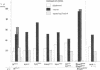Abstract
In embryos of Drosophila melanogaster the development of a pluripotent cell in the neurogenic ectoderm as a member of either a neural or an epidermal lineage depends on its interactions with neighboring cells. Certain genes, designated neurogenic, participate in this process in that there is a deficiency of epidermal histotypes in mutant embryos lacking neurogenic gene functions. To test the cell autonomy of expression of the neurogenic phenotype, individual cells were transplanted from the neurogenic ectoderm of mutant donor embryos into wild-type host embryos. Cells transplanted from donors homozygous for any of several mutant alleles of the neurogenic genes amx, N, bib, mam, neu, and Dl were found to give rise to clones exhibiting a distribution of neural and epidermal histotypes similar to that of the wild type. By contrast, cells transplanted from donors homozygous for loss of the neurogenic E(spl) gene gave rise exclusively to clones of neural histotypes. Thus, only the expression of E(spl) is autonomous, with that of all of the other tested neurogenic genes being nonautonomous. These results are consistent with the inference that the nonautonomous genes provide a source and the autonomous gene provides a receptor of a hypothetical intercellular regulatory signal that is necessary for cell commitment to an epidermal rather than neural fate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artavanis-Tsakonas S., Muskavitch M. A., Yedvobnick B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1977–1981. doi: 10.1073/pnas.80.7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich U., Campos-Ortega J. A. The expression of neurogenic loci in imaginal epidermal cells of Drosophila melanogaster. J Neurogenet. 1984 Dec;1(4):315–332. doi: 10.3109/01677068409107094. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Goodman C. S. Early events in insect neurogenesis. I. Development and segmental differences in the pattern of neuronal precursor cells. Dev Biol. 1985 Sep;111(1):193–205. doi: 10.1016/0012-1606(85)90445-2. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Goodman C. S. Early events in insect neurogenesis. II. The role of cell interactions and cell lineage in the determination of neuronal precursor cells. Dev Biol. 1985 Sep;111(1):206–219. doi: 10.1016/0012-1606(85)90446-4. [DOI] [PubMed] [Google Scholar]
- Hoppe P. E., Greenspan R. J. Local function of the Notch gene for embryonic ectodermal pathway choice in Drosophila. Cell. 1986 Aug 29;46(5):773–783. doi: 10.1016/0092-8674(86)90353-3. [DOI] [PubMed] [Google Scholar]
- Kidd S., Kelley M. R., Young M. W. Sequence of the notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol. 1986 Sep;6(9):3094–3108. doi: 10.1128/mcb.6.9.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Knust E., Dietrich U., Tepass U., Bremer K. A., Weigel D., Vässin H., Campos-Ortega J. A. EGF homologous sequences encoded in the genome of Drosophila melanogaster, and their relation to neurogenic genes. EMBO J. 1987 Mar;6(3):761–766. doi: 10.1002/j.1460-2075.1987.tb04818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P., Struhl G. Different requirements for homeotic genes in the soma and germ line of Drosophila. Cell. 1983 Nov;35(1):27–34. doi: 10.1016/0092-8674(83)90204-0. [DOI] [PubMed] [Google Scholar]
- Poulson D. F. Chromosomal Deficiencies and the Embryonic Development of Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1937 Mar;23(3):133–137. doi: 10.1073/pnas.23.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M. P. Characterization of the female-sterile mutant almondex of Drosophila melanogaster. Genetica. 1972;43(2):244–256. doi: 10.1007/BF00123632. [DOI] [PubMed] [Google Scholar]
- Vässin H., Vielmetter J., Campos-Ortega J. A. Genetic interactions in early neurogenesis of Drosophila melanogaster. J Neurogenet. 1985 Nov;2(5):291–308. doi: 10.3109/01677068509102325. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Zalokar M., Erk I. Phase-partition fixation and staining of Drosophila eggs. Stain Technol. 1977 Mar;52(2):89–95. doi: 10.3109/10520297709116753. [DOI] [PubMed] [Google Scholar]