Abstract
Line bisection performance in children has been hypothesized to be a measure of corpus callosum maturation. Several previous studies have shown that normal prepubescent children bisect lines to the right of true center with their right hand and to the left with their left hand (symmetrical neglect). In contrast, children entering puberty reportedly bisect lines to the left with both the right and left hands (pseudoneglect). The shift from symmetrical to pseudoneglect has been hypothesized to reflect corpus callosum maturation and its involvement in the transfer of attention-based visuospatial processes. In the current study, line bisection performance and MR quantitative corpus callosum volumes were examined in 46 healthy children ages 8–18 years. A linear relationship between corpus callosum volume and age was found. However, the expected age-contingent line bisection performance pattern was not observed. In addition to the expected two patterns of line bisection bias, pseudoneglect and symmetric neglect, two additional distinct patterns of line bisection were identified. These findings, and other findings in the literature, raise important questions about the reliability and validity of the line bisection test. No relationship was found between corpus callosum volume and amount or direction of line bisection deviation. Our findings do not support previous hypotheses regarding line bisection-corpus callosum relationship.
Keywords: MRI, corpus callosum, spatial attention, line bisection
Introduction
The corpus callosum (CC) is the largest neural pathway connecting the two cerebral hemispheres (Giedd et al., 1999; Musiek, 1986), and is generally considered to play an important role in the interhemispheric transfer of information (Ptito, 2003). Although the line bisection task is traditionally used to assess hemispatial neglect, a number of studies have used it as a measure of the hemispheric distribution of attentional resources and as a behavioral correlate of callosal integrity (Barnett, 2006; Yazgan, Wexler, Kinsbourne, Peterson, & Leckman, 1995; McCourt et al., 2008). Hausmann, Waldie, and Corballis (2003) specificially hypothesized that performance changes on the line bisection task seen from childhood to adolescence may reflect maturation of the CC. However, there are currently no empirical data on this issue in healthy children. It is important to determine the validity of this hypothesis, as an increasing number of studies are using the line bisection task as a measure of callosal maturity. These results may also have important clinical implications in using the task to assess callosal functioning in neurodevelopmental disorders.
Normal right-handed adults frequently bisect horizontal lines to the left of objective center with both their right and left hands. The term pseudoneglect was coined to describe this leftward bias whereby healthy individuals “neglect” right hemispace (Bowers & Heilman, 1980). In contrast, some studies report that healthy children bisect lines to the right of true center with their right hand and to the left with their left hand, a phenomenon known as symmetrical neglect (Hausmann et al., 2003a). Cross-sectional studies in children have indicated that children transition from symmetrical neglect to pseudoneglect sometime between the ages of 5 and 12 years, suggesting a developmental pattern associated with the distribution of spatial attention (Bradshaw, Spataro, Harris, Nettleton, & Bradshaw, 1988; Hausmann et al., 2003a). Panel A of Figure 1A shows examples of pseudoneglect and symmetrical neglect. It is important to note, however, that variable patterns of line bisection performance have been reported in the literature, although with less consistency (Bowers & Heilman, 1980; Bradshaw et al., 1985; Levander et al., 1993; Manning et al., 1990; Nichelli, Rinaldi, & Cubelli, 1989; Schenkenberg et al., 1980).
Figure 1.
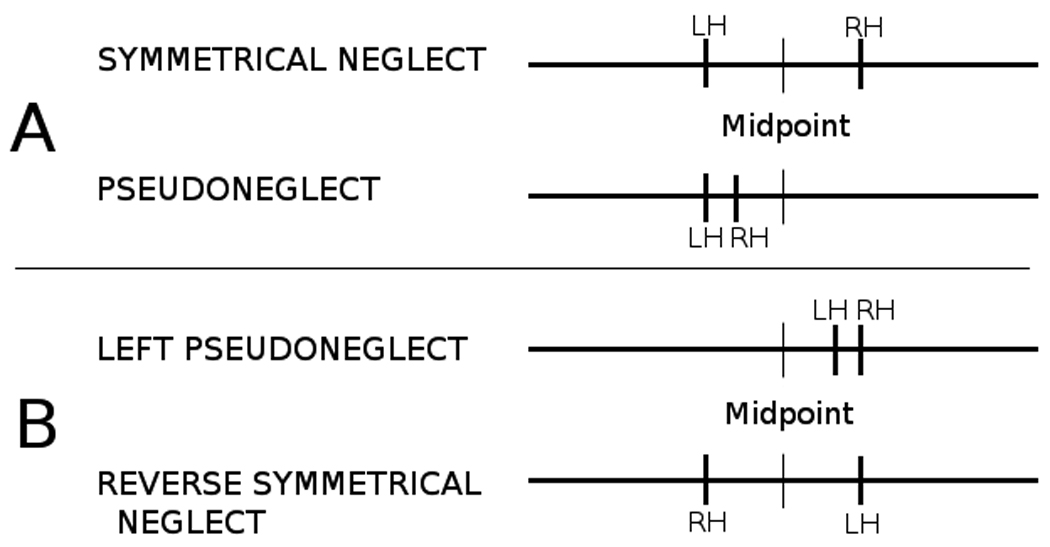
A) Symmetrical neglect and pseudoneglect patterns. B) Additional two patterns of performance observed in this study.
Two contrasting hypotheses regarding the relationship between CC integrity and changes on line bisection performance patterns have been posed (Yazgan et al., 1995; Hausmann et al., 2003a). Hausmann et al. (2003a) proposed that individuals with larger CCs, particularly the posterior section (e.g., splenium), would demonstrate increased leftward bias with the right hand (i.e., pseudoneglect). Yazgan et al. (1995) proposed that individuals with larger CCs would demonstrate increased rightward bias with their right hand, consistent with symmetrical neglect.
Hausmann et al. (2003a) argued that substantial posterior CC growth during childhood and the CC’s involvement in the transfer of attention-based visuospatial processes would produce a right-handed leftward shift on the task. Thus, they hypothesized that a larger CC, particularly the posterior section, facilitates the transfer of the right hemisphere’s attention-biased perceptual dominance to the contralateral hemisphere, overcoming the left hemisphere’s visuospatial attention. Conversely, a smaller CC prevents the transfer of spatial dominance from the right hemisphere and allows each hemisphere to exert its own dominance on the contralateral hemispace. This hypothesis suggests that a larger CC produces pseudoneglect and a smaller CC produces symmetrical neglect. Initial support for this hypothesis is provided by studies of adult epileptic patients with varying levels of callosal section, although these studies included only a small number of subjects. The posterior region of the CC is believed to be involved in the control of spatial attention and line bisection performance (Goldenberg, 1986; Hausmann, Corballis, & Farbi, 2003b). Patients with anterior callosotomies did not differ from controls on line bisection, whereas subjects with posterior callosotomies showed aberrant patterns of line bisection performance (Hausmann, Corballis, & Farbi, 2003b).
In contrast, Yazgan et al. (1995) suggested that a smaller CC favors increased spatial attention control by the specialized (i.e., right) hemisphere, whereas a larger CC distributes this role more equitably between hemispheres. In a study of 11 normal adult right-handed subjects, they found that all but one subject showed a leftward deviation with their right hand. Increased midsagittal CC area was significantly associated with an increased rightward bias, although most subjects bisected lines to the left of objective midpoint. These findings are consistent with the notion that pseudoneglect is associated with smaller CC area and symmetrical neglect is associated with larger CC area. That is, a larger CC allows each hemisphere to exert its own spatial dominance over the contralateral hemispace.
In this study, we examined patterns of line bisection performance in 46 normal children and quantitatively measured (MRI) CC volumes. Specifically, we examined (1) the relationship between age and CC volume, (2) the relationship of age to observed patterns of line bisection bias, and (3) the relationship of CC volume to line bisection performance.
Materials and Methods
Participants
Forty-six healthy right-handed children ages 8–18 years (19 males and 27 females) underwent MRI scanning and completed the line bisection task. Subjects were recruited as control subjects for a study examining the neurodevelopmental effects of pediatric epilepsy. All children were screened by the study coordinator for a history of learning disability, developmental disability, or significant medical conditions. All subjects also completed a full neuropsychological test battery. The test battery, including the line bisection test, was administered and scored by an advanced graduate student or psychometrist. Preferred hand for writing was used to determine handedness. Left-handed subjects were excluded because of confounding variables such as distribution of spatial attention abilities. See Table 1 for demographics and frequencies of ages. Subjects were divided into four age groups, 8–9 years, 10–12 years, 13–15 years, and 16–18 years, which approximate those used by Hausmann et al. (2003a).
Table 1.
Demographic and age distribution information
| Gender | Frequencies |
||
| Males (M) | 19 | ||
| Females (F) | 27 | ||
| Age (years) | Mean & Range |
||
| Mean (SD) | 13.1 (3.2) | ||
| Range | 8–18 |
||
| Age Groups | Frequencies |
||
| 8–9 (n = 9) |
8 | 4 | 4 M |
| 9 | 5 | 5 F | |
| 10–12 (n = 16) |
10 | 4 | 9 M 7 F |
| 11 | 10 | ||
| 12 | 2 | ||
| 13–15 (n = 11) |
13 | 3 | 2 M 9 F |
| 14 | 1 | ||
| 15 | 7 | ||
| 16–18 (n = 10) |
16 | 2 | 4 M 6 F |
| 17 | 5 | ||
| 18 | 3 | ||
The mean WISC-III Full Scale IQ score of the sample was 108 (S.D. = 12.5). All MRIs were screened by a board certified neuroradiologist for evidence of cortical lesions or neurological/developmental abnormalities. This study was reviewed and approved by the Institutional Review Boards of both institutions and on the day of study participation families and children gave informed consent and assent.
Line Bisection Test
Line bisection tasks have been frequently used in research and clinical practice, but no manualized protocol exists and the precise administration varies across studies. In this study, the line bisection task consisted of 21 horizontal black lines of 1 mm width on a white sheet of paper or transparency placed on a white sheet of paper. The lines ranged from 117 to 233 mm in their length with a mean length of 168 mm. Lines were pseudorandomly positioned so that seven lines appeared near the left margin, center, and near the right margin. The sheet was placed in front of the subject’s midline and they were instructed to cut each line in half by placing a small mark as close to the middle of the line as possible. A cardboard sheet that exposed one line at a time was used. The exposure of a single line has been used previously (Bradshaw et al., 1988), but not in all studies. We felt that exposure of a single line would reduce bias associated with line position or cuing effects caused by other lines. All subjects completed the task with the right hand first and then repeated it with the left hand. There were no time restrictions, but subjects were encouraged to mark the line promptly. Deviations to the left or right were measured to 1 mm accuracy. The equation for determining the percentage of deviation relative to line length was the same as used in previous studies (Scarisbrick, Tweedy, & Kulansky, 1987; Schenkenberg et al., 1980; Hausmann et al., 2003a):
The average percent deviation for each line position (left, center, middle) was calculated by summing the percent deviations for each line position and dividing the sum by 7 (the number of lines in each position on the page). A total average percent deviation was calculated by summing the percent deviations for all lines and dividing the sum by 21 (the total number of lines). Additionally, the difference in the amount of deviation between hands was calculated by subtracting the left hand bias from right hand bias.
In their meta-analysis, Jewell and McCourt (2003) reported that subjects tend to perceive the midpoint of lines towards the horizontal spatial position of the line stimulus (i.e., right-sided deviations on right-sided lines and left-sided deviations on left-sided lines). Therefore, classification of line bisection performance was determined by performances on center lines only, as they provide the least biased measurement of line bisection.
Corpus Callosum Measurement
MR images were obtained on a 1.5 Tesla GE Signa MRI scanner. Sequences acquired for each subject included: 1) T1-weighted, three-dimensional SPGR acquired with the following parameters: TE = 5, TR = 24, flip angle = 40, NEX = 2, FOV =26, slice thickness = 1.5 mm, slice plane = coronal, matrix = 256×192; 2) Proton Density (PD), and 3) T2-weighted images acquired with the following parameters: TE = 36 msec (for PD) or 96 msec (for T2), TR = 3000 msec, NEX = 1, FOV = 26, slice thickness = 3.0 mm, slice plane = coronal, matrix = 256×192, and echo train length = 8. All images were reviewed by a board-certified neuroradiologist to determine the absence of lesions and other abnormalities.
Images were initially post-processed at the University of Wisconsin Hospital using the semi-automated software program Brain Research: Analysis of Images, Networks, and Systems (BRAINS2; Magnotta, Harris, O'Leary, Andreasen, & Heckel, 2002). T1-weighted (T1-w), T2-weighted (T2-w), and PD-weighted (PD-w) images were realigned to a standard orientation, coregistered, and resampled to 1.0 mm cubic voxels.
Using a previously established tracing protocol (Hermann et al., 2003), three co-registered image sets were used to delineate CC volume. Boundary identification was based on a pre-manipulated trimodal image as the primary image set, with reference to the continuously segmented image and a discrete segmented image in areas of poorly defined borders. The trimodal image (a composite of the T1-w, T2-w, and PD-w images) enhanced visual tissue discrimination. The manual trace of the CC began in the midsagittal plane and extended two slices laterally in each direction from the midsagittal view. The CC trace was then divided into the seven Witelson (1989) areas in which the inner convexity of the genu was used as a landmark in conjunction with the rostral-caudal axis length to subdivide the callosum. Witelson areas were summed to create anterior (1, 2, & 3), midbody (4 & 5), and posterior (6 & 7) regions. Figure 2 shows a manual trace of the CC after combining the regions. Three independent raters completed all tracing with an inter-rater agreement (kappa) of .99.
Figure 2.
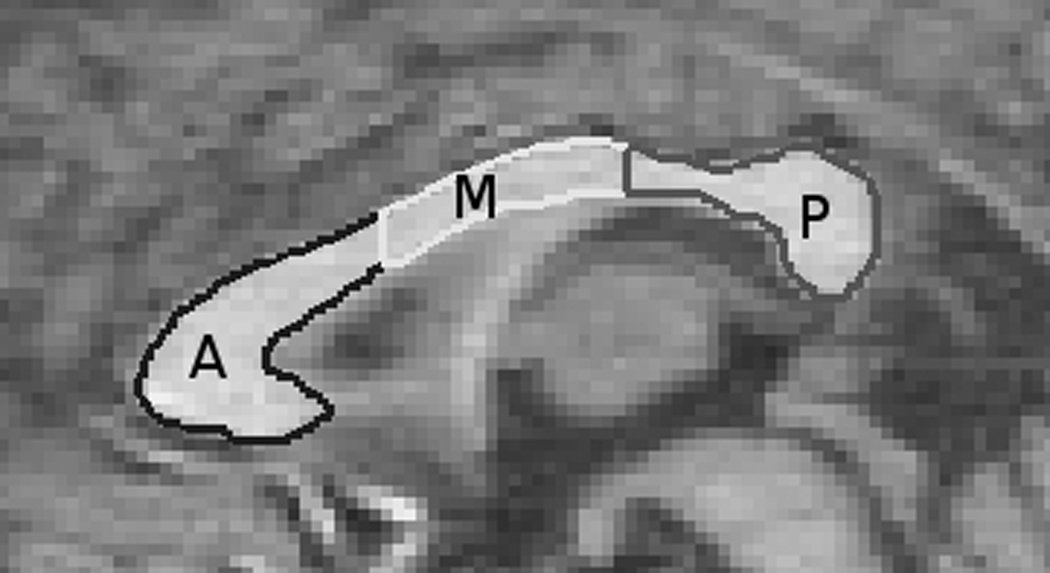
Manual trace of the corpus callosum with combined Witelson region divisions.
Statistical Analyses
Statistics were conducted using SPSS 13 (Chicago, IL). All brain volume analyses were corrected for age and intracranial volume (ICV) by covarying each group’s mean and ICV using multiple analysis of covariance (MANCOVA) or partial correlations. In other words, raw volumes were entered into each analysis with age and ICV entered as covariates. ICV was calculated via an automated process which included total brain tissue, ventricular CSF, and surrounding CSF, with the exclusion of bone, dura, or sinuses. Images were then manually inspected and corrected for inclusion of inappropriate tissues.
Linear and nonlinear (i.e., quadratic, logarithmic, and compound) correlations (controlling for ICV) were conducted between CC and age, line bisection and age, and CC and line bisection. MANCOVA, ICV as a covariate, was used to determine group differences on CC volume. MANOVA was used to determine group differences on the line bisection test. Distributions were examined for outliers in all age groups on both line bisection performance and callosal volumes. No outliers were found in any group.
Results
Corpus Callosum Development
Volumes for all subjects are provided in Table 2. CC volumes (by region) did not differ between males and females for the whole sample [F (4, 40) = .76, p = .56; partial η2 = .07]. MANCOVA revealed age group differences in the midbody [F (3, 46) = 3.47, p = .03; partial η2 = .20] and posterior CC [F (3, 46) = 3.59, p = .02; partial η2 = .21]. Post-hoc tests showed that the 13–15 group had larger midbodies than the 8–9 year old group (p = .04), while the 16–18 group had larger midbodies than the 8–9 (p = .005) and 10–12 group (p = .04). Both the 13–15 and 16–18 groups had larger posterior sections than the 8–9 (p = .009 for 13–15; p = .05 for 16–18) and 10–12 (p = .007 for 13–15; p = .02 for 16–18) groups. Similarly, increased age was significantly linearly associated with increased CC volumes in the midbody (r = .44, p = .002) and posterior regions (r = .29, p = .05) across the combined sample of subjects.
Table 2.
Corpus callosum volumes (least squares means), ICV corrected (cm3)
| All Subjects (n = 46) |
8–9 years (n = 9) |
10–12 years (n = 16) |
13–15 years (n = 11) |
16–18 years (n =10) |
Effect Size (partial η2) |
|
|---|---|---|---|---|---|---|
| Anterior | 1.15 (.17) | 1.06 (.19) | 1.11 (.19) | 1.23 (.18) | 1.18 (.19) | .12 |
| Midbody | .54 (.09) | .48 (.09) | .52 (.09) | .56 (.09) | .60 (.09) | .20 |
| Posterior | .95 (.17) | .87 (.19) | .88 (.18) | 1.09 (.18) | .97 (.18) | .21 |
| Total | 2.63 (.37) | 2.40 (.40) | 2.52 (.41) | 2.81 (.39) | 2.74 (.39) | .16 |
All values are Mean (SD).
Line Bisection Performance
Table 3 shows the mean amount of deviation for each age group at each line position, as well as the difference in deviation between hands. No significant linear correlation (r = .20, p = .18) or nonlinear correlational models (all p’s > .05) were found between mean right-handed center line bisection and age for the combined sample, indicating that subjects did not demonstrate an age-contingent right hand effect previously reported in the literature (Bradshaw et al., 1988; Hausmann et al., 2003). No differences between the age groups were found on mean amount of deviation with the left hand, right hand, or with the amount of difference between hands for any line position [F (21, 104) = 1.49, p = .10; partial η2 = .22]. Although no differences in mean amount of deviation were found between age groups, all groups conformed to the typical pattern of deviating towards the horizontal spatial position of the line stimulus for right- and left-sided lines, except for the 13–15 year old group that deviated mildly to the left with their left hand on right-sided lines. Based upon mean scores on the center line for each age group, all groups demonstrated a symmetrical neglect pattern of bias. Additionally, no performance differences were found between gender groups for the whole sample [F (2, 43) = 1.96, p = .15; partial η2 = .08].
Table 3.
Directional deviation of line bisection (mm)
| All Subjects (n = 46) |
8–9 years (n = 9) |
10–12 years (n = 16) |
13–15 years (n = 11) |
16–18 years (n =10) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Line Position |
Left Hand |
Right Hand |
R-L Diff. |
Left Hand |
Right Hand |
R-L Diff. |
Left Hand |
Right Hand |
R-L Diff. |
Left Hand |
Right Hand |
R-L Diff. |
Left Hand |
Right Hand |
R-L Diff. |
| Left | −4.47 (.80) |
−2.49 (.51) |
1.97 (.83) |
−1.01 (1.72) |
−1.77 (1.18) |
.76 (1.82) |
−4.88 (1.29) |
−3.05 (.89) |
1.83 (1.36) |
−7.14 (1.56) |
−2.13 (1.07) |
5.01 (1.64) |
−3.97 (1.63) |
−2.67 (1.12) |
1.30 (1.72) |
| Center | −1.90 (.63) |
1.95 (.64) |
3.84 (.80) |
−1.30 (1.47) |
1.79 (1.46) |
3.08 (1.84) |
−2.35 (1.10) |
.75 (1.11) |
3.11 (1.38) |
−2.15 (1.33) |
3.05 (1.32) |
5.20 (1.67) |
−1.43 (1.39) |
2.79 (1.39) |
4.22 (1.75) |
| Right | .93 (.88) |
4.37 (.81) |
3.44 (.79) |
2.84 (2.01) |
3.51 (1.89) |
.67 (1.77) |
.001 (1.51) |
4.67 (1.41) |
4.67 (1.33) |
−.53 (1.82) |
3.91 (1.71) |
4.44 (1.60) |
2.32 (1.91) |
5.18 (1.79) |
2.86 (1.68) |
| Total | −1.81 (.52) |
1.27 (.46) |
3.08 (.62) |
.18 (1.13) |
1.18 (1.06) |
1.00 (1.39) |
−2.41 (.85) |
.79 (.80) |
3.20 (1.04) |
−3.26 (1.02) |
1.61 (.96) |
4.87 (1.26) |
−1.03 (1.07) |
1.76 (1.01) |
2.79 (1.32) |
All values are Mean (SE).
Note: For left and right hand deviation, negative values indicate a deviation to the left, positive values indicate a deviation to the right. R-L Diff (Right-Left Difference) was calculated by subtracting the amount of left hand bias from right hand bias.
Important differences were found when individual line bisection scores for all subjects were examined. As determined by deviation on center lines, we observed the two expected patterns of line bisection performance for the entire sample, symmetrical bias (n = 18) and pseudoneglect (n = 13). However, two other patterns of line bisection performance emerged (left pseudoneglect and reverse symmetrical neglect). The left pseudoneglect group consisted of children that bisected center lines to the right with both hands, thereby “neglecting” left hemispace (n = 11). The reverse symmetrical neglect group consisted of children that deviated to the left with the right hand and to the right with the left hand (n = 4). Figure 1B shows these patterns of line bisection. It is important to reiterate that classification of performance pattern was based on direction of bias of the center line, not amount of bias. Usage of direction of deviation for classification is the same criterion that has been published in previous studies (Hausmann et al., 2003a; Hausmann et al., 2003b). Table 4 shows the frequency of line bisection performance patterns for all subjects and each age group. A chi-square analysis showed that the four age groups did not differ on the frequencies which line bisection performance patterns were observed [χ2 (9) = 11.02, p = .27]. Caution should be used in interpreting this statistic, as the number of subjects in each cell is small.
Table 4.
Frequencies of line bisection performance patterns*
| All Subjects (n = 46) |
8–9 years (n = 9) |
10–12 years (n = 16) |
13–15 years (n = 11) |
16–18 years (n =10) |
|
|---|---|---|---|---|---|
| Symmetrical Neglect | 18 | 2 | 7 | 6 | 3 |
| Pseudoneglect | 13 | 4 | 5 | 1 | 3 |
| Left Pseudoneglect | 11 | 3 | 1 | 3 | 4 |
| Reverse Symmetrical Neglect | 4 | 0 | 3 | 1 | 0 |
Classification based upon direction of deviation of center lines only
Supplementary analyses were conducted to determine if the line bisection performance groups differed on demographic variables. There were no significant age [F (3, 45) = .60, p = .62; partial η2 = .04], education [F (3, 45) = .66, p = .58; partial η2 = .05], or gender [χ2 (3) = 1.22, p = .75] differences between the four line bisection groups.
Corpus Callosum and Line Bisection
No significant linear or nonlinear correlations were found between any CC region and right hand deviation, left hand deviation, or differences between hands on the center line for the entire sample (r’s = −.29 to .18, p’s = .13 to .90) or within any of the age groups (r’s = −.58 to .54, p’s = .07 to .79). Correlations between the other two line positions (i.e., right and left) and CC regions were also conducted for each hand. Using a Bonferroni corrected alpha level of .001 for multiple correlations, no significant correlations were found. Supplementary analyses showed that there were no CC volume differences (age- and ICV-corrected) between the four line bisection groups [F (12, 98.18) = .61, p = .83; partial η2 = .06].
Discussion
The objective of this study was to examine line bisection performance and CC volume with respect to two hypotheses (Hausmann et al. 2003a; Yazgan et al., 1995). Contrary to both hypotheses, no relationship between line bisection performance and CC volume was found. These findings have important implications pertaining to the continued usage of the line bisection task as a measure of callosal maturation or integrity. There were several additional findings that are of significance regarding the psychometric properties of the line bisection test. This study is the first to directly quantitatively examine CC development (i.e., volume) and line bisection performance with both hands in healthy children. These findings demonstrate that the relationship between CC volume and line bisection is not straightforward and cannot be characterized by a single pattern.
Corpus Callosum Volumes
Although variable findings were observed on the line bisection task, a hypothesized age-associated increase in CC volume was evident for the midbody and posterior CC regions, but not the anterior region. These findings are consistent with previous studies that have shown that the CC grows in an anterior to posterior gradient, with more posterior sections maturing later (Giedd, Rumsey, & Castellanos, 1996). The CC grows markedly from ages 5–18 years and such changes are believed to be a result of increased myelination (Giedd et al., 1999). Individual studies continue to report conflicting findings regarding sex differences in the corpus callosum (Sullivan et al., 2001; Mitchell et al., 2003), although reviews and meta-analyses indicate no significant sex differences (Bishop & Wahlsten, 1997). Similarly, we found no sex differences in CC volumes when correcting for ICV. Reports of sex differences in the CC illustrate considerable variability in measuring this structure. Differences may be attributable to scan resolution, anatomical variability, and sample bias.
Line Bisection Performance Patterns
Multiple studies have reported symmetrical neglect in younger children and pseudoneglect in older children (Bradshaw et al., 1988; Failla et al., 2003; Dellatolas, Coutin, De Agostini, 1996; Hausmann et al., 2003a). However, an examination of the literature indicates a wide range of findings for neurologically normal and abnormal subjects. Some authors have found a small but consistent rightward bias (Bowers & Heilman, 1980; Bradshaw et al., 1985; Hu et al., 2007; Nichelli, Rinaldi, & Cubelli, 1989; Patston et al., 2006; Schenkenberg et al., 1980), some have found both leftward and rightward deviations (Levander et al., 1993; Manning et al., 1990), and yet others have found a consistent leftward bias (Bradshaw et al., 1985; Hausmann, Ergun, Yazgan, & Gunturkun, 2002; Milner, Brechmann, & Pagliarini, 1992). Although a shift from symmetrical neglect to pseudoneglect with increasing age in children has been reported in several studies, it may be premature to define that pattern of findings as “typical.” The varied findings across studies appear to be a result of different methodologies and moderating contributory performance factors that are discussed in greater detail below. Regardless of the factors accounting for these varied performances, these findings suggest that line bisection performance cannot be easily characterized according to age when examining different cohorts across studies.
Our findings indicate a pattern of substantial between-subject variability in the magnitude and direction of line bisection deviation. Two previous studies have recognized the unreliability of line bisection results reported from group studies and have attempted to examine it (Halligan, Manning, & Marshall, 1990; Manning, Halligan, & Marshall, 1990). These two studies examined healthy adults and specifically focused on individual variability on line bisection performance. Lines were presented one per page and were centered horizontally and vertically Healthy adult subjects in both studies showed both leftward and rightward deviations on horizontally oriented lines. The authors concluded that “there is considerable between-subject variation in whether transection errors will be placed to the right or the left of centre in the line-bisection task” (p. 652, Manning, Halligan, & Marshall, 1990). The heterogeneity of findings suggests that usage of the terms pseudoneglect and symmetrical neglect may be better applied to individual performance patterns rather than groups.
One possible explanation for the current pattern of line bisection findings reported here may reflect differences from other studies in the design and administration procedures for the line bisection task. As we indicated earlier, there is no standardized protocol for the administration of the line bisection test. Instead, there is considerable variability in procedural format across studies. Our test was modeled after Schenkenberg et al. (1980) and Hausmann et al.’s (2003a) detailed descriptions, although administration differed slightly. In the current study, subjects were presented 21 lines pseudorandomly positioned on the page with varying line lengths in each position and were prohibited from viewing more than one line at a time. Hausmann et al (2003a) presented 17 lines, and the experimenter covered each line after it was marked to ensure that the participants were not biased by their previous choices. That is, subjects were not allowed to see the line they just bisected, but they could see all lines below it. Additionally, bisection with the right and left hand was counterbalanced. Schenkenberg et al. (1980) presented 20 lines, but their administration guidelines did not specify if lines were presented one at a time. Yazgan et al. (1995) presented only five lines to adults and did not specify line length, line presentation, or number of lines viewable at one time. Exposure of multiple lines may affect perceptual cues associated with patterns of bias (Jewell & McCourt, 2000). By limiting exposure to a single line, perceptual cues associated with bisection were presumably removed. For example, subjects were not able to use surrounding lines or the edge of the paper to assist them in better approximating the midpoint. Previous research has identified that the presence of cues may bias the direction of deviation (Jewell & McCourt, 2000). Our administration using the right hand first followed by the left hand could potentially contribute in part to differences compared to Hausmann et al.’s findings, although there are presently no data to indicate that counterbalancing hand usage significantly contributes to any pattern of bias or the extent of the bias.
The pattern of findings on the line bisection task reported here and in other papers suggests the need for greater examination of the line bisection test in both healthy controls and clinical populations. Specifically, considerable caution in interpretation of findings related to line bisection performance in healthy children should be used given the variability in reported findings across a large number of studies. Furthermore, a detailed characterization of reliability and validity should be conducted to determine the psychometric soundness of the instrument. Test-retest reliability is rarely reported and is an important issue in characterizing typical versus atypical patterns of performance. No data regarding the test-rest reliability of the task in children have been reported, although Yazgan et al. (1995) reported test-reliability for seven adult subjects tested two weeks apart to be 0.68. Pierce et al. (2003) reported two week test-retest reliability for 58 subjects ages 18–85 to be 0.44, although age group correlations ranged from 0.29 to 0.71.
A careful inspection of factors that contribute to reliability and validity, as well as procedures to minimize their effects, should also be carefully and systematically studied. Jewell and McCourt (2000) identified 26 factors that can affect performance on line bisection tasks. Additional factors that influence line bisection performance, such as menstruation cycle, also have been identified as performance moderating factors (Hausmann, 2005). With such a large number of factors that can contribute to performance on a relatively “simple” task, a standardized method controlling for as many of these factors as possible would be required to validate the usage of the line bisection task as a measure of spatial attention or interhemispheric transfer. Although it is beyond the scope of this paper to review all of these performance factors, it is possible to systematically rule out the contribution of some of these factors in the present study. In the present study, gender, handedness, hand usage, line length, cuing effects, and scanning direction are not likely contributors due to their control in administration or the absence of statistically significant differences in these areas between groups.
Line Bisection and Its Neural Substrates
Neural structures other than the CC likely play a critical role in line bisection performance, but it is unknown to what extent. Dennis et al. (2005) showed that larger retrocallosal volumes were associated with smaller leftward bias using the preferred hand in healthy children (n = 10), although no significant relationship between retrocallosal volume and line bisection performance was found for a group with spina bifida. An fMRI study found increased activity in the bilateral superior and inferior parietal lobes, cerebellar vermis, anterior cingulate, and prefrontal cortex bilaterally while performing a bisection task (Fink et al., 2001). Additionally, a recent electrical source imaging study found that the right middle occipital gyrus, right superior posterior parietal cortex, bilateral inferior occipital gyrus, and right inferior posterior parietal cortex were significantly active during performance on a line bisection judgment task (Waberski et al., 2008). Because none of these studies (or the present study) has identified corpus callosum involvement, this further supports the notion that the line bisection test may not be an accurate representation of callosal maturity or integrity.
Although Yazgan et al. (1995) found a linear relationship between CC area and line bisection performance, it is important to note that there are also some important differences between their study and ours. First, our study obviously examined a much younger (and larger) sample. Thus, it is difficult to compare these studies due to age differences and the hypothesized contribution of age on performance. Second, our study examined performance with the right hand, left hand, and the difference between hands, while Yazgan et al. only reported right hand performance. Left hand performance is unknown in their study and may have provided potentially important information regarding the characterization of line bisection performance patterns. Third, we examined more lines at multiple spatial positions on the page. However, the determination of line bisection performance pattern was based on the center lines. Analysis of all line positions and examination of bias of left and right lines in the present study provided no additional information, although it is unknown what contribution this information may have provided in adults. Finally, it is unclear it Yazgan et al. corrected their CC analyses for ICV or age. Corrected CC results may have yielded a potentially different relationship with line bisection.
While our findings do not support Hausmann et al.’s (2003a) hypothesis of callosal maturation as underlying line bisection performance, several issues should be noted. They pointed out that various growth factors affect callosal structure and functioning. For example, testosterone is positively related to morphological changes in the posterior section of the CC (Moffat, Hampson, Wickett, Vernon, & Lee, 1997), although increases in callosal size over time are attributed to increased myelination (Giedd et al., 1996). The influence of testosterone or other hormones may affect callosal morphology or connectivity without affecting overall size that would not be reflected by volumetric measurements. Future studies using other neuroimaging modalities, such as diffusion tensor imaging and magnetization transfer imaging, may provide insight into the relationship between callosal microstructure and line bisection performance.
Limitations
Although this was the first study to test the neurodevelopmental relationship between CC growth and line bisection performance, several limitations should be noted. First, our determination of handedness was based upon self-report of preferred handedness. Thus, we did not measure degree of handedness, and as such, may have included subjects with varying degrees of “right handedness.” Second, the age groups in this study approximated those used by Hausmann et al. (2003a). Although we reported correlations between line bisection performance and callosal volumes for the entire group, it is possible that a different grouping of ages may have altered the findings in a meaningful way. However, several age groupings were examined in post-hoc analyses (data not presented above) and the pattern of findings remained the same.
Conclusion
In summary, our current findings, placed in context with previous findings reported in the literature, indicate that a simple relationship does not exist between CC structure and line bisection performance in children. A critical evaluation of the line bisection task and methods for standardization of development and administration are necessary to substantiate continued use of the task as a measure of the hemispheric distribution of spatial attention.
ACKNOWLEDGEMENTS
This project was supported by NIH NINDS RO1 44351 and 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. We thank Michelle Szomi for overall project coordination; Dr. Jana Jones for participant interviews; and Kevin Dabbs, Leslie Guidotti, and Jared Morton for MR processing.
References
- Barnett KJ. Schizophrenia and rightward bias in line bisection. Laterality. 2006;11:36–42. doi: 10.1080/13576500500233628. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Wahlsten D. Sex differences in the human corpus callosum: myth or reality? Neuroscience and Biobehavioral Reviews. 1997;21:581–601. doi: 10.1016/s0149-7634(96)00049-8. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Nettleton NC, Nathan G, Wilson L. Bisecting rods and lines: effects of horizontal and vertical posture on left-side underestimation by normal subjects. Neuropsychologia. 1985;23:421–425. doi: 10.1016/0028-3932(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Spataro JA, Nettleton NC, Bradshaw J. Crossing the midline by four to eight year old children. Neuropsychologia. 1988;26:221–235. doi: 10.1016/0028-3932(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Dellatolas G, Coutin T, De Agostini M. Bisection and perception of horizontal lines in normal children. Cortex. 1996;32:705–715. doi: 10.1016/s0010-9452(96)80040-2. [DOI] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Frederick J, Copeland K, Francis D, Blaser SE, et al. Peripersonal spatial attention in children with spina bifida: Associations between horizontal and vertical line bisection and congenital malformations of the corpus callosum, midbrain, and posterior cortex. Neuropsychologia. 2005;43:2000–2010. doi: 10.1016/j.neuropsychologia.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Failla CV, Sheppard DM, Bradshaw JL. Age and responding-hand related changes in performance of neurologically normal subjects on the line-bisection and chimeric faces tasks. Brain and Cognition. 2003;52:353–363. doi: 10.1016/s0278-2626(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marhsall JC, Weiss PH, Zilles K. The neural basis of vertical and horizontal line bisection judgments: an fMRI study of normal volunteers. Neuroimage. 2001;14:S59–S67. doi: 10.1006/nimg.2001.0819. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX. A quantitative MRI study of the corpus callosum in children and adolescents. Developmental Brain Research. 1996;92:274–280. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, et al. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Progress in Neuro-Psychopharmacological and Biological Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Neglect in a patient with partial callosal disconnection. Neuropsychologia. 1986;24:397–403. doi: 10.1016/0028-3932(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Manning L, Marshall JC. Individual variation in line bisection: a study of four patients with right hemisphere damage and normal controls. Neuropsychologia. 1990;28:1043–1051. doi: 10.1016/0028-3932(90)90139-f. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Ergun G, Yazgan Y, Gunturkun O. Sex differences in line bisection as a function of hand. Neuropsychologia. 2002;40:235–240. doi: 10.1016/s0028-3932(01)00112-9. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Waldie KE, Corballis MC. Developmental changes in line bisection: A result of callosal maturation? Neuropsychology. 2003a;17:155–160. [PubMed] [Google Scholar]
- Hausmann M, Corballis MC, Farbi M. Line bisection in the split brain. Neuropsychology. 2003b;17:602–609. doi: 10.1037/0894-4105.17.4.602. [DOI] [PubMed] [Google Scholar]
- Hermann B, Hansen R, Seidenberg M, Magnotta V, O'Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003;18:284–292. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- Hu X, Liu Y, Liu X, Shen M, Drake RA, Wang W. Line bisection performance in right-handed primary headache sufferers. Neurology India. 2007;55:333–337. doi: 10.4103/0028-3886.37091. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Levander M, Tegner R, Caneman G. Tactile line-bisection in normal subjects. Perceptual and Motor Skills. 1993;76:831–836. doi: 10.2466/pms.1993.76.3.831. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Shpaner M, Javitt DC, Foxe JJ. Hemispheric asymmetry and callosal integration of visuospatial attention in schizophrenia: A tachistoscopic line bisection study. Schizophrenia Research. 2008;102:189–196. doi: 10.1016/j.schres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, O'Leary DS, Andreasen NC, Heckel D. BRAINS2: A structural imaging process toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Manning L, Halligan PW, Marshall JC. Individual variation in line bisection: a study of normal subjects with application to the interpretation of visual neglect. Neuropsychologia. 1990;28:647–655. doi: 10.1016/0028-3932(90)90119-9. [DOI] [PubMed] [Google Scholar]
- Milner AD, Brechmann M, Pagliarini L. To halve and to halve not: an analysis of line bisection judgements in normal subjects. Neuropsychologia. 1992;30:515–526. doi: 10.1016/0028-3932(92)90055-q. [DOI] [PubMed] [Google Scholar]
- Mitchell TN, Free SL, Merschhemke M, Lemieux L, Sisodiya SM, Shorvon SD. Reliable callosal measurement: population normative data confirm sex-related differences. American Journal of Neuroradiology. 2003;24:410–418. [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Wickett JC, Vernon PA, Lee DH. Testosterone is correlated with regional morphology of the human corpus callosum. Brain Research. 1997;767:297–304. doi: 10.1016/s0006-8993(97)00614-8. [DOI] [PubMed] [Google Scholar]
- Musiek FE. Neuroanatomy, neurophysiology, and central auditory assessment. Part III: Corpus callosum and efferent pathways. Ear and Hearing. 1986;7:349–357. doi: 10.1097/00003446-198612000-00001. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Rinaldi M, Cubelli R. Selective spatial attention and length representation in normal subjects and in patients with unilateral spatial neglect. Brain and Cognition. 1989;9:57–70. doi: 10.1016/0278-2626(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Patston LLM, Corballis MC, Hogg SL, Tippett LJ. The neglect of musicians: line bisection reveals an opposite bias. Psychological Science. 2006;17:1029–1031. doi: 10.1111/j.1467-9280.2006.01823.x. [DOI] [PubMed] [Google Scholar]
- Pierce CA, Jewell G, Mennemeier M. Are psychophysical functions derived from line bisection reliable? Journal of the International Neuropsychological Society. 2003;9:72–78. doi: 10.1017/s1355617703910083. [DOI] [PubMed] [Google Scholar]
- Ptito M. Functions of the corpus callosum as derived from split-chiasm studies in cats. In: Zaidel E, Iacoboni M, editors. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. Cambridge, MA: The MIT Press; 2003. [Google Scholar]
- Scarisbrick DJ, Tweedy JR, Kulansky G. Hand preference and performance effects on line bisection. Neuropsychologia. 1987;25:695–699. doi: 10.1016/0028-3932(87)90061-3. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–517. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiology of Aging. 2001;22:603–611. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Narr KL, Blanton RE, Toga AW. Mapping structural alterations of the corpus callosum during brain development and degeneration. In: Zaidel E, Iacoboni M, editors. The Parallel Brain: The Cognitive Neuroscience of the Corpus Callosum. Cambridge, MA: The MIT Press; 2003. [Google Scholar]
- Waberski TD, Gobbele R, Lamberty K, Buchner H, Marshall JC, Fink GR. Timing of visuo-spatial information processing: electrical source imaging related to line bisection judgements. Neuropsychologia. 2008;46:1201–1210. doi: 10.1016/j.neuropsychologia.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Yazgan MY, Wexler BE, Kinsbourne M, Peterson B, Leckman JF. Functional significance of individual variations in callosal area. Neuropsychologia. 1995;33:769–779. doi: 10.1016/0028-3932(95)00018-x. [DOI] [PubMed] [Google Scholar]


