Abstract
Objective
Previous research on the physical health consequences of childhood abuse and other adversities has been based on data from young or middle-aged adults. This study addressed the question of whether childhood abuse and other adversities have lasting, detectable consequences for inflammation and cell aging late in life, and whether the effects are large enough to be discernible beyond that of a major chronic stressor, dementia family caregiving.
Method
In this community sample of 132 healthy older adults (mean age = 69.70, SD=10.14), including 58 dementia family caregivers and 74 noncaregivers, blood samples were analyzed for interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and telomere length, a measure of cell aging. Depressive symptoms were assessed by the Center for Epidemiological Studies Depression Scale (CES-D).
Results
After controlling for age, caregiving status, gender, body mass index, exercise, and sleep, the presence of multiple childhood adversities was related to both heightened IL-6 (.37 ± .03 vs. .44 ± .03 log10 pg/mL) and shorter telomeres (6.51 ± .17 vs. 5.87 ± .20 Kb), compared to the absence of adversity; the telomere difference could translate into a 7–15 year difference in lifespan. Abuse was associated with heightened IL-6 and TNF-α levels, and, for TNF-α, this relationship was magnified in caregivers compared to controls. Moreover, abuse and caregiving status were significantly and independently associated with higher levels of depressive symptoms.
Conclusions
Adverse childhood events are related to continued vulnerability among older adults, enhancing the impact of chronic stressors. Childhood adversities cast a very long shadow.
Keywords: psychoneuroimmunology, IL-6, TNF-α, depression, cell aging, trauma
Adverse events during childhood have been associated with a higher prevalence of several mental disorders, including DSM-IV-defined mood disorders, disruptive behavior, and substance abuse disorders (1). In fact, simulation data suggested that adversities were associated with 44.6% of all childhood-onset disorders and 25.9% to 32% of adult-onset disorders (1).
Adults who experienced abuse or neglect as children appear to have an enhanced emotional sensitivity to stress; they are more likely to develop psychiatric disorders when confronting subsequent stressors than adults who did not have a similarly troubled history (2). Indeed, childhood maltreatment is a particularly potent risk factor for depression in adults, especially when individuals encounter stressful life events (3).
In addition to the enhanced emotional stress sensitivity associated with childhood adversity, heightened physiological stress sensitivity has also been documented (3). Alterations in hypothalamic-pituitary-adrenal (HPA) axis and autonomic stress responses have been found in adult survivors of childhood abuse compared to similar individuals without a history of abuse (4, 5). Findings from trauma survivors consistent with neuroendocrine stress response sensitization include enhanced glucocorticoid resistance, increased central corticotropin-releasing factor (CRF) activity, and reduced hippocampal volume (3).
The evidence that glucocorticoid resistance has been associated with childhood adversity is particularly important because cortisol is a very powerful anti-inflammatory hormone (6, 7). Depression and psychological stressors can markedly enhance the production of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) (6, 8–11); these cytokines play a central role in a range of age-related diseases, and thus long-term dysregulation could be very important for health (12–14).
In fact, there is good evidence that early adversity has longer-term consequences for inflammation as well as other aspects of immune system regulation; for example, childhood maltreatment is a predictor of elevated C-reactive protein (CRP) in young adults (15). Furthermore, adolescent girls who experienced either early deprivation through institutionalization or physical abuse had higher antibody titers to herpes simplex virus type 1 than normal controls; because the prevalence of HSV infection was similar across the groups, the elevated antibody titers suggested that the cellular immune response was less able to limit herpesvirus reactivation in the former (16). Other studies showed that childhood adversities increased the risk for adult onset arthritis (17); similarly, men and women who had experienced traumatic stress in childhood were more likely to be hospitalized for a spectrum of autoimmune diseases decades later than individuals without a similar stress history (18).
Furthermore, adults who had experienced socioeconomic disadvantage, maltreatment, or social isolation as children were at increased risk for major depression, elevated CRP, and metabolic risk markers including obesity, high blood pressure, high cholesterol, and high glycated hemoglobin (19). Given these findings, it is not surprising that child abuse has been associated with many of the leading causes of death among adults including heart disease, cancer, chronic lung disease, skeletal fractures, and liver disease (20).
A recent study suggested that early maltreatment might even accelerate cell aging; young adults with an average age of 27 who reported childhood maltreatment had shorter telomeres in peripheral blood mononuclear cells (PBMCs) than those who reported no maltreatment (21). Telomeres, DNA/protein repeats that are located at the end of chromosomes, promote chromosomal stability and also regulate the cells’ cellular replicative lifespan (22, 23). Indeed, a growing literature has linked shorter telomeres with health behaviors including physical activity, obesity, and smoking, as well as aging and age-related diseases including cancer, coronary heart disease, diabetes, and heart failure (24–29). Furthermore, there is evidence that shorter telomeres are associated with mortality (25, 28–34), although there are also some null findings, particularly among the oldest old (35, 36).
Although a number of researchers have reported poorer physical health in adults who experienced childhood abuse and/or early adverse events compared to adults without a similarly troubled history, the evidence has primarily come from young or middle-aged adults, with some exceptions (20, 37–45). Poorer health outcomes are likely to be more obvious among older samples, and thus data from younger cohorts may underestimate effect sizes (37). In fact, in a recent meta-analysis of studies that assessed the longer-term medical outcomes of childhood abuse, only a handful had participants whose mean age was greater than 45 (37). In this study we addressed the question of whether childhood abuse and other adversities have lasting, detectable consequences for inflammation and cell aging late in life, and whether the effects are large enough to be discernible beyond that of a major chronic stressor, dementia family caregiving.
Methods
Participants
Participants were 58 caregivers for a spouse or parent with Alzheimer’s disease or another progressive dementia (mean age=70.10, SD=9.41), and 74 demographically similar controls (mean age=69.37, SD=10.73) who had no caregiving responsibilities. Participants were recruited for a caregiving stress and health study via notices placed in community and university newspapers, senior citizen centers, a collaborating neurologist, as well as through the local Alzheimer’s Disease Association between September, 2004, and August, 2009. Individuals with immunologically-related health problems such as cancer or recent surgeries or diabetes were excluded, as well as those taking any medications with broad immunological or anti-inflammatory consequences, e.g., statins, systemic steroids, or antibiotics.
The full sample was predominately female, 72%, reflecting the proportion of women in other caregiver studies (8, 46–48), with no difference between caregivers (71%) and controls (73%). The median education was partial college in both groups, and 7.5% were non-white. The groups did differ in terms of marital status; 41% of controls were married compared to 84% of caregivers, p=.01, and thus marital status was included as a covariate.
Telomere length data were collected on a subsample of individuals recruited between September 2004 and October 2006 by using frozen PBMCs for all caregivers and controls, as described previously (23). The number of caregivers and controls in this sub-cohort were individually matched on age (± 5 years) and sex (23). The average age of the 22 men and 60 women in this subsample was 65.90 (SD=9.71); the median education was partial college; all were married. The Ohio State University Biomedical Research Review Committee approved the project; all participants gave written informed consent prior to participation.
Protocol
Eligible individuals were scheduled for a laboratory or home appointment. To minimize the impact of diurnal cytokine changes, all participants were scheduled between 8 to 10 AM. Participants had their blood drawn, and were weighed before completing self-reported questionnaires about childhood abuse and other adversities, depression, health, and health behaviors.
Measures
The Center for Epidemiological Studies Depression Scale (CES-D) was used to assess the severity of depressive symptoms (49, 50). Studies have shown acceptable test-retest reliability and excellent construct validity (50). Widely used, the CES-D has distinguished depressed from non-depressed participants in community and clinical samples (50).
The Childhood Trauma Questionnaire (CTQ) is a 28-item self-report inventory that assesses abuse and neglect during childhood (51). The CTQ includes 5 subscales: emotional, physical, and sexual abuse, and emotional and physical neglect. The high-sensitivity cut-off scores were used to identify individuals reporting different types of childhood abuse. Participants were classified as having experienced childhood abuse if they reported any form of emotional, physical, or sexual abuse. We focused on the three abuse scales for several reasons; first, the literature on physical health outcomes is drawn primarily from studies of abuse, rather than neglect (37). In addition, the abuse scale items highlight more severe behaviors; examples of emotional abuse items include “People in my family called me things like stupid, lazy, or ugly,” and “I thought my parents wished I had never been born.” Representative emotional neglect scale items include “I felt loved,” and “There was someone in my family who help me feel that I was important or special.” The CTQ possesses excellent 4-month test-retest reliability (52). The questionnaire had adequate convergent validity since it correlated highly with interview-based rating of childhood abuse as well as therapists’ ratings of abuse (51, 52).
Six types of childhood adversity were assessed by asking participants to respond “Yes/No” to experiences that may have occurred before they were 16 years old (53). The adversities included 1) death of the mother; 2) death of the father; 3) severe parental marital problems; 4) an immediate family member suffering from a mental illness; 5) an immediate family member abusing alcohol; and 6) lack of at least one close relationship with an adult. Because the effects of childhood adversity on adult mental health are known to be additive, a categorical adversity variable was created (54). Participants were classified according to whether they had experienced no adversities, one adversity, or more than one adversity.
Health behaviors were evaluated in several ways. The frequency of chronic medical conditions and medications use was assessed with the Physical Health section of the Older Adult Resources and Services (OARS) Multidimensional functional Assessment Questionnaire (55). Assessment of health-related behaviors included body mass index (BMI), smoking, and alcohol intake (56). Questions from Baecke et al. (57) quantified recent physical activity. The Pittsburgh Sleep Quality Index provided data on sleep quality and sleep disturbances in the past month (58).
Cytokine and Telomere Length Assays
Serum levels of IL-6 and TNF-α were assayed using Quantikine High Sensitivity Immunoassay kits (R&D), per kit instructions. Samples were run undiluted in duplicate. Those samples that fell out of range of the standard curve were retested, diluted 1:10 with diluent buffer included with the kit. Plates were read at a wavelength of 490nm with a correction wavelength of 690nm using a Multiscan MCC/340 plate reader (Labsystems). Sample concentrations were extrapolated from a standard curve calculated using a four parameter logistic fit and then multiplied by the dilution factor if necessary.
Telomere length was measured using frozen PBMCs by Southern blot in this subset of our caregiver and control participants as previously described (23). In brief, T cells and monocytes were isolated from PBMC by anti-CD2 and CD14 conjugated beads, respectively. Genomic DNA from PBMCs, T cells, and monocytes were then isolated using a DNA isolation kit (Gentra Systems, Research Triangle Park, NC) and digested with HinfI and RsaI (Roche Molecular Biochemicals) (40U of each enzyme/5 μg DNA). Digested DNA was loaded at 1 μg/well on a 0.6% agarose gel and separated by electrophoresis. The gel was dried at 65°C for 2 hr, denatured and neutralized. The hybridization was performed with a 32P-end-labeled oligonucleotide (CCCTAA)4 probe, at 43°C overnight. The gels were washed three times (5X SSC/0.1%SDS, 2X SSC/0.1% SDS, and 3.2M tetramethylammonium chloride/0.1% SDS) at 45°C, followed by analysis with a phosphor imager (Typhoon 9410; Amersham Biosciences). Mean terminal telomere restriction fragment (TRF) was calculated as described previously (59).
We have previously reported the significant differences in telomere length between caregivers and controls in this subsample of 82 (23). For these additional analyses, we were interested in whether the contributions of childhood abuse and adversity would be evident beyond that of caregiving status.
Statistical Analyses
Analyses consisted of mixed linear models. Independent variables assessed were caregiving status (control vs. caregiver), childhood abuse, childhood adversities, and the 2-way interactions of caregiving status by abuse and caregiving status by adversity. Dependent variables were telomere length, serum interleukin-6 (IL-6) and tumor-necrosis factor-α (TNF-α) levels, and depressive symptoms; age, sex, BMI, and marital status were all included as covariates. Health status and health behaviors were also included in subsequent models to assess their role as potential confounding variables. Tobacco use was not included in the statistical model because of the low prevalence of smokers in the subsample of participants with telomere length data (N=5). Log-transformations were used to normalize right skewed cytokine distributions. A two-sided significance level of α = .05 was used for all tests.
Results
Childhood Abuse and Other Adversities
In this sample, 42 (31.8%) participants reported some form of physical, emotional, or sexual abuse during childhood, while 90 (68.2%) participants did not experience any kind of childhood abuse; 28 participants had 1, while 14 had 2 or 3. Among the 58 caregivers, 19 reported a history of childhood abuse and 39 were not abused. Among the 74 noncaregiving controls, 23 reported a history of abuse, and 51 were not abused. There were no differences between caregivers and controls in the likelihood of being abused, χ2=.04, p = .84.
The absence of childhood adversities was reported by 58 (43.9%) of the sample, 43 (32.6%) participants reported one childhood adversity, and 31 (23.5%) participants experienced multiple childhood adversities. Among the 58 caregivers, 28 had no childhood adversity, 18 reported one adversity, and 12 experienced multiple adversities. Among the 74 noncaregiving controls, 30 had no childhood adversities, 25 reported one adversity, and 19 experienced multiple adversities. There were no differences between caregivers and controls in the likelihood of experiencing one or multiple childhood adversities, χ2=.86, p = .65.
A similar distribution of childhood abuse and adversity was found in the subsample of participants with telomere data. The relative proportions of caregivers and controls who had experienced abuse or other adversities did not differ in the subsample.
Other research has suggested that abuse and other adversities are highly intercorrelated and tend to cluster (53, 60), and this was true in our sample as well. Participants who reported one or more childhood adversities were more likely to have a history of childhood abuse compared to individuals with no childhood adversities, χ2(2)= 23.98, p < .001.
Telomere Length
In a model adjusting for differences in age, sex, BMI, and caregiving status, the presence of childhood adversity was significantly associated with telomere length, t(73) = 1.95, p = .05. Post-hoc tests revealed that participants who had experienced 2 or more childhood adversities had significantly shorter telomeres than those who reported no childhood adversities, p =.01, but did not differ from those who reported only one adversity, p =.12. Childhood abuse was not related to telomere length, t(74) = .67, p = .50. The interactions of caregiving status and childhood adversity, t(74) = 1.48, p = .14, and caregiving status and childhood abuse, t(73) = .27, p = .79, were not significant. Caregivers had significantly shorter telomere length than noncaregiving controls, t(74) = 2.07, p = .04. Telomere length was negatively correlated with IL-6, r = −.32, p = .03, but was not significantly related to TNF-α, r = −.12, p =.45. Figure 1 depicts the relationship between childhood adversity and telomere length.
Figure 1.
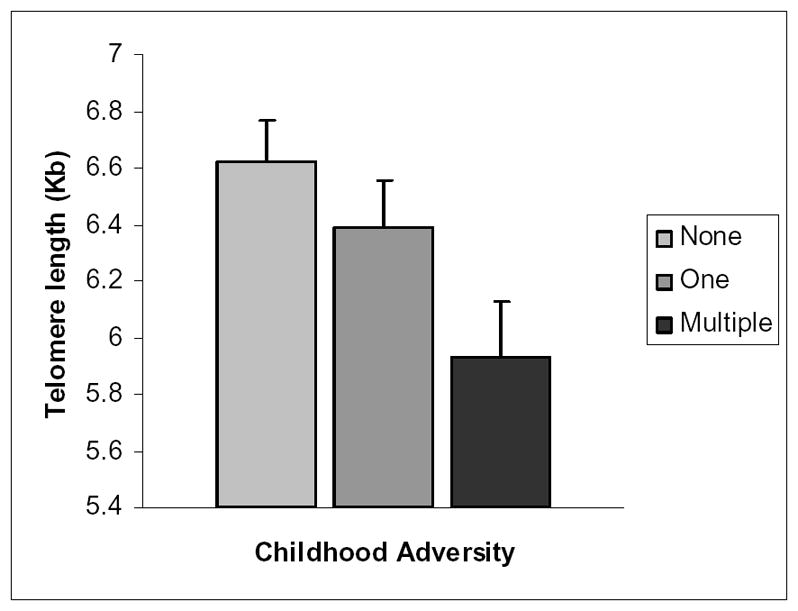
Mean (±SEM) telomere length as a function of childhood adversity. The three groups do not differ in age, with means of 66.06 (0 adversities), 67.20 (1 adversity) and 64.03 (2 or more adversities).
IL-6 and TNF-α
In a model adjusting for differences in age, sex, BMI, marital status, and caregiving status, the presence of any type of childhood abuse was associated with IL-6 levels, F(1,126) = 9.51, p = .003. Individuals who reported at least one type of childhood abuse had greater IL-6 levels than participants who did not experience childhood abuse. Childhood adversity was also significantly related to IL-6 levels, F(1,125) = 1.96, p = .05. Post-hoc tests revealed that individuals who experienced multiple childhood adversities had higher IL-6 levels than participants reporting no adversity, p = .05. The caregiving status by childhood abuse interaction, F(1,24) = 1.03, p = .30, and the caregiving status by childhood adversity interaction, F(1,122) = 1.37, p = .17, were not significant. Although caregivers had higher average IL-6 levels than controls, the difference was not statistically significant, F(1,122) = 1.37, p = .17. Figure 2 illustrates the relationships between IL-6 and childhood abuse, and other adversities.
Figure 2.
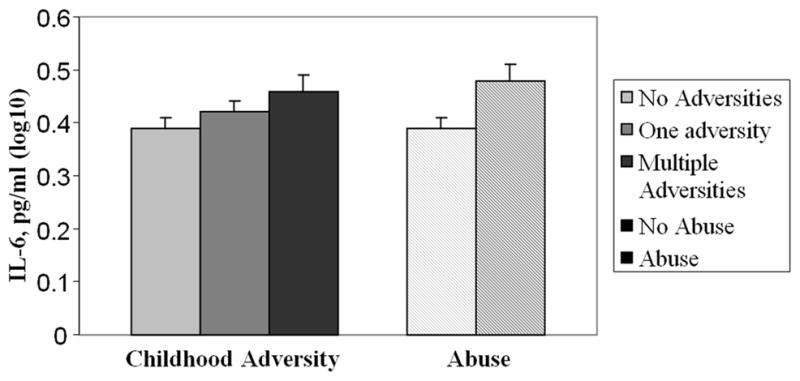
Mean (±SEM) IL-6 levels as a function of childhood abuse and childhood adversity.
In a model adjusting for differences in age, sex, BMI, marital status, and caregiving status, the presence of any type of childhood abuse was marginally associated with TNF-α levels, F(1,124) = 1.86, p = .06. Individuals who had experienced some form of childhood abuse had marginally higher TNF-α than participants who reported no abuse. Furthermore, there was a significant caregiving status by childhood abuse interaction, F(1,123) = 3.48, p = .05. Post-hoc tests revealed that abused caregivers had higher TNF-α levels than non-abused caregivers, p = .009, abused controls, p = .01, and non-abused controls, p = .002. Childhood adversity was not significantly related to TNF-α levels, F(1,123) = 1.16, p = .25. The adversity by caregiving interaction was not significant, F(1,123) = 1.05, p = .30. Caregivers had marginally higher TNF-α than noncaregiving controls, F(1,124) = 1.75, p = .08. Figure 3 depicts TNF-α as a function of the interaction between childhood abuse and caregiving status.
Figure 3.
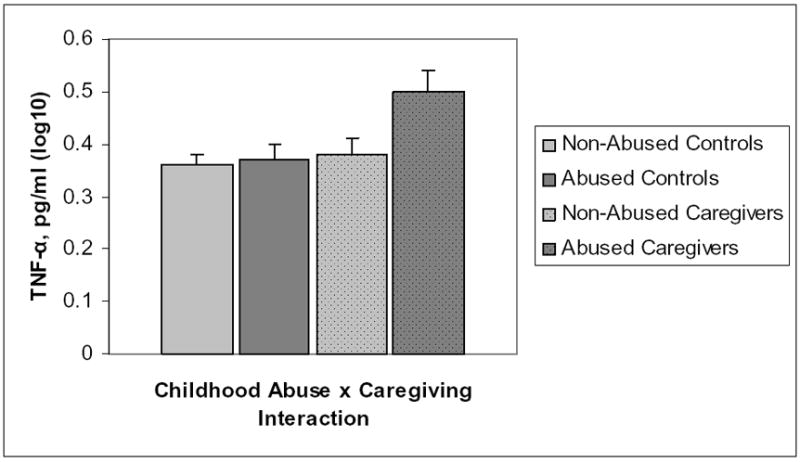
Mean (±SEM) TNF-α levels as a function of the interaction between childhood abuse and caregiving status.
Additive and multiplicative effect of childhood abuse and adversity
The unique contribution of childhood abuse and adversity was tested by including both variables in the same model. Furthermore, the multiplicative effect of childhood abuse and adversity was tested by adding an interaction term composed of the two childhood maltreatment variables to the models. Table 1 presents the results of the additive and multiplicative effects of childhood abuse and adversity. Results show that there was no additive effect of childhood abuse and adversity. For IL-6 only, there was a marginally significant childhood abuse by adversity interaction. Post-hoc tests showed that individuals who reported no childhood abuse or adversities had lower IL-6 than participants reporting childhood abuse and two or more adversities, t = 3.40, p = .01. Among participants who reported two or more childhood adversities, those who did not experience childhood abuse had also lower IL-6 than participants who reported some form of abuse, t = 3.22, p = .02.
Table 1.
Additive and multiplicative effects of childhood abuse and adversity on telomere length and cytokine levels. Results were adjusted for age, sex, BMI, marital status, and caregiving status.
| Telomere length | IL-6 | TNF-α | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Abuse | 2.26 | .14 | 2.45 | .003 | 1.90 | .17 |
| Adversity | 3.13 | .05 | .81 | .42 | 3.58 | .03 |
| Abuse* Adversity | 1.43 | .25 | 2.56 | .08 | .09 | .92 |
Depressive symptoms
After adjusting for age, sex, BMI, and caregiving status, the presence of childhood abuse was associated with greater depressive symptoms, F(1,125) = 2.45, p = .02. Childhood adversities were not related to current depressive symptoms, F(1,124) = .49, p = .69. Interactions were nonsignificant between abuse and caregiving, F(1,124) = .25, p = .80, and adversity by caregiving, F(1,122) = .24, p = .81, in the prediction of current depressive symptoms. Caregivers reported more depressive symptoms than controls, F(1,124) = 4.27, p <.001.
Because depression levels were higher in those who had experienced childhood abuse, we also addressed the question of whether the associations between childhood abuse and cytokines were significant over and above any relationships between depression and cytokine levels. In a hierarchical regression model, depression was entered in the step preceding the childhood abuse variable. Even after including depression in the model, childhood abuse remained significantly associated with IL-6, F(1,124) = 8.67, p = .005, and the caregiving by abuse interaction for TNF-α levels became marginally significant F(1,122) = 3.50, p = .06. However, in the latter case, because caregivers had significantly higher levels of depressive symptoms than controls, we are also attenuating the magnitude of the difference between caregivers and noncaregivers by controlling for depression, and thus diminishing the significance of the interaction term.
Health Behaviors
Health behaviors were included to assess their contribution to telomere length and inflammation. After adjusting for differences in weekly physical activity, alcohol consumption, and sleep quality in the past month, the presence of multiple childhood adversities was still significantly associated with telomere length. Similarly, childhood abuse and adversity remained significantly associated with IL-6 and TNF-α even after adjusting for the aforementioned health behaviors.
The two most common medications used were NSAIDs (n=36) and antidepressants (n=31), and both may affect inflammation. After adjusting for the use of NSAIDs and antidepressants, the impact of abuse on IL-6 remained significant, F(1,123) = 8.50, p = .004, adversity became marginally associated with IL-6, F(2,122) = 3.53, p = .06 and the abuse × caregiving interaction remained significantly associated with TNF-a, F(2,121) = 3.82, p = .05. However, further examination of the data showed that caregivers were marginally more likely to be taking antidepressants than noncaregivers, χ2(1)= 3.28, p = .07, such that controlling for antidepressant use was functionally attenuating the contribution of group membership to the TNF-α group by abuse interaction.
Discussion
Childhood adversity was associated with both shorter telomeres and heightened serum IL-6 levels after controlling for age, caregiving status, gender, body mass index, exercise, and sleep. Childhood abuse was associated with heightened IL-6 and TNF-α levels, and, for TNF-α, this relationship was magnified in caregivers compared to controls. Moreover, childhood abuse and caregiving status were significantly and independently associated with higher levels of depressive symptoms on the CES-D. These data demonstrate that adverse childhood experiences have lasting, measurable consequences later in life, producing effects that were large enough to be perceptible beyond a major chronic stressor, dementia family caregiving.
These effects are particularly noteworthy because the mental and physical health consequences of dementia family caregiving have been well-documented; caregivers have higher rates of depression and poorer health than their counterparts without caregiving responsibilities (61). Caregivers also heal wounds more slowly, respond more poorly to influenza and pneumococcal pneumonia vaccines, show larger age-related increases in inflammation, and have higher rates of mortality than non-caregivers (8, 46–48, 62–64). In accord with their higher rates of mortality, caregivers also have shorter telomeres than their contemporaries without caregiving responsibilities (22, 23). Thus, the finding that childhood adversities are associated with shorter telomeres and greater inflammation even after controlling for age and caregiving status in older adults highlights the importance of childhood maltreatment.
Indeed, a growing literature has documented the clinical significance of cell aging measures for health; shorter telomeres have been linked with aging, age-related diseases, and mortality (65). Reported rates of telomere attrition in PBMCs range from 31 bp to 67 base pairs (bp) per year (23); in the current study the 640 bp difference between individuals reporting no adversities and those reporting multiple adversities could thus translate into a 7–15 year difference in lifespan. However, considering the highly polymorphic nature of telomere length in human, a longitudinal assessment of the rate of telomere attrition of these two groups of individuals will be necessary.
Prior research showed that young adults (average age=27) who reported childhood maltreatment had shorter telomeres compared to those who did not experience maltreatment (21). The fact that we also found differences in cell aging among our older adult participants speaks to both the persistence and the robust nature of this finding.
We found that adversity was associated with higher IL-6 levels as well as shorter telomeres. Inflammation triggers T-cell proliferation and enhances the leukocyte turnover rate, one known cause of telomere shortening (24), and higher IL-6 levels were associated with shorter telomeres in our data.
The findings on inflammation are also important because sustained overproduction of IL-6 and TNF-α has been linked to a spectrum of age-associated health problems including cardiovascular disease, osteoporosis, arthritis, type 2 diabetes, certain cancers, and Alzheimer’s disease (66). In fact, more globally, chronic inflammation has been suggested as one key biological mechanism that may fuel declines in physical function leading to frailty, disability, and, ultimately, death. Prior work has shown that childhood maltreatment predicts elevated CRP in young adults (15). Our data suggest that adverse early events are related to continuing vulnerability even among older adults, enhancing the impact of chronic stressors like caregiving on inflammation.
If dementia family caregivers were genetically predisposed to higher levels of inflammation and/or to shorter telomeres, we might see a similar pattern of risk. In our prior caregiver studies we used data from structured clinical interviews to assess both past (lifetime) and current mood disorders (67, 68). We found that caregivers experienced significantly more depressive disorders during the years they had been providing care than matched controls experienced during the same developmental and historical time periods. In addition, caregivers’ incidence of syndromal depression, particularly major depression, was higher than reports from epidemiological surveys of older community-dwelling adults, while the number of cases among comparison participants was comparable to base rates found in epidemiological studies. Most noteworthy, however, was the fact that neither depressive disorders prior to caregiving nor family history was even weakly related to identification of caregivers at risk. Caregivers with no personal or family mood disorder history developed depressive disorders concomitant with caregiving. These data suggest that the chronic strains of caregiving can provoke syndromal mood disorders in older adults with no prior evidence of vulnerability, as well as subsyndromal elevations in depressive symptoms. Moreover, most of our studies have focused on spousal caregivers who have no genetic predisposition for Alzheimer’s. Thus, in considering the higher levels of proinflammatory cytokines and shorter telomeres observed in caregivers compared to their noncaregiving counterparts in this and other studies (8, 46, 69, 70), we assume that these differences largely reflect the strains of caregiving, rather than genetic predispositions to inflammation or shorter telomeres.
However, psychiatric disorders have a clear genetic component, and our list of adversities includes conditions such as family mental illness and alcohol abuse. Thus, it is possible that there are genetic links between some adversities and the biomarkers we studied.
We found that reports of abuse and other adversities were significantly related, consistent with evidence that early stressful circumstances are likely to co-occur (37). We did not have a sufficiently large sample to allow us to assess the unique effects of particular kinds of abuse or other adversities, one limitation of the study. In addition, conclusions about gender effects are limited by the preponderance of female participants in the sample (68%). Furthermore, individuals with a history of maltreatment are more likely to develop harmful health behaviors like excessive alcohol consumption (37), and are more likely to become obese (71); the fact that we controlled for these behaviors means that our data may underestimate the impact of childhood abuse and adversity.
Other studies have provided solid evidence that childhood abuse and other adversities can affect adults’ mental and physical health. Our data confirm the prior findings and extend them by showing differences may be measurable in older adults, and are of sufficient magnitude to be discernible even beyond the effects of a notable chronic stressor, dementia caregiving. Childhood adversity casts a very long shadow.
Acknowledgments
The study was supported in part by NIH grants AG025732, UL1RR025755, CA16058, a doctoral training award from the Fonds de la Recherche en Santé du Québec, and the Intramural Research Programs of the National Institute on Aging, NIH. We appreciate the helpful assistance of Cathie Atkinson and Bryon Laskowski with the study, as well as the Central Ohio Alzheimer’s Association.
Acronyms
- BMI
body mass index
- CES-D
Center for Epidemiological Studies Depression Scale
- CRP
C-reactive protein
- IL-6
interleukin 6
- PBMC
peripheral blood mononuclear cells
- TNF-α
tumor necrosis factor-alpha
References
- 1.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: associations with persistence of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:124–32. doi: 10.1001/archgenpsychiatry.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 5.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 6.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappa B signaling. Biol Psychiatry. 2008;64:266–72. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong S, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol A Biol Sci Med Sci. 1999;54:M434–9. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. The American journal of psychiatry. 2004;161:271–7. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 10.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–84. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 11.Miller GE, Backwell E. Turning up the heat: Inflammation as a mechanism linking chronic stress, depression, and heart disease. Curr Dir Psychol Sci. 2006;15:269–72. [Google Scholar]
- 12.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in persons>65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–24. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 15.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proceedings of the National Academy of Sciences. 2009;106:2963–7. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Korff M, Alonso J, Ormel J, Angermeyer M, Bruffaerts R, Fleiz C, de Girolamo G, Kessler RC, Kovess-Masfety V, Posada-Villa J, Scott KM, Uda H. Childhood psychosocial stressors and adult onset arthritis: broad spectrum risk factors and allostatic load. Pain. 2009;143:76–83. doi: 10.1016/j.pain.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–50. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–43. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 21.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67:531–4. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng N-p. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aviv A. Telomeres and human aging: facts and fibs. Science of Aging, Knowledge, and Environment. 2004;51:pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- 25.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 26.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte Telomere Length and Cardiovascular Disease in the Cardiovascular Health Study. Am J Epidemiol. 2006;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 28.van der Harst P, de Boer RA, Samani NJ, Wong LS, Huzen J, Codd V, Hillege HL, Voors AA, van Gilst WH, Jaarsma T, van Veldhuisen DJ. Telomere length and outcome in heart failure. Ann Med. 2010;42:36–44. doi: 10.3109/07853890903321567. [DOI] [PubMed] [Google Scholar]
- 29.Astrup AS, Tarnow L, Jorsal A, Lajer M, Nzietchueng R, Benetos A, Rossing P, Parving HH. Telomere length predicts all-cause mortality in patients with type 1 diabetes. Diabetologia. 53:45–8. doi: 10.1007/s00125-009-1542-1. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60:174–80. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, Hjelmborg JVB, Gardner JP, Bathum L, Brimacombe M, Lu XB, Christiansen L, Vaupel JW, Aviv A, Christensen K. Telomere length and mortality: A study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 33.Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease - Data from the heart and soul study. Arteriosclerosis Thrombosis and Vascular Biology. 2008;28:1379–84. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu KD, Orme LM, Shaughnessy J, Jacobson J, Barlogie B, Moore MAS. Telomerase and telomere length in multiple myeloma: correlations with disease heterogeneity, cytogenetic status, and overall survival. Blood. 2003;101:4982–9. doi: 10.1182/blood-2002-11-3451. [DOI] [PubMed] [Google Scholar]
- 35.Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, Harris TM, Cummings SR, Cawthon RM. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–4. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RGJ. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–90. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 37.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71:805–12. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- 38.White HR, Widom CS. Does childhood victimization increase the risk of early death? A 25-year prospective study. Child Abuse & Neglect. 2003;27:841–53. doi: 10.1016/s0145-2134(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 39.Sachs-Ericsson N, Blazer D, Plant EA, Arnow B. Childhood sexual and physical abuse and the 1-year prevalence of medical problems in the national comorbidity survey. Health Psychol. 2005;24:32–40. doi: 10.1037/0278-6133.24.1.32. [DOI] [PubMed] [Google Scholar]
- 40.Leserman J, Li Z, Drossman DA, Hu YJB. Selected symptoms associated with sexual and physical abuse history among female patients with gastrointestinal disorders: the impact on subsequent health care visits. Psychol Med. 1998;28:417–25. doi: 10.1017/s0033291797006508. [DOI] [PubMed] [Google Scholar]
- 41.Kendall-Tackett KA, Marshall R. Victimization and diabetes: An exploratory study. Child Abuse & Neglect. 1999;23:593–6. doi: 10.1016/s0145-2134(99)00033-2. [DOI] [PubMed] [Google Scholar]
- 42.Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med. 2004;34:509–20. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- 43.Drossman DA, Leserman J, Nachman G, Li ZM, Gluck H, Toomey TC, Mitchell CM. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–33. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 44.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–54. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- 45.Walker EA, Gelfand A, Katon WJ, Koss MP, Von Korff M, Bernstein D, Russo J. Adult health status of women with histories of childhood abuse and neglect. Am J Med. 1999;107:332–9. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- 46.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Kanel R, Dimsdale JE, Mills PJ, Ancoli-Israel S, Patterson T, Mausbach BT, Grant I. Effect of Alzheimer caregiving stress and age on frailty markers interleukin-6, c-reactive protein, and d-dimer. J Gerontol. 2006;61A:963–9. doi: 10.1093/gerona/61.9.963. [DOI] [PubMed] [Google Scholar]
- 48.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. JAMA. 1999;282:2215–9. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 49.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 50.Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. Washington D. C: American Psychological Association; 1997. pp. 207–45. [Google Scholar]
- 51.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-report. San Antonio, Texas: The Psychological Corporation; 1998. [Google Scholar]
- 52.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 53.Kessler RC, Magee WJ. Childhood adversities and adult depression: Basic patterns of association in a US national survey. Psychol Med. 1993;23:679–90. doi: 10.1017/s0033291700025460. [DOI] [PubMed] [Google Scholar]
- 54.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 55.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS Multidimensional Functional Assessment Questionnaire. J Gerontol. 1981;36:428–34. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 56.Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- 57.Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 58.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 59.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 60.Schilling EA, Aseltine RH, Gore S. The impact of cumulative childhood adversity on young adult mental health: Measures, models, and interpretations. Social Science & Medicine. 2008;66:1140–51. doi: 10.1016/j.socscimed.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–62. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–6. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 63.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–7. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glaser R, Sheridan JF, Malarkey WB, MacCallum RC, Kiecolt-Glaser JK. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom Med. 2000;62:804–7. doi: 10.1097/00006842-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Epel ES. Telomeres in a Life-Span Perspective: A New "Psychobiomarker"? Curr Dir Psychol Sci. 2009;18:6–10. [Google Scholar]
- 66.Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2009.09.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dura JR, Stukenberg KW, Kiecolt-Glaser JK. Chronic stress and depressive disorders in adult children caring for demented parents. Psychology of Aging. 1990;6:467–73. doi: 10.1037//0882-7974.6.3.467. [DOI] [PubMed] [Google Scholar]
- 68.Dura JR, Stukenberg KW, Kiecolt-Glaser JK. Anxiety and depressive disorders in adult children caring for demented parents. Psychol Aging. 1991;6:467–73. doi: 10.1037//0882-7974.6.3.467. [DOI] [PubMed] [Google Scholar]
- 69.Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J Clin Oncol. 2009;27:2909–15. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- 70.von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc. 2006;54:431–7. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 71.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54:330–7. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]


