Abstract
Intrarenal angiotensinogen (AGT) is expressed highly in renal proximal tubular cells (RPTCs) and contributes to the regulation of intrarenal angiotensin II levels. Inhibition of nuclear factor (NF)-κB suppressed human (h)AGT expression in human RPTCs. However, the presence and localization of an NF-κB binding site in the hAGT promoter region have not been determined. Therefore, this study was performed to demonstrate that an NF-κB binding site in the hAGT promoter region contributes to hAGT promoter activity in human RPTCs. The hAGT promoter region was cloned from −4358 to +122 and deletion analysis was performed. A possible NF-κB binding site was removed from the hAGT promoter region (M1) and mutated (M2). Human RPTCs were transfected, and hAGT promoter activity was determined by luciferase assay. The identity of DNA binding proteins from binding assays were determined by Western blot. Progressive 5′-end deletions demonstrated removal of a distal promoter element in hAGT_−2414/+122 reduced promoter activity (0.61±0.12, ratio to hAGT_−4358/+122). Inhibition of NF-κB suppressed promoter activity in hAGT_−4358/+122 (0.51±0.14, ratio to control) and hAGT_−3681/+122 (0.48±0.06, ratio to control) but not in the construct without the NF-κB binding site. Promoter activity was reduced in the domain mutants M1 (0.57±0.08, ratio to hAGT_−4358/+122) and M2 (0.61±0.16, ratio to hAGT_−4358/+122). DNA binding levels of NF-κB protein were reduced in M1. These data demonstrate the functional importance of an NF-κB binding site in the hAGT promoter region, which contributes to hAGT promoter activity in human RPTCs.
Keywords: angiotensinogen, NF-κB, renal proximal tubular cells
The intrarenal renin–angiotensin system (RAS) plays important roles in regulating sodium excretion and blood pressure.1 Angiotensinogen (AGT) is the only known substrate for renin which is the rate-limiting enzyme for RAS activity.1,2 The plasma concentration of AGT is close to the Michaelis–Menten constant of the enzymatic reaction between renin and AGT.3,4 For this reason, increases in AGT, as well as renin, can lead to increases in angiotensin (Ang) II production.1 Recent studies using experimental animal models including transgenic mice have documented the involvement of AGT in RAS activity.5–14 Genetic manipulations that lead to overexpression of the AGT gene have consistently been shown to cause hypertension.15,16 In human genetic studies, a linkage between the AGT gene and hypertension has been established.17–24 Therefore, expression levels of AGT plays a role in blood pressure regulation.
Several studies have demonstrated that the kidney possesses all of the components of the RAS.1 Furthermore, intrarenal Ang II has been shown to be elevated in many forms of hypertension and blockade of the components of the RAS control hypertension.1 In the kidney, AGT is expressed mainly in renal proximal tubular cells (RPTCs).25–27 Intrarenal AGT expression is enhanced by chronic Ang II infusion and in various models such as human renin/kidney-specific human (h)AGT double transgenic mice.11 Thus, AGT production in RPTCs regulates intrarenal RAS activity.
Several studies have demonstrated the importance of regulatory elements in the proximal promoter region of the hAGT gene.28–31 The mechanisms of the proximal regulatory elements in the hAGT gene have been clarified by studies of polymorphisms in the hAGT promoter region.17,22 Thus, the proximal promoter elements are important in regulation of hAGT expression.
In rodent tissues and cells, nuclear factor (NF)-κB has been demonstrated to mediate rat AGT transcription.32–34 An acute-phase response element was identified in the rat AGT promoter region, which consists of a composite NF-κB and CCAAT/enhancer protein binding site.35 Previous studies have demonstrated high basal NF-κB activity in human RPTCs.36 Furthermore, we recently reported in human RPTCs that parthenolide, an inhibitor of NF-κB activity, suppressed basal hAGT mRNA expression.37 However, the existence of an NF-κB binding site in the hAGT promoter region has not been reported,31,38 although data indicate NF-κB may play a role in hAGT gene expression. Therefore, this study was performed to demonstrate that an NF-κB binding site in the hAGT promoter region contributes to hAGT promoter activity in human RPTCs.
Methods
Materials
An NF-κB inhibitor, parthenolide, was purchased from MP Biomedicals and used as described previously.37,39 The pGL4.14 firefly luciferase reporter vector plasmid and the pRL-TK Renilla luciferase reporter vector plasmid (internal control for transfection efficiency) were obtained from Promega. Rabbit anti-p65 antibody was obtained from Cell Signaling Technology. IRDye labeled anti-rabbit IgG antibody was obtained from Li-Cor as secondary antibodies in Western blot analysis.
Cell Culture
Human kidney (HK)-2 cells, immortalized human RPTCs, were obtained from the American Type Culture Collection and cultured in 24-well plates (Costar) as described previously.37,40
Bioinformatics Analysis
DNA sequence of hAGT promoter region in human chromosome 1 was obtained from National Center for Biotechnology Information (NCBI) accession no. NG008836.1. A possible NF-κB binding site in hAGT promoter region was searched by GENETYX software based on the human NF-κB binding motif.41
Plasmid Construction
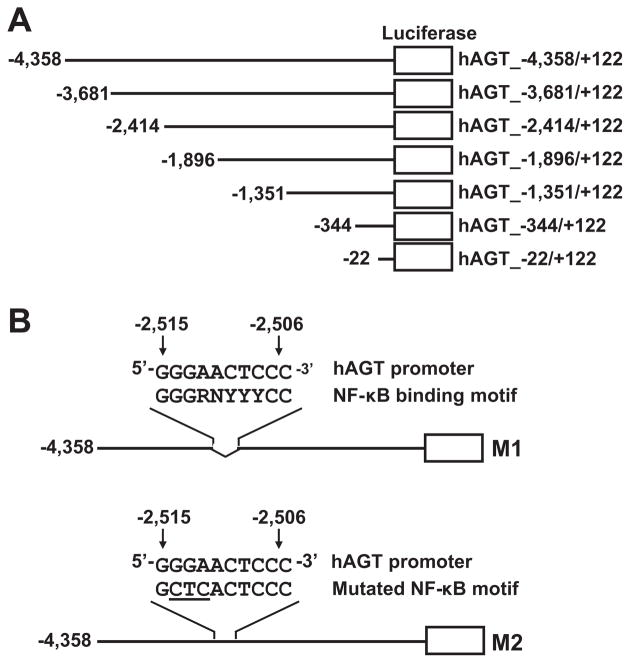
Human genomic DNA was isolated from human keratinocyte cells (Lonza) using a genomic DNA isolation kit (Invitrogen). Using the genomic DNA as a template, the 5′-flanking hAGT promoter region was isolated by PCR using a high-fidelity polymerase kit (Clontech). Sense (5′-CAG CTC ATG GAC AGC TAT GTC TGA ATC AAC AG-3′) and antisense (5′-ATA AGC TTA TCA CAG CTC AGT TAC ATC TGA G-3′) primers for PCR amplification of the hAGT 5′-flanking area corresponded to the hAGT gene nucleotides −5223 to +122 (relative to the transcription start site of the hAGT gene). Each PCR cycle consisted of a 10-second denaturing step at 98°C, a 15-second annealing step at 65°C, and a 7-minute extension step at 72°C, for a total of 30 cycles. The PCR product was then cloned into the pGL4.14 luciferase vector plasmid using XbaI (Invitrogen) and HindIII (Invitrogen) restriction enzymes to generate the construct hAGT_−4358/+122 (NCBI HQ287930). Progressive deletion mutants (Figure 1A) were constructed using a Deletion kit for Kilo Sequencing (TaKaRa) according to the protocol of the manufacturer. The deletion of the possible NF-κB binding site sequence 5′-GGG AAC TCC C-3′ (−2515 to −2506) in mutant-1 (M1) (Figure 1B) was generated by PCR amplification of hAGT_−4358/+122 using sense (5′-CTT TAT AAA ACC ATC AGA TCT CAT GAG-3′) and antisense (5′-CTG AAC ATG CTT TCT CTC TTT TGC C-3′) primers. The domain mutant-2 (M2) (Figure 1B) was generated from hAGT_−4358/+122 with a QuickChange site-directed mutagenesis kit (Stratagene) according to the protocol of the manufacturer. The possible NF-κB binding site sequence was changed from 5′-GGG AAC TCC C-3′ to 5′-GCT CAC TCC C-3′ in M2 (The underlined sequence represents the mutated bases of the NF-κB binding site in M2.). The full sequence of all plasmid constructs were sequenced by an autosequencer (ABI Prism 3130x1) and confirmed as expected.
Figure 1.
The hAGT promoter region constructs used in this study. A, Deletion mutant constructs of the hAGT promoter region. Thick horizontal lines represent hAGT promoter region sequences; open boxes, the pGL4.14 luciferase reporter vector sequence. B, Domain mutations of the possible NF-κB binding site in the hAGT promoter region. M1 indicates the mutant-1 with a 10-bp removal of the possible NF-κB binding site; M2, the mutant-2 with point mutations in the possible NF-κB binding site. Nucleotides: G, guanine; A, adenine; C, cytosine; T, thymine; R, purine (A or G); Y, pyrimidine (C or T); N, any nucleotide.
Luciferase Assay
Transient DNA transfections were performed as described previously42 using Lipofectamine 2000 (Invitrogen). Luciferase assays were performed using a commercially available kit (Promega) and an automatic plate reader (Optima) as described previously.42 Data were normalized based on the pRL-TK luciferase activity.
DNA–Protein Binding Assay
The hAGT promoter region constructs, hAGT_−4358/+122 and M1, were biotinylated using a commercially available kit (Pierce). DNA affinity purification was performed using biotinylated constructs as described previously43 with minor modifications of the binding buffer and elution buffer components. Plasmid DNA, which does not contain an NF-κB binding site, and a no-DNA control were also biotinylated and used as negative controls in the binding studies. The binding buffer consisted of 10 mmol/L Tris-HCl, pH 7.5, 50 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40, 2 mmol/L Na3VO4, 5 mmol/L NaF, and 0.25% protease inhibitor cocktail (Sigma). An elution buffer consisting of 10 mmol/L Tris-HCl, pH 7.5, 250 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40, 2 mmol/L Na3VO4, 5 mmol/L NaF, and 0.25% protease inhibitor cocktail was used to elute proteins for Western blot analysis.
Western Blot Analysis
Western blot analysis was performed as described previously.37,44
Statistical Analysis
An unpaired t test was used to compare values between 2 groups and 1-way ANOVA, followed by Dunnett test, was used to compare values between more than 2 groups. All of the data are presented as means±SD. P<0.05 was considered significant.
Results
Deletion Analysis of the 5′-Upstream hAGT Promoter Region
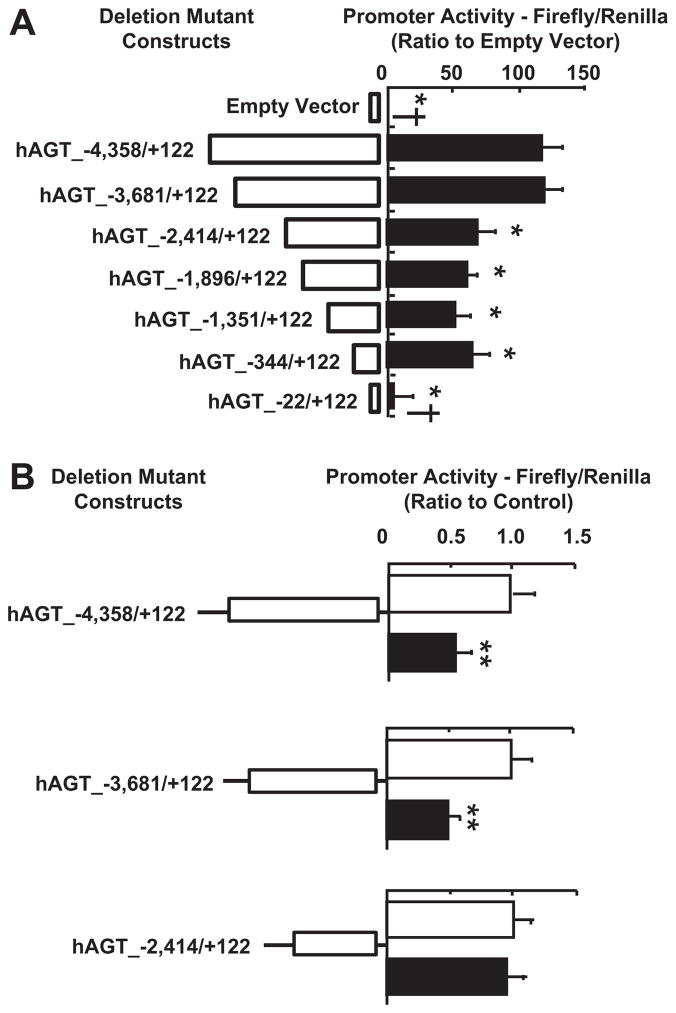
In HK-2 cells transfected with hAGT_−4358/+122, the promoter activity was higher than that in cells transfected with the empty parent pGL4.14 vector (119.8±12.4, ratio to empty vector; Figure 2A). hAGT_−3681/+122 did not change promoter activity when compared to hAGT_−4358/+122 (Figure 2A). Further deletion to hAGT_−2414/+122 resulted in a reduction in promoter activity (67.6±14.0, ratio to empty vector; Figure 2A). Further 5′-end deletion to hAGT_−344/+122 did not change the promoter activity significantly when compared to hAGT_−2414/+122 (Figure 2A). Removal of proximal promoter elements by 5′-end deletion to hAGT_−22/+122 completely reduced the promoter activity to levels of the empty luciferase vector (Figure 2A). Inhibition of NF-κB activity with 10 μmol/L parthenolide reduced basal hAGT promoter activity in cells transfected with hAGT_−4358/+122 (0.51±0.14, ratio to hAGT_−4358/+122 without parthenolide treatment; Figure 2B) and hAGT_−3681/+122 (0.48±0.06, ratio to hAGT_−3681/+122 without parthenolide treatment; Figure 2B). However, parthenolide treatment did not demonstrate the inhibitory effect on the promoter activity in cells transfected with hAGT_−2414/+122 (0.93±0.18, ratio to hAGT_−2414/+122 without parthenolide treatment; Figure 2B).
Figure 2.
A, 5′-End deletion reduces hAGT promoter activity. Deletion of a distal region from −3681 to −2414 reduced hAGT promoter activity (n=6). B, Parthenolide treatment reduced hAGT promoter activity in promoter constructs possessing region −3681 to −2414 (n=6). Open bars represent control values; filled bars, 10 μmol/L parthenolide treatment for 24 hours. These data are expressed as luminometric values and represent means±SD. *P<0.05 vs hAGT_−4358/+122; †P<0.05 vs hAGT_−2414/+122; **P<0.05 vs controls without parthenolide treatment.
Bioinformatics Analysis of the Distal hAGT Promoter Region
To identify possible NF-κB binding sites, the hAGT promoter region from −4358 to +122 was analyzed. An NF-κB binding site (5′-GGG AAC TCCC C-3′) was found from −2515 to −2506, which matches the known consensus NF-κB motif.41
NF-κB Binding Site at −2515 to −2506 Is Required to Maintain High hAGT Promoter Activity
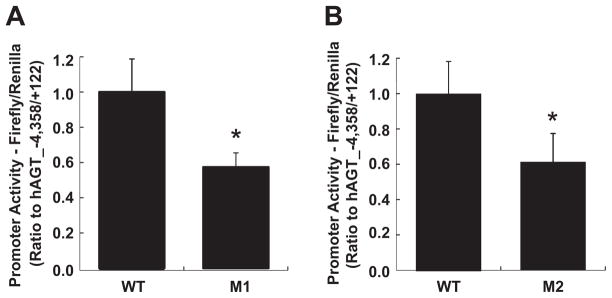
Deletion of the 10-bp NF-κB binding site sequence in M1 reduced basal hAGT promoter activity (0.57±0.08, ratio to hAGT_−4358/+122; Figure 3A). Furthermore, site-directed mutagenesis of the 10-bp NF-κB binding site in M2 reduced basal hAGT promoter activity (0.61±0.16, ratio to hAGT_−4358/+122; Figure 3B).
Figure 3.
The possible NF-κB binding site contributes to hAGT promoter activity. A, Removal of the possible NF-κB binding site in M1 reduced hAGT promoter activity (n=6). B, Site-directed mutagenesis of the possible NF-κB binding site in M2 reduced hAGT promoter activity (n=6). These data are expressed as relative luminometric units compared with hAGT_−4358/+122 and represent means±SD. *P<0.05 vs hAGT_−4358/+122. WT indicates hAGT_−4358/+122; M1, hAGT promoter region construct lacking the possible NF-κB binding site; M2, hAGT promoter region construct with mutated possible NF-κB binding site.
The NF-κB Binding Site Is Required for NF-κB Protein Binding to the hAGT Promoter Region
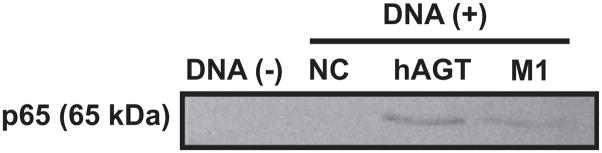
To confirm the functional importance of the NF-κB binding site to NF-κB protein binding in the hAGT promoter region, a nonquantitative DNA–protein binding assay was performed. NF-κB proteins bind to the hAGT_−4358/+122. On the other hand, when the 10-bp NF-κB binding site was removed in M1, NF-κB protein binding to the hAGT promoter region was reduced (Figure 4).
Figure 4.
Deletion of the possible NF-κB binding site reduces NF-κB binding activity to hAGT promoter. Removal of the NF-κB binding site in M1 reduced the binding of NF-κB protein to the hAGT promoter region compared with hAGT_−4358/+122. DNA (−) indicates no-DNA control; NC, negative control DNA lacking an NF-κB binding site; hAGT, hAGT_−4358/+122; M1, mutant-1.
Discussion
Although the proximal promoter elements within −1000 bp from the transcription start site of many genes have been demonstrated to be very important in gene expression, there are many exceptions.45–47 Previous studies have demonstrated that proximal promoter elements of the hAGT gene located from −1222 to +44 are sufficient for hAGT gene promoter activity in hepatocytes and in the liver of transgenic mice.31,48 Although the production of AGT in hepatocytes is higher than in RPTCs,22 RPTCs are the main source of AGT in the kidney.25–27 Therefore, the hAGT promoter region construct hAGT_−4358/+122 is sufficient for hAGT gene promoter activity in RPTCs, as demonstrated in the present study. The importance of these proximal regulatory elements has been demonstrated by deletion analyses of the proximal promoter elements of the hAGT gene promoter region.31 In agreement with the previous findings, hAGT promoter activity was completely reduced to the negligible levels of the empty, promoter-less luciferase vector by the deletion of proximal promoter elements in the hAGT promoter construct hAGT_−22/+122 (Figure 2A), indicating that the activity is hAGT promoter–dependent. However, previous experiments characterizing the hAGT promoter region used hAGT promoter region constructs from −1222 to +44 of the hAGT gene that have identified several transcription factor binding sites that contribute to hAGT gene promoter activity.30,31,49 We have recently demonstrated the contribution of signal transducer and activator of transcription-3 to hAGT expression,44 which indicates that hAGT expression is regulated by more than 1 factor. In this study, a hAGT promoter region clone from −4358 to +122 bp was used to investigate hAGT promoter activity. The data demonstrate that the longer hAGT promoter region construct used in this study possesses a distal regulatory element which contributes to hAGT promoter activity in human RPTCs, indicating that distal regulatory elements in the hAGT promoter region contribute to hAGT gene expression in human RPTCs.
The NF-κB signaling pathway has been shown to play a pivotal role in several animal models of hypertension and kidney disease.39,50,51 NF-κB is detected in most cell types and binds to the NF-κB consensus binding sequence.41 The most common NF-κB protein isoform is the p65/p50 heterodimer, which is a potent activator of transcription.52 Our previous studies demonstrated that parthenolide, an NF-κB inhibitor, suppressed basal AGT mRNA expression in HK-2 cells, indicating that NF-κB plays a role in hAGT expression in these cells.37 In this study, we also examined the dose-dependent response to parthenolide treatment on hAGT mRNA expression and demonstrated sufficient inhibition at 10 μmol/L parthenolide treatment in HK-2 cells (data not shown). Furthermore, several in vitro studies have demonstrated dose-dependent reductions in NF-κB –DNA binding activity in response to parthenolide treatment.53–55 We previously demonstrated that suppression of p65 NF-κB protein by small interfering RNA reduced hAGT expression in HK-2 cells,40 which provides further support to the pharmacological studies with parthenolide. Although an NF-κB binding site in the hAGT promoter region has not been described previously,31,38 we demonstrated that tumor necrosis factor-α reduced hAGT expression in HK-2 cells via the NF-κB signaling pathway.40 The suppression of AGT expression was attributable to excess formation of the p50/p50 homodimer by tumor necrosis factor-α. In the present study, inhibition of the NF-κB pathway reduced hAGT promoter activity in HK-2 cells through an NF-κB binding site at −2515 to −2506 in the distal hAGT promoter region. These data demonstrate the contribution of NF-κB signaling via the NF-κB binding site in the hAGT promoter region to hAGT promoter activity in these cells. Mutation of the 5′-end half-site of the possible NF-κB consensus sequence has been demonstrated to reduce binding of NF-κB proteins.56–58 In agreement with these findings, our mutational studies demonstrate the importance of the NF-κB binding site in the hAGT promoter region to the transcriptional activation of the hAGT gene. Furthermore, our binding studies demonstrated that the NF-κB binding site in the hAGT promoter region is important for NF-κB protein binding to the hAGT promoter region. Similar mutational analyses and binding studies in the rat AGT promoter region revealed the contribution of the NF-κB binding site to rat AGT expression.33 Although the removal of the NF-κB binding site did not completely abolish binding of NF-κB proteins to the hAGT promoter region (Figure 4), the results of these studies demonstrate the functional importance of the NF-κB binding site to hAGT gene promoter activity in human RPTCs. However, to quantify the results of the DNA binding activity of NF-κB to the hAGT promoter region, other methods such as the development of a yeast 2-hybrid system59 or use of microarray and plasmon resonance technologies60 would be required. Furthermore, the incomplete abolition of NF-κB protein binding to the hAGT promoter region is likely attributable to nonspecific binding of NF-κB proteins to the hAGT promoter region. Our deletion analysis and inhibition studies, however, demonstrate the importance of NF-κB protein binding activity to the NF-κB binding site at −2515 to −2506 in the hAGT promoter region to hAGT promoter activity in human RPTCs.
It has been established that the location of binding sites of transcriptional factors plays an important role in the mechanism of gene regulation.45 In the rat AGT promoter region, the composite NF-κB and C/EBP binding site (−531 to −557) is flanked by 1 full GRE site (−570 to 584) and a half GRE site (−470 to −477).38,61 The results of the present study indicate a functional NF-κB binding site at −2515 to −2506, which is located further upstream of the transcription start site in the hAGT gene promoter region compared to its more proximal location in the rat AGT gene promoter region. These data suggest that the mechanism of regulation of hAGT gene expression via the NF-κB signaling pathway is different from that of rat AGT gene expression when considering the large difference in location of the NF-κB binding site in the respective gene promoter region.
Perspectives
The present study adds to our knowledge regarding the regulation of hAGT gene expression in human RPTCs because it identifies a distal promoter element, possessing a binding site for the transcription factor NF-κB, in the hAGT promoter region. These findings indicate that NF-κB contributes to hAGT promoter activity in human RPTCs through a functional NF-κB binding site in the hAGT promoter region. This is important considering only modest changes in AGT expression result in detectable changes in blood pressure.16 The results of this study suggest that in vivo NF-κB controls the level of hAGT mRNA, hAGT protein, and ultimately intrarenal Ang II levels. Therefore, the possibility of targeting the intrarenal NF-κB signaling pathway may provide results that will aid in the development of novel and more effective approaches to the treatment of hypertension.
Acknowledgments
We acknowledge critical discussion and/or excellent technical assistance from Maki Urushihara, MD, PhD; Masumi Kamiyama, PhD; Kayoko Miyata, PhD; Akemi Katsurada, MS; G. Michael Upchurch, MS; Nina A. Perrault, BS; Jessica L. Mucci, MS; and Salem I. Elkhayat, MS (Tulane University).
Sources of Funding
This study was supported by grants from the National Institute of Diabetic and Digestive Kidney Diseases (R01DK072408) and the National Center for Research Resources (P20RR017659).
Footnotes
Disclosures
None.
References
- 1.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 3.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Davisson RL, Hardy DO, Zhu LJ, Merrill DC, Catterall JF, Sigmund CD. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272:28142–28148. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 6.Kimura S, Mullins JJ, Bunnemann B, Metzger R, Hilgenfeldt U, Zimmermann F, Jacob H, Fuxe K, Ganten D, Kaling M. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J. 1992;11:821–827. doi: 10.1002/j.1460-2075.1992.tb05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo MS, Takahashi S, Hatae T, Kajiwara N, Yagami K, Murakami K. Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem. 1993;268:11617–11621. [PubMed] [Google Scholar]
- 8.Bohlender J, Menard J, Ganten D, Luft FC. Angiotensinogen concentrations and renin clearance: implications for blood pressure regulation. Hypertension. 2000;35:780–786. doi: 10.1161/01.hyp.35.3.780. [DOI] [PubMed] [Google Scholar]
- 9.Smithies O. Theodore cooper memorial lecture. A mouse view of hypertension. Hypertension. 1997;30:1318–1324. doi: 10.1161/01.hyp.30.6.1318. [DOI] [PubMed] [Google Scholar]
- 10.Merrill DC, Thompson MW, Carney CL, Granwehr BP, Schlager G, Robillard JE, Sigmund CD. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ang II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol. 2007;293:F938–F945. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, Guo DF, Filep JG, Ingelfinger JR, Sigmund CD, Hamet P, Chan JS. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–1023. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 13.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol. 2004;286:F965–F971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 14.Falcao S, Stoyanova E, Cloutier G, Maurice RL, Gutkowska J, Lavoie JL. Mice overexpressing both human angiotensinogen and human renin as a model of superimposed preeclampsia on chronic hypertension. Hypertension. 2009;54:1401–1407. doi: 10.1161/HYPERTENSIONAHA.109.137356. [DOI] [PubMed] [Google Scholar]
- 15.Smithies O, Kim HS. Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci U S A. 1994;91:3612–3615. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue I, Nakajima T, Williams CS, Quackenbush J, Puryear R, Powers M, Cheng T, Ludwig EH, Sharma AM, Hata A, Jeunemaitre X, Lalouel JM. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest. 1997;99:1786–1797. doi: 10.1172/JCI119343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 19.Zhao YY, Zhou J, Narayanan CS, Cui Y, Kumar A. Role of C/A polymorphism at −20 on the expression of human angiotensinogen gene. Hypertension. 1999;33:108–115. doi: 10.1161/01.hyp.33.1.108. [DOI] [PubMed] [Google Scholar]
- 20.Ishigami T, Umemura S, Tamura K, Hibi K, Nyui N, Kihara M, Yabana M, Watanabe Y, Sumida Y, Nagahara T, Ochiai H, Ishii M. Essential hypertension and 5′ upstream core promoter region of human angiotensinogen gene. Hypertension. 1997;30:1325–1330. doi: 10.1161/01.hyp.30.6.1325. [DOI] [PubMed] [Google Scholar]
- 21.Lalouel JM, Rohrwasser A. Genetic susceptibility to essential hypertension: insight from angiotensinogen. Hypertension. 2007;49:597–603. doi: 10.1161/01.HYP.0000257145.20363.9c. [DOI] [PubMed] [Google Scholar]
- 22.Dickson ME, Zimmerman MB, Rahmouni K, Sigmund CD. The −20 and −217 promoter variants dominate differential angiotensinogen haplotype regulation in angiotensinogen-expressing cells. Hypertension. 2007;49:631–639. doi: 10.1161/01.HYP.0000254350.62876.b1. [DOI] [PubMed] [Google Scholar]
- 23.Pilbrow AP, Palmer BR, Frampton CM, Yandle TG, Troughton RW, Campbell E, Skelton L, Lainchbury JG, Richards AM, Cameron VA. Angiotensinogen M235T and T174M gene polymorphisms in combination doubles the risk of mortality in heart failure. Hypertension. 2007;49:322–327. doi: 10.1161/01.HYP.0000253061.30170.68. [DOI] [PubMed] [Google Scholar]
- 24.Pereira TV, Nunes AC, Rudnicki M, Yamada Y, Pereira AC, Krieger JE. Meta-analysis of the association of 4 angiotensinogen polymorphisms with essential hypertension: a role beyond M235T? Hypertension. 2008;51:778–783. doi: 10.1161/HYPERTENSIONAHA.107.100370. [DOI] [PubMed] [Google Scholar]
- 25.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 27.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nibu Y, Takahashi S, Tanimoto K, Murakami K, Fukamizu A. Identification of cell type-dependent enhancer core element located in the 3′-downstream region of the human angiotensinogen gene. J Biol Chem. 1994;269:28598–28605. [PubMed] [Google Scholar]
- 29.Nibu Y, Tanimoto K, Takahashi S, Ono H, Murakami K, Fukamizu A. A cell type-dependent enhancer core element is located in exon 5 of the human angiotensinogen gene. Biochem Biophys Res Comm. 1994;205:1102–1108. doi: 10.1006/bbrc.1994.2779. [DOI] [PubMed] [Google Scholar]
- 30.Yanai K, Nibu Y, Murakami K, Fukamizu A. A cis-acting DNA element located between TATA box and transcription initiation site is critical in response to regulatory sequences in human angiotensinogen gene. J Biol Chem. 1996;271:15981–15986. doi: 10.1074/jbc.271.27.15981. [DOI] [PubMed] [Google Scholar]
- 31.Fukamizu A, Takahashi S, Seo MS, Tada M, Tanimoto K, Uehara S, Murakami K. Structure and expression of the human angiotensinogen gene. Identification of a unique and highly active promoter. J Biol Chem. 1990;265:7576–7582. [PubMed] [Google Scholar]
- 32.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem. 2000;212:155–169. [PubMed] [Google Scholar]
- 33.Jamaluddin M, Meng T, Sun J, Boldogh I, Han Y, Brasier AR. Angiotensin II induces nuclear factor (NF)-kappaB1 isoforms to bind the angiotensinogen gene acute-phase response element: a stimulus-specific pathway for NF-kappaB activation. Mol Endocrinol. 2000;14:99–113. doi: 10.1210/mend.14.1.0400. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Brasier AR. Angiotensinogen gene activation by angiotensin II is mediated by the Rel A (nuclear factor-kappaB p65) transcription factor: one mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol Endocrinol. 1996;10:252–264. doi: 10.1210/mend.10.3.8833654. [DOI] [PubMed] [Google Scholar]
- 35.Brasier AR, Ron D, Tate JE, Habener JF. A family of constitutive C/EBP-like DNA binding proteins attenuate the IL-1 alpha induced, NF kappa B mediated trans-activation of the angiotensinogen gene acute-phase response element. EMBO J. 1990;9:3933–3944. doi: 10.1002/j.1460-2075.1990.tb07614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Haij S, Adcock IM, Bakker AC, Gobin SJ, Daha MR, van Kooten C. Steroid responsiveness of renal epithelial cells. Dissociation of transrepression and transactivation. J Biol Chem. 2003;278:5091–5098. doi: 10.1074/jbc.M209836200. [DOI] [PubMed] [Google Scholar]
- 37.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, Kobori H. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol. 2008;295:F283–F289. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain S, Li Y, Patil S, Kumar A. HNF-1alpha plays an important role in IL-6-induced expression of the human angiotensinogen gene. Am J Physiol Cell Physiol. 2007;293:C401–C410. doi: 10.1152/ajpcell.00433.2006. [DOI] [PubMed] [Google Scholar]
- 39.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-kappaB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol. 2007;293:F100–F109. doi: 10.1152/ajprenal.00520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satou R, Miyata K, Katsurada A, Navar LG, Kobori H. Tumor necrosis factor-{alpha} suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells. Am J Physiol Cell Physiol. 2010;299:C750–C759. doi: 10.1152/ajpcell.00078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baeuerle PA. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991;1072:63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- 42.Kobori H, Hayashi M, Saruta T. Thyroid hormone stimulates renin gene expression through the thyroid hormone response element. Hypertension. 2001;37:99–104. doi: 10.1161/01.hyp.37.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pazdrak K, Shi XZ, Sarna SK. TNFalpha suppresses human colonic circular smooth muscle cell contractility by SP1- and NF-kappaB-mediated induction of ICAM-1. Gastroenterology. 2004;127:1096–1109. doi: 10.1053/j.gastro.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, Navar LG, Kobori H. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol. 2009;311:24–31. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maston GA, Evans SK, Green MR. Transcriptional regulatory elements in the human genome. Ann Rev Gen Hum Gen. 2006;7:29–59. doi: 10.1146/annurev.genom.7.080505.115623. [DOI] [PubMed] [Google Scholar]
- 46.Strait KA, Stricklett PK, Kohan RM, Kohan DE. Identification of two nuclear factor of activated T-cells (NFAT)-response elements in the 5′-upstream regulatory region of the ET-1 promoter. J Biol Chem. 2010;285:28520–28528. doi: 10.1074/jbc.M110.153189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agarwal S, Rao A. Long-range transcriptional regulation of cytokine gene expression. Curr Opin Immunol. 1998;10:345–352. doi: 10.1016/s0952-7915(98)80174-x. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi S, Fukamizu A, Hasegawa T, Yokoyama M, Nomura T, Katsuki M, Murakami K. Expression of the human angiotensinogen gene in transgenic mice and transfected cells. Biochem Biophys Res Comm. 1991;180:1103–1109. doi: 10.1016/s0006-291x(05)81180-5. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt H, Aulchenko YS, Schweighofer N, Schmidt R, Frank S, Kostner GM, Ott E, van Duijn C. Angiotensinogen promoter B-haplotype associated with cerebral small vessel disease enhances basal transcriptional activity. Stroke. 2004;35:2592–2597. doi: 10.1161/01.STR.0000144646.96121.d2. [DOI] [PubMed] [Google Scholar]
- 50.Rangan G, Wang Y, Harris D. NF-kappaB signalling in chronic kidney disease. Front Biosci. 2009;14:3496–3522. doi: 10.2741/3467. [DOI] [PubMed] [Google Scholar]
- 51.Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 52.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Ann Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 53.Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 54.Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- 55.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 56.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivanov V, Merkenschlager M, Ceredig R. Antioxidant treatment of thymic organ cultures decreases NF-kappa B and TCF1(alpha) transcription factor activities and inhibits alpha beta T cell development. J Immunol. 1993;151:4694–4704. [PubMed] [Google Scholar]
- 58.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3–E1 cells. J Biol Chem. 1995;270:31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 59.Coates PJ, Hall PA. The yeast two-hybrid system for identifying protein-protein interactions. J Pathol. 2003;199:4–7. doi: 10.1002/path.1267. [DOI] [PubMed] [Google Scholar]
- 60.Linnell J, Mott R, Field S, Kwiatkowski DP, Ragoussis J, Udalova IA. Quantitative high-throughput analysis of transcription factor binding specificities. Nucleic Acids Res. 2004;32:e44. doi: 10.1093/nar/gnh042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brasier AR, Han Y, Sherman CT. Transcriptional regulation of angiotensinogen gene expression. Vitam Horm. 1999;57:217–247. doi: 10.1016/s0083-6729(08)60645-7. [DOI] [PubMed] [Google Scholar]