Abstract
An influx of experimental and theoretical studies of ion transport protein structure has inspired efforts to understand underlying determinants of ionic selectivity. Design principles for selective ion binding can be effectively isolated and interrogated using simplified models composed of a single ion surrounded by a set of ion-ligating molecular species. While quantum mechanical treatments of such systems naturally incorporate electronic degrees of freedom, their computational overhead typically prohibits thorough dynamic sampling of configurational space, and thus, requires approximations when determining ion-selective free energy. As an alternative, we employ dynamical simulations with a polarizable force field to probe the structure and K+/Na+ selectivity in simple models composed of one central K+/Na+ ion surrounded by 0–8 identical model compounds – N-methylacetamide, formamide, or water. In the absence of external restraints, these models represent gas-phase clusters displaying relaxed coordination structures with low coordination number. Such systems display Na+ selectivity when composed of more than ~3 organic carbonyl-containing compounds, and always display K+ selectivity when composed of water molecules. Upon imposing restraints that solely enforce specific coordination numbers, we find all models are K+-selective when ~7–8-fold ion coordination is achieved. However, when models composed of the organic compounds provide ~4–6-fold coordination, they retain their Na+-selectivity. From these trends, design principles emerge that are of basic importance in the behavior of K+ channel selectivity filters, and suggest a basis not only for K+ selectivity, but also modulation of block and closure by smaller ions.
Recent growth in knowledge of biological ion transport protein structure has motivated investigations to identify principles underlying ion-selective binding site design. A potentially incisive means of isolating and directly probing specific design principles lies in the construction of simplified ion-ligating model systems whose ionic selectivity (taking bulk water as a reference) can be determined computationally1–10. Such systems are composed of a single ion, such as K+ or Na+, interacting with a set of surrounding molecular species. The functional groups of these surrounding species are typically chosen to interrogate the effect of ligand chemistry on specific local interactions. For example, to model ion interactions with the peptide backbone, simple uncharged compounds like formamide, formaldehyde, or N-methylacetamide (NMA) are chosen to surround the ion.
Despite the simplicity of such models, subtleties in their design and analysis can lead to quantitatively or even qualitatively different ion-selective behavior. This notion manifests itself most apparently in simplified model studies aiming to probe mechanistic aspects of K+ selectivity over Na+ in carbonyl-lined K+ channel binding sites. For example, one such set of studies concluded that the eight carbonyl (C=O) ligands of a canonical K+ channel binding site, by virtue of their electrostatic properties alone (especially their large dipole moment), are intrinsically suited to select K+ over Na+4,5,11. Moreover, it was suggested that K+ selectivity is entirely lost or even reversed when replacing a model’s C=O ligands with water molecules5,11. Thus, it was argued to be unlikely that a Na+-selective site could be formed exclusively by backbone C=O ligands11. However, other simplified model studies found this trend (i.e., loss or reversal of K+ selectivity) upon C=O/water replacement could not be reproduced1,2,8–10, and closer investigation suggested external restraints (i.e., architectural, topological, or structural forces) from the protein are crucial for K+ selectivity by the C=O ligands1–3,7–10,12–15.
More recent works16,17, though oppositional, do not present evidence or theory that conflicts the conclusions of the latter studies1–3,7–10,12–15, but vigorously question the methods used in their simplified model calculations. While such questioning is mostly abated by the literature1,3, the outstanding issue pertains to those works7–10,15 that employ quantum mechanical (QM) methods to investigate selectivity in simplified models17. Although such approaches are attractive because they explicitly account for electronic degrees of freedom, questions are raised16,17 because their free energy estimates are based on optimized molecular configurations around K+/Na+, and only implicitly consider the effects of dynamic sampling.
In this study, we consider simple models, comprised of one ion and 0–8 surrounding model compounds – NMA, water, or formamide – using the AMOEBA polarizable force field18 (see Supporting Information (SI)). These models are more computationally accessible and simultaneously model polarizability, as available in QM studies. As such, we are able to sample configurational space using molecular dynamics simulation to obtain precise free energy measures of K+/Na+ selectivity. Furthermore, we can probe the models’ emergent coordination structure to see how it affects K+/Na+ selectivity.
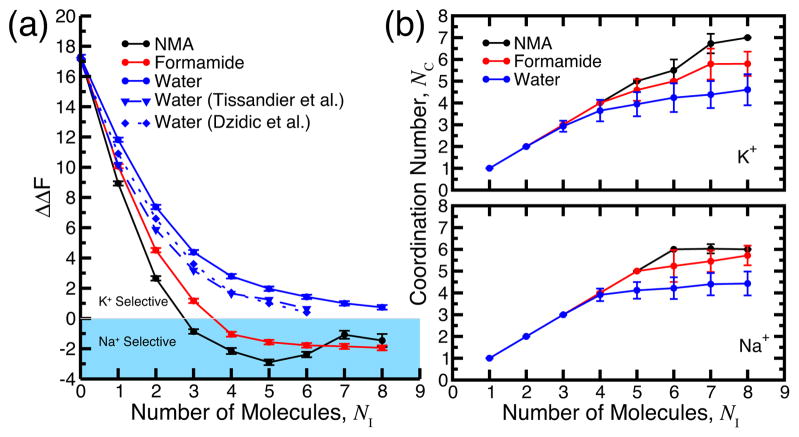
Figure 1a displays the Helmholtz free energy of selectivity with respect to bulk water, ΔΔF, for models where surrounding compounds are unrestrained (i.e., gas-phase). For 0–2 molecules, all such models produce K+ selectivity (positive ΔΔF). However, as the number of included molecules, NI, is increased toward eight, all models produce ΔΔF approaching the expected bulk liquid values for their respective compositions. As NI becomes very large, ΔΔF is expected to reach exact bulk liquid values. For example, bulk water is definitively nonselective (ΔΔF ≡ 0), and prior work (as pointed out by many3,7,8) suggests that ΔΔF in the organic solvents is either Na+-selective (ΔΔF < 0) or nonselective18,21. In contrast, widely-used pairwise additive models of liquid NMA and Formamide are known to provide K+ selectivity in the range of ~1.3–3.8 kcal/mol, depending on the model4,18. However, it is of particular interest that all models composed of water molecules in Figure 1a (blue data) are invariably more K+-selective than the C=O-containing models.
Figure 1.
Analysis of unrestrained (gas-phase) simplified ion-ligating models. (a) Helmholtz free energy of selectivity versus the number of (NMA, formamide, or water) molecules, NI, in the cluster – calculated as where and are free energies to alchemically transform K+→Na+ in the cluster and in bulk water, respectively. ΔΔF >0 and ΔΔF < 0 indicate K+ and Na+ selectivity, respectively. For comparison we show experimental values for water molecules19,20 (blue dashed/dotted lines). Error bars were obtained by block averaging (see SI). (b) Average coordination number, NC, versus the number of molecules, NI, included in the cluster. Vertical bars represent standard deviation of the sample.
Analyzing ion-oxygen coordination in these models (see SI), we find that the number of molecules included in each model, NI, is not equal to the number of molecules actually coordinating the ion, NC (Figure 1b). Thus, one may not necessarily use such gas-phase models to draw conclusions about the effect of NC on ΔΔF as suggested by recent work16. Figure 1b shows that, upon including more than ~4 (water, NMA, or formamide) molecules around K+ or Na+, second and/or third solvation shells form (see also Figure S1). When NI = 8, water provides the lowest NC (~4–5 for K+ and Na+) of all compounds. Under the same conditions, formamide provides ~5–6-fold coordination, and NMA provides ~7-fold and ~6-fold coordination for K+ and Na+, respectively.
If an external potential (see SI) is imposed to ensure ion-oxygen coordination by all included molecules (i.e., NI Δ NC), the selectivity trend in unrestrained models (Figure 1a) is qualitatively reproduced for ~0–6 molecules (see Figure 2a). However, ΔΔF is increased drastically with ~7–8 coordinating molecules (Figure 2a) in comparison with the unrestrained models. Coordinating models composed of the organic C=O-containing compounds become K+-selective when more than ~6 oxygen atoms coordinate both K+ and Na+. Following prior studies4,5,11,16, we tested selectivity in models using a “generic” harmonic volume-confinement that restrains all ion-oxygen distances to be less than ~3.5 Å (Figure 2b). Figures 2a and 2b show that the qualitative trend in such models is the same. However the generic confinement models (Figure 2b) provide lower (but still positive) ΔΔF for ~7–8 included molecules. The quantitative difference in K+-selectivity between the models is explained by observing the dependence of NC on NI. Beyond 6–7 molecules, full coordination (i.e., NI = NC) is not necessarily enforced by the generic confinement (Figure S4). The difference between NI and NC is largest in the 3.5 Å generic confinement model when NI = 8 water molecules. This model provides ~6–7 and ~5–6 coordinating water molecules for K+ and Na+, respectively (Figure S5), and as a result, ΔΔF is lower than that provided by the C=O-containing compounds of the corresponding model (Figure 2b, NI = 8).
Figure 2.
Simplified models with external restraints. Helmholtz free energy of selectivity as a function of the number of molecules (a) coordinating K+/Na+ and (b) included in a model imposing 3.5 Å volume-confinement. Error bars obtained by block averaging.
These unrestrained and restrained models indicate that, despite the caveats17 suggested for QM-based studies, the qualitative conclusion of prior works1–3,7–10,12–15 – that sole enforcement of >6-fold coordination by the organic C=O-containing compounds or water is a sufficient, though not a necessary, condition for K+/Na+ selectivity. This result is in stark contrast with inferences from pairwise-additive models of C=O-containing ligands4,5,11, which are shown to provide a residual K+-selectivity both in bulk liquids and in simplified models “across the board” (i.e., for all NI)1,2,4,5,11,18. Taken together, Figures 1 and 2 suggest that the imposed external restraints applied in this study “cause” K+ selectivity in models including ~7–8 C=O-containing compounds. More to the point, the K+ selectivity in these models (Fig. 2) is not “caused” or “controlled” by the C=O ligands, themselves. Nor is it caused by specified (e.g., ion-ligand, ligand-ligand, et al.) interaction energy or entropic contributions (SI Discussion). Instead, the external potential applied to the ligands generally determines all such contributions (Table S1 and SI Discussion) to yield a positive net ΔΔF. In fact, without the external potential (i.e., “topological” control1–3,7–10,12–15), these contributions would yield net Na+-selectivity (Fig. 1a).
Our findings have implications for permeant ion effects on K+ channel behavior22–25. Both Na+ and K+ are known to bind the K+ channel selectivity filter, but at different sites, and with different specific interactions with C=O and water13,14,22. If C=O ligands were always K+ selective, then interactions with smaller ions like Na+ or Li+ would not modulate filter block22,23 or closure25. Some modes of Na+ binding in the filter can involve ~4–6 C=O ligands13,14,22, and are favorable. Others will impose larger coordination numbers4,10,12,13 on both K+ and Na+, will be K+ selective, and provide barriers to Na+ permeation. These concepts appear rudimentary.
Supplementary Material
Acknowledgments
This material is based upon work supported by the National Science Foundation (NSF) under grant number 0434578. Additional NSF (PHYS0216576 and MCB-0413858) and National Institutes of Health (RR06009) support are also acknowledged.
Footnotes
Supporting Information Available: Discussion, Methods, and additional figures from simplified model analyses. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Bostick DL, Arora K, Brooks CL., III Biophys J. 2009;96:3887–3896. doi: 10.1016/j.bpj.2008.12.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostick DL, Brooks CL., III Proc Natl Acad Sci U S A. 2007;104:9260–9265. doi: 10.1073/pnas.0700554104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostick DL, Brooks CL., III Biophys J. 2009;96:4470–4492. doi: 10.1016/j.bpj.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noskov SY, Bernèche S, Roux B. Nature. 2004;431:830–834. doi: 10.1038/nature02943. [DOI] [PubMed] [Google Scholar]
- 5.Noskov SY, Roux B. J Gen Physiol. 2007;129:135–143. doi: 10.1085/jgp.200609633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noskov SY, Roux B. J Mol Biol. 2008;377:804–818. doi: 10.1016/j.jmb.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas M, Jayatilaka D, Corry B. Biophys J. 2007;93:2635–2643. doi: 10.1529/biophysj.107.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varma S, Rempe SB. Biophys J. 2007;93:1093–1099. doi: 10.1529/biophysj.107.107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma S, Rempe SB. J Am Chem Soc. 2008;130:15405–15419. doi: 10.1021/ja803575y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudev T, Lim C. J Am Chem Soc. 2009;131:8092–8101. doi: 10.1021/ja900168k. [DOI] [PubMed] [Google Scholar]
- 11.Noskov SY, Roux B. Biophys Chem. 2006;124(124):279–291. doi: 10.1016/j.bpc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Baştuğ T, Kuyucak S. Biophys J. 2009;96(10):4006–4012. doi: 10.1016/j.bpj.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler PW, Tai K, Sansom MSP. Biophys J. 2008;95:5062–5072. doi: 10.1529/biophysj.108.132035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miloshevsky GV, Jordan PC. Biophys J. 2008;95:3239–3251. doi: 10.1529/biophysj.108.136556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varma S, Sabo D, Rempe SB. J Mol Biol. 2008;376:13–22. doi: 10.1016/j.jmb.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, Noskov SY, Roux B. J Phys Chem B. 2009;113:8725–8730. doi: 10.1021/jp901233v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Roux B. Biophys J. 2009;97:L15–L17. doi: 10.1016/j.bpj.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossfield A, Ren P, Ponder JW. J Am Chem Soc. 2003;125:15671–15682. doi: 10.1021/ja037005r. [DOI] [PubMed] [Google Scholar]
- 19.Dzidic I, Kebarle P. J Phys Chem. 1970;74(7):1466–1474. [Google Scholar]
- 20.Tissandier MD, Cowen KA, Feng WY, Gundlach E, Cohen MH, Earhard AD, Coe JV, Tuttle TR. J Phys Chem. 1998;102:7787–7794. [Google Scholar]
- 21.Marcus Y. Pure Appl Chem. 1983;55:977–1021. [Google Scholar]
- 22.Thompson AN, Kim I, Panosian TD, Iverson TM, Allen TW, Nimigean CM. Nat Struct and Mol Biol. 2009;16(22):1317–1324. doi: 10.1038/nsmb.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimigean CM, Miller C. J Gen Physiol. 2002;120:323–335. doi: 10.1085/jgp.20028614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loboda A, Melishchuk A, Armstrong C. Biophys J. 2001;80(6):2704–2714. doi: 10.1016/S0006-3495(01)76239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swenson RP, Armstrong CM. Nature. 1981;291:427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.