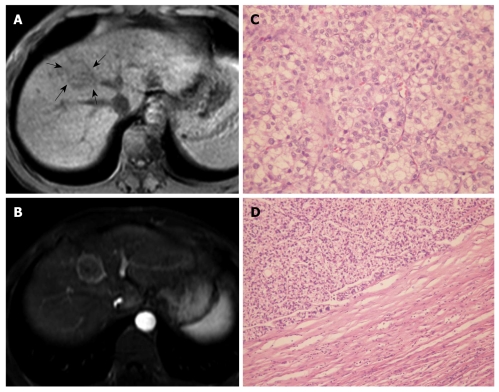
Figure 2.
Primary clear cell carcinoma of the liver in a 29-year-old man. A: On T1WI, the mass shows slightly hypo-intense signals (arrows); B: At portal venous phase, the mass presents with rim enhancement (pseudocapsule); C: Pathologically, the mass is mainly composed of clear cells (HE, × 200); D: Pathologically, the mass shows a pseudocapsule (HE, × 100).
A higher percentage of tumor rupture was found in patients with PCCCL (15.0%, 3/20) than in patients with CHCC (3.1%, 4/127); however, there was no significant difference between the two groups (P > 0.05). Of the 20 PCCCL cases, three showed tumor ruptures. The ruptured tumors were 15.9 cm, 10.9 cm and 9.3cm in diameter and were located at the periphery of the liver with protruding contours. Two cases presented as discontinuities of the liver surface on CT scan (Figure 1). The remaining case presented a local hematoma at the rupture site on MRI, which appeared as mixed iso-/hypo-intense signals on T1WI and hypo-intense signals on T2WI with no enhancement after injection of contrast agent.
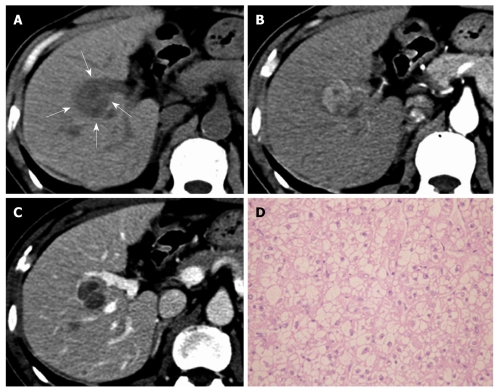
Typical HCC enhancement patterns were noted in 72.2% (13/18) of PCCCLs and 83.6% (97/116) of CHCCs; however, no significant difference was found between the two groups (P > 0.05) (Figures 1 and 3). The other five PCCCL cases showed atypical CT features on dynamic scan: two cases showed minimal enhancement and remained hypo-attenuated at HAP and PVP, while the other three cases showed gradual contrast enhancement during the portal phase.
Figure 3.
Primary clear cell carcinoma of the liver in a 62-year-old man. A: On pre-contrast computed tomography scan, the mass shows hypo-attenuation (arrows); B: At hepatic arterial phase, the mass shows early enhancement; C: At portal venous phase, the mass shows hypo-attenuation and thin rim enhancement (pseudocapsule); D: Microscopically, the mass is mainly composed of clear cells (HE, × 200).
Four patients (20.0%) with PCCCL had portal vein tumor thrombosis: one located at the left branch of the portal vein, one at the right branch, and one at the right anterior branch and main portal vein. Compared with CHCC patients, PCCCL patients showed a slightly higher incidence of portal vein tumor thrombosis (17.3% and 20.0%, respectively); however, there was no significant difference between the two groups (P > 0.05). No PCCCL patients and 2.4% (3/127) CHCC patients showed sign of lymph node metastasis (P > 0.05).
DISCUSSION
PCCCL is a specific and rare subtype of primary HCC. The reported incidence of PCCCL is 0.4%-37%; inconsistent diagnostic criteria may be responsible for the variable reports[1,3,4,7,8,12,13]. Lai et al[7] suggested that the diagnosis of PCCCL could be made even when the proportion of clear cells was < 30%, while Buchanan et al[8] suggested that PCCCL should be diagnosed when the proportion of clear cells was > 30%. Most studies diagnosed PCCCL when the proportion of clear cells was > 50%[1,3,4,12]. Using this criteria, PCCCL only accounts for 2.2%-6.7% of all resectable HCCs in most reports[3,4]. Among the 570 cases of primary HCC resected in our hospital, only 3.5% patients had PCCCL. The clear cell development is presumed to involve metabolic disorders and abnormalities of sugar metabolism[14,15].
The clinicopathological presentations of PCCCL were different from those of CHCC. The rates of hepatitis C infection and capsule formation were higher in PCCCL patients than in those with CHCC; however, no remarkable differences in patients’ age, sex, AFP-positive rate or the location, number, size and grade of tumors were observed between the two groups[3]. Both tumor types were prone to occur in patients with hepatitis B, mostly on the basis of liver cirrhosis[3]. PCCCL had a better prognosis than CHCC, mainly related to capsule formation, vascular invasion, preoperative liver function and clear cell proportion[3,4,12]. Surgical resection is an effective treatment for patients with PCCCL[3,4,7].
The presence of clear cells and fatty changes characterizes well-differentiated HCC in the early stage, and their ratio is presumed to decrease as the tumor enlarges[15]. In 1999, Monzawa et al[16] analyzed the pathologic and imaging changes of well-differentiated HCC; and found that some well-differentiated HCCs showed clear cell formation and/or fatty changes, which presented as high echo on ultrasound and hyper-intense signals on T1WI. However, in their study, the proportion of clear cells in the recruited HCC was less than 10%, or only 10%-50%, which did not meet the diagnosis criteria for PCCCL. In 2008, Takahashi et al[11] described CT, MR and angiographic findings of PCCCL in a woman with a normal liver. To our knowledge, no further research on the imaging manifestations of PCCCL has been conducted.
Pseudocapsule formation (consisting mainly of peritumoral hepatic sinusoids and/or fibrosis) is an important gross pathologic feature of HCC. Pseudocapsule indicates a relatively positive prognosis after tumor resection[17]. Liu et al[3] found a higher ratio of pseudocapsule formation in PCCCL than in CHCC microscopically (88.4% vs 68.0%, P < 0.05); and pseudocapsule formation might be related to a relatively lower degree of malignancy and a better prognosis for PCCCL. CT and MRI are reliable imaging examinations for the detection of HCC pseudo capsules. The pseudocapsule presents as rim enhancement on dynamic contrast scanning, and MRI is more sensitive than CT in identifying pseudocapsule[17-19]. Among the 20 cases of PCCCL in our study, 15 (75.0%) had pseudocapsule, all of which were confirmed pathologically. The percentage of pseudocapsule formation was higher in PCCCL patients than in CHCC patients (P < 0.05).
Because of hypervascular blood supply, typical HCC showed early enhancement at HAP, and rapid contrast medium washout at PVP or EP with hypo-attenuation/intense signal or iso-attenuation/intense signal[9,10]. Among the 18 PCCCL cases in our study that underwent dynamic contrast CT or MRI examination, 13 presented a typical HCC enhancement pattern, indicating that the tumor is rich of blood supply. The enhancement pattern of PCCCL is not different from that of CHCC (P > 0.05). This imaging characteristic may be useful in differentiating PCCCL from other liver tumors, such as hemangioma and hepatic metastases. The other five PCCCL cases presented atypical enhancement on dynamic CT scans: two cases showed minimal enhancement with hypo-attenuation at HAP and PVP, indicating hypovascularity, and three cases showed gradual contrast enhancement during the portal phase, which may be attributable to the difference in blood supply (such as existence of small arterioportal shunts), tumor differentiation or liver cirrhosis background[20,21].
Spontaneous rupture of HCC is usually life-threatening but relatively uncommon, with a reported incidence of 3%-15%[22]. CT is a valuable imaging technique for diagnosing HCC ruptures. The imaging findings include: discontinuity or disruption of the liver capsule adjacent to the liver mass and hematoma with hyper-attenuation at the rupture site. The enucleation sign is a specific sign for diagnosing HCC rupture[23,24]. To our knowledge, no report on PCCCL rupture is available for review. Among the 20 PCCCL cases in our study, only three had tumor rupture: two showed discontinuity of the liver capsule on CT scans, and the other showed a hematoma at the rupture site on MRI, with iso-/hypo-intense signals on T1WI and hypo-intense signals on T2WI.
Portal vein thrombosis, the characteristic growth pattern of HCC, occurs in 12.5%-39.7% of HCC patients[25]. Liu et al[3] reported that the microscopic vascular invasion rates are similar between PCCCL and CHCC (53.4% vs 65.0%, P > 0.05). In our study, the incidence of macroscopic portal vein tumor thrombus in PCCCL and CHCC detected on imaging examination was not significantly different (P > 0.05). Portal vein invasion was an independent risk factor for the prognosis of patients with PCCCL[12].
Chemical shift imaging is valuable for characterizing lesions with a mixture of water and fat[26]. Renal clear cell carcinomas usually contain fat, and present focal and diffused signal loss on chemical shift imaging. This imaging technique is helpful for differentiating renal clear cell carcinoma from other types of renal cancer[27,28]. The cell morphology of PCCCL is similar to that of renal clear cell carcinoma, with cytoplasmic accumulation of glycogens and/or fat. The signal reduction of HCC during chemical shift imaging may help identify intratumoral fatty components and confirm a diagnosis of PCCCL[2].
In summary, the imaging characteristics of PCCCL are similar to those of CHCC, including early enhancement and rapid washout of contrast agent on dynamic contrast scans, and presence of portal vein thrombus or tumor rupture. These imaging features may help differentiate PCCCL from other liver tumors, such as hemangioma and hepatic metastases. Pseudocapsule formation is more likely to occur in PCCCL than in CHCC and may be related to PCCCL’s relatively lower degree of malignancy and better prognosis.
COMMENTS
Background
Primary clear cell carcinoma of the liver (PCCCL) is a specific and rare subtype of primary hepatocellular carcinoma (HCC), with a frequency varying between 2.2% and 6.7% among HCCs in the published literatures. PCCCL may pose a diagnostic dilemma even with histological sections because the morphology of PCCCL cells is similar to that of metastatic clear cell tumors. As a result of the paucity of cases, available data about its imaging findings are limited.
Research frontiers
Imaging modalities [computed tomography (CT) and magnetic resonance imaging (MRI)] are important for the detection and characterization of liver tumors. The imaging characteristics of common type hepatocellular carcinoma (CHCC) are well documented; for example, CHCC is usually associated with liver cirrhosis, typical enhancement pattern on dynamic contrast scanning (early enhancement at hepatic arterial phase and rapid contrast medium washout at portal venous phase or equilibrium phase) and the presence of pseudocapsule. However, the imaging features of PCCCL have not been unequivocally addressed. This study clarifies the CT or MRI findings of PCCCL.
Innovations and breakthroughs
The authors presented 20 surgically confirmed PCCCL cases and retrospectively analyzed their imaging findings. This study revealed that the imaging characteristics of PCCCL are similar to those of CHCC. PCCCLs are more likely to form pseudo capsules than CHCCs.
Applications
With a better understanding of the imaging features of PCCCL, further investigations should determine how to use imaging modalities, especially MRI, to differentiate PCCCL from CHCC or metastatic clear cell cancer. Chemical shift imaging with an MR scanner may help detect lipid component in the cytoplasm of clear cells in PCCCL.
Terminology
PCCCL is a rare variant of HCC. Due to the accumulation of large amounts of glycogen and/or lipids that are dissolved by routine histological processing (hematoxylin-eosin staining), the cytoplasm of PCCCL cells is clear. PCCCL can be diagnosed when the tumor cells are predominantly or wholly composed of clear cell cytoplasm (a proportion of clear cells > 50%). The prognosis of PCCCL is generally considered better than that of the CHCC.
Peer review
It is a well written paper, with interesting results.
Footnotes
Peer reviewers: C Triantopoulou, MD, PhD, Head of Radiology Department, Konstantopouleio general Hospital, 3-5, Agias Olgas street, 14233 N. Ionia, Athens, Greece; Hiroshi Yoshida, Associate Professor, Department of Surgery, Nippon Medical School Tama Nagayama Hospital, 1-7-1 Nagayama, Tama-city, Tokyo, 206-8512, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Lin YP
References
- 1.Cong WM, Zhang SH. Introduction of the rare types of HCC. Chin J Pathol. 2002;31:457–460. [Google Scholar]
- 2.Chung YE, Park MS, Park YN, Lee HJ, Seok JY, Yu JS, Kim MJ. Hepatocellular carcinoma variants: radiologic-pathologic correlation. AJR Am J Roentgenol. 2009;193:W7–W13. doi: 10.2214/AJR.07.3947. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Ma W, Li H, Li Q. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res. 2008;38:291–299. doi: 10.1111/j.1872-034X.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 4.Lao XM, Zhang YQ, Jin X, Lin XJ, Guo RP, Li GH, Li JQ. Primary clear cell carcinoma of liver--clinicopathologic features and surgical results of 18 cases. Hepatogastroenterology. 2006;53:128–132. [PubMed] [Google Scholar]
- 5.Murakata LA, Ishak KG, Nzeako UC. Clear cell carcinoma of the liver: a comparative immunohistochemical study with renal clear cell carcinoma. Mod Pathol. 2000;13:874–881. doi: 10.1038/modpathol.3880156. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira AM, Erickson LA, Burgart LJ, Lloyd RV. Differentiation of primary and metastatic clear cell tumors in the liver by in situ hybridization for albumin messenger RNA. Am J Surg Pathol. 2000;24:177–182. doi: 10.1097/00000478-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Lai CL, Wu PC, Lam KC, Todd D. Histologic prognostic indicators in hepatocellular carcinoma. Cancer. 1979;44:1677–1683. doi: 10.1002/1097-0142(197911)44:5<1677::aid-cncr2820440522>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan TF Jr, Huvos AG. Clear-cell carcinoma of the liver. A clinicopathologic study of 13 patients. Am J Clin Pathol. 1974;61:529–539. doi: 10.1093/ajcp/61.4.529. [DOI] [PubMed] [Google Scholar]
- 9.Willatt JM, Hussain HK, Adusumilli S, Marrero JA. MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology. 2008;247:311–330. doi: 10.1148/radiol.2472061331. [DOI] [PubMed] [Google Scholar]
- 10.Jeong YY, Yim NY, Kang HK. Hepatocellular carcinoma in the cirrhotic liver with helical CT and MRI: imaging spectrum and pitfalls of cirrhosis-related nodules. AJR Am J Roentgenol. 2005;185:1024–1032. doi: 10.2214/AJR.04.1096. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi A, Saito H, Kanno Y, Abe K, Yokokawa J, Irisawa A, Kenjo A, Saito T, Gotoh M, Ohira H. Case of clear-cell hepatocellular carcinoma that developed in the normal liver of a middle-aged woman. World J Gastroenterol. 2008;14:129–131. doi: 10.3748/wjg.14.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji SP, Li Q, Dong H. Therapy and prognostic features of primary clear cell carcinoma of the liver. World J Gastroenterol. 2010;16:764–769. doi: 10.3748/wjg.v16.i6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashala LO, Conne B, Kalengayi MM, Kapanci Y, Frei PC, Lambert PH. Histopathologic features of hepatocellular carcinoma in Zaire. Cancer. 1990;65:130–134. doi: 10.1002/1097-0142(19900101)65:1<130::aid-cncr2820650126>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Yang SH, Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int. 1996;46:503–509. doi: 10.1111/j.1440-1827.1996.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 15.Kojiro M. Pathology of early liver cancer and similar lesions. 1st ed. Tokyo: Igaku-Shoin Ltd; 1996. pp. 35–37. [Google Scholar]
- 16.Monzawa S, Omata K, Shimazu N, Yagawa A, Hosoda K, Araki T. Well-differentiated hepatocellular carcinoma: findings of US, CT, and MR imaging. Abdom Imaging. 1999;24:392–397. doi: 10.1007/s002619900521. [DOI] [PubMed] [Google Scholar]
- 17.Ishigami K, Yoshimitsu K, Nishihara Y, Irie H, Asayama Y, Tajima T, Nishie A, Hirakawa M, Ushijima Y, Okamoto D, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009;250:435–443. doi: 10.1148/radiol.2501071702. [DOI] [PubMed] [Google Scholar]
- 18.Grazioli L, Olivetti L, Fugazzola C, Benetti A, Stanga C, Dettori E, Gallo C, Matricardi L, Giacobbe A, Chiesa A. The pseudocapsule in hepatocellular carcinoma: correlation between dynamic MR imaging and pathology. Eur Radiol. 1999;9:62–67. doi: 10.1007/s003300050629. [DOI] [PubMed] [Google Scholar]
- 19.Ebara M, Ohto M, Watanabe Y, Kimura K, Saisho H, Tsuchiya Y, Okuda K, Arimizu N, Kondo F, Ikehira H. Diagnosis of small hepatocellular carcinoma: correlation of MR imaging and tumor histologic studies. Radiology. 1986;159:371–377. doi: 10.1148/radiology.159.2.3008213. [DOI] [PubMed] [Google Scholar]
- 20.Efremidis SC, Hytiroglou P, Matsui O. Enhancement patterns and signal-intensity characteristics of small hepatocellular carcinoma in cirrhosis: pathologic basis and diagnostic challenges. Eur Radiol. 2007;17:2969–2982. doi: 10.1007/s00330-007-0705-z. [DOI] [PubMed] [Google Scholar]
- 21.Hayashida M, Ito K, Fujita T, Shimizu A, Sasaki K, Tanabe M, Matsunaga N. Small hepatocellular carcinomas in cirrhosis: differences in contrast enhancement effects between helical CT and MR imaging during multiphasic dynamic imaging. Magn Reson Imaging. 2008;26:65–71. doi: 10.1016/j.mri.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 23.Choi BG, Park SH, Byun JY, Jung SE, Choi KH, Han JY. The findings of ruptured hepatocellular carcinoma on helical CT. Br J Radiol. 2001;74:142–146. doi: 10.1259/bjr.74.878.740142. [DOI] [PubMed] [Google Scholar]
- 24.Kim HC, Yang DM, Jin W, Park SJ. The various manifestations of ruptured hepatocellular carcinoma: CT imaging findings. Abdom Imaging. 2008;33:633–642. doi: 10.1007/s00261-007-9353-7. [DOI] [PubMed] [Google Scholar]
- 25.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valls C, Iannacconne R, Alba E, Murakami T, Hori M, Passariello R, Vilgrain V. Fat in the liver: diagnosis and characterization. Eur Radiol. 2006;16:2292–2308. doi: 10.1007/s00330-006-0146-0. [DOI] [PubMed] [Google Scholar]
- 27.Outwater EK, Bhatia M, Siegelman ES, Burke MA, Mitchell DG. Lipid in renal clear cell carcinoma: detection on opposed-phase gradient-echo MR images. Radiology. 1997;205:103–107. doi: 10.1148/radiology.205.1.9314970. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimitsu K, Honda H, Kuroiwa T, Irie H, Tajima T, Jimi M, Kuroiwa K, Naito S, Masuda K. MR detection of cytoplasmic fat in clear cell renal cell carcinoma utilizing chemical shift gradient-echo imaging. J Magn Reson Imaging. 1999;9:579–585. doi: 10.1002/(sici)1522-2586(199904)9:4<579::aid-jmri12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]