Abstract
Permeabilized anterior byssus retractor muscles (ABRM) from Mytilus edulis were used as a simple system to test whether there is a stretch dependent activation of a kinase as has been postulated for titin and the mini-titin twitchin. The ABRM is a smooth muscle that shows catch, a condition of high force maintenance and resistance to stretch following stimulation when the intracellular Ca++ concentration has diminished to sub-maximum levels. In the catch state twitchin is unphosphorylated, and the muscle maintains force without myosin crossbridge cycling through what is likely a twitchin mediated tether between thick and thin filaments. In catch, a small change in length results in a large change in force. The phosphorylation state of an added peptide, a good substrate for molluscan twitchin kinase, with the sequence KKRAARATSNVFA was used as a measure of kinase activation. We find that there is about a two-fold increase in phosphorylation of the added peptide with a 10% stretch of the ABRM in catch. The increased phosphorylation is due to activation of a kinase rather than to an inhibition of a phosphatase. The extent of phosphorylation of the peptide is decreased when twitchin is phosphorylated and catch force is not present. However, there is also a large increase in peptide phosphorylation when the muscle is activated in pCa 5, and the catch state does not exist. The force-sensitive kinase activity is decreased by ML-9 and ML-7 which are inhibitors of twitchin kinase, but not by the Rho kinase inhibitor Y-27632. There is no detectable phosphorylation of myosin light chains, but the phosphorylation of twitchin increases by a small, but significant extent with stretch. It is possible that twitchin senses force output resulting in a force-sensitive twitchin kinase activity that results in autophosphorylation of twitchin on site(s) other than those responsible for relaxation of catch.
Keywords: Twitchin, Force sensor, Smooth muscle, Kinase
Introduction
Both titin and the mini-titin twitchin are thought to act as force-bearing tethers in muscle. Titin extends from the M-line to the Z-line in striated muscle and is responsible for most of the passive resistance of the muscle to stretch (for review see Tskhovrebova and Trinick 2010). Recent studies show that titin interaction with the thin filament is regulated (Yamasaki et al. 2001; Kulke et al. 2001; Linke et al. 2002; Nagy et al. 2004; Bianco et al. 2007), and it has been postulated that such binding to the thin filament results in a viscous load that resists the relative sliding of thick and thin filaments (Nagy et al. 2004; Bianco et al. 2007). It is also possible that the interaction of titin with the thin filament modifies the spring length of titin thereby changing its contribution to total force output from the muscle (Leonard and Herzog 2010). The mini-titin twitchin is a central player in the mechanism of catch force maintenance in invertebrate smooth muscle. Catch is a state in which force is maintained for long periods of time with little or no myosin crossbridge turnover and associated energy utilization. Recent evidence suggests that twitchin is a force-bearing tether between thick and thin filaments, and as such is responsible for the extended force maintenance and resistance to lengthening exhibited during the catch state (for review see Butler and Siegman 2010). Protein kinase A mediated phosphorylation of twitchin rapidly relaxes catch force most likely by causing detachment of the twitchin mediated tether between thick and thin filaments (Siegman et al. 1998; Shelud’ko et al. 2007; Funabara et al. 2007; Butler et al. 2010).
In addition to tethering functions, titin and twitchin molecules have kinase domains whose functions are not clear. Both twitchin and titin have autoinhibited kinase domains situated near the C terminus of the molecule. The kinase domains are homologous to smooth muscle myosin light chain kinase, and they share a similar organization of Ig and Fn domains around the kinase domain. Autoinhibition of the kinase results from an autoinhibitory tail sequence C-terminal to the catalytic core (Olson et al. 1990). In twitchin, the autoregulatory sequence interacts with binding sites for the protein substrate, the ATP and the Mg++ binding sites as well as with the catalytic activation loop (Hu et al. 1994; Kobe et al. 1996). Titin kinase is autoinhibited by a C-terminal autoinhibitory tail in a manner similar to twitchin kinase (Kobe et al. 1996; Mayans et al. 1998; Grater et al. 2005). Both of these enzymes require release of the autoinhibitory tail for activation of kinase activity. An elegant mechanism by which this might occur is for strain of the molecule to physically pull the autoinhibitory domain away from its inhibitory contacts, resulting in activation of the kinase activity. It has been postulated that both the twitchin kinase and titin kinase domains are activated by stretch of the molecule (Grater et al. 2005; Greene et al. 2008; Puchner et al. 2008; Puchner and Gaub 2010), and that the resulting phosphorylation of a substrate serves as a reporter of force output of the muscle (Lange et al. 2005). This could provide a signaling pathway by which changes in mechanical force cause a graded substrate phosphorylation that is part of a signaling pathway that leads to changes in gene and protein expression of muscle (Lange et al. 2005).
The experiments described here tested the possibility that stretch of muscles in the catch state and associated elongation of the twitchin tether between thick and thin filaments leads to an activation of kinase activity. Permeabilized anterior byssus retractor muscles (ABRM) from Mytilus edulis were used as a simple test system since the force that is developed by active crossbridge cycling is maintained in the catch state (without crossbridge cycling) and can be varied by small changes in muscle length. This means that any force-dependent change in phosphorylation of an exogenously added peptide can be measured under very low [Ca+2] (pCa > 8) where the only intervention necessary to change force output is a relatively small change in muscle length. Importantly, the same experiment can be performed when twitchin is pre-thiophosphorylated. In this condition, there is no catch force maintenance, total force output from the muscle is low, and the force response to stretch is substantially reduced. The sequence of the added peptide whose phosphorylation state was monitored was KKRAARATSNVFA. This peptide, which is related to the region of phosphorylation of the smooth muscle myosin light chain, is a good substrate for molluscan twitchin kinase activity (Heierhorst et al. 1996).
We find that there is a kinase whose activity is increased not only when the muscle is stretched while in the catch state, but also when force in increased by activation of the muscle in the absence of catch. This force-sensitive kinase activity is decreased by ML-9 and ML-7 which are inhibitors of twitchin kinase, but not by the rho-kinase inhibitor Y-27632. There is no detectable phosphorylation of myosin light chains, but twitchin phosphorylation increases a small, but significant extent with stretch. It is possible that twitchin, which is tightly bound to the thick filament, senses force output resulting in a force-sensitive twitchin kinase that results in autophosphorylation of twitchin.
Methods
Mytilus edulis were obtained from the Marine Biological Laboratory, Woods Hole MA. Bundles (0.2–0.3 mm in diameter and about 1 cm in length) of the anterior byssus retractor muscle were removed from the mussel and maintained at in vivo length. The muscles were permeabilized by incubation in a rigor solution containing 1% Triton X-100 for 30 min, and then rinsed in rigor solution before further experimental manipulation (Siegman et al. 1997). All experiments were performed at 20 °C. Mechanical measurements on muscle bundles of less than 5 mm in length were made as described earlier (Siegman et al. 1997; Franke et al. 2007). Changes of muscle length in the stretch and release protocol were made via a micrometer and were complete in less than 1 s. The volume of incubation solution was 125 µl for the solutions containing 32P-ATP.
Relaxing solution contained the following: 1 mM MgATP; 20 mM EGTA; 3 mM free Mg2+; 1 mM dithiothreitol; 30 mM piperazine-N,N′-bis[2-ethanesulfonic acid] (PIPES); and 0.5 mM leupeptin. Ionic strength was maintained at 202 mM with 1,6-diaminohexane-N,N,N′,N′-tetraacetic acid (HDTA), and the pHwas 6.8. In activating solutions, the total EGTAwas maintained at 20 mM,butCaEGTAwas added to bring the calcium concentration to pCa 5. Rigor solution was similar to relaxing solution except that no MgATP was added. (±)-Blebbistatin (Calbiochem) was dissolved in 90% DMSO, 10% water for stock solutions and then diluted into muscle solutions for a final concentration of 50 µM. γ32P-ATP (Perkin Elmer Life and Analytical Sciences) was added to solutions to give a 32P activity of 40 µCi/ml, and the peptide with the sequence KKRAARATSNVFA (Biomol International) was used at a concentration of 200 µM. The inhibitors ML-7, ML-9 and Y27632 were obtained from Sigma–Aldrich. In some cases the twitchin in muscles was thiophosphorylated by incubation in a rigor solution containing ATPγS (100 µM) and cAMP for 10 min (Siegman et al. 1997; Butler et al. 1998; Butler et al. 2001). Thiophosphorylated twitchin is not susceptible to dephosphorylation and results in relaxation of catch force (Siegman et al. 1997; Butler et al. 1998).
In experiments in which muscles were frozen, the muscles were directly immersed into a vial containing liquid nitrogen. The muscle was then pulverized in frozen 0.5 N HClO4, and the protein precipitate was taken up in SDS sample buffer and subjected to SDS-PAGE. The gel was stained with Coomassie Blue and scanned on a laser densitometer. This was followed by drying and exposure to a storage phosphor screen (GE Healthcare) which was read on a Typhoon imager (Amersham Biosciences) and analyzed with ImageQuant software. The primary antibody used for detecting phosphorylated myosin light chain in Western Blots was obtained from GenScript, and it was made against the immunogen A-T-SP-N-V. The secondary antibody was anti-rabbit IgG peroxidase-linked whole antibody from donkey (GE Healthcare). The Amersham ECL Plus Western Blotting Detection System (GE Healthcare) was used along with a FluorChem imager.
For analysis of the extent of phosphorylation of the peptide in the muscle incubation solution, an equal volume of 50 mM phosphoric acid was added to the solution. The sample was then subjected to liquid chromatography using an SPFF strong cation exchange column (GE Healthcare) with a mobile phase of 50 mM NaH2PO4 and a gradient from 0 to 1 M NaCl. The radioactivity in the effluent fractions was determined by liquid scintillation counting.
The 32P labeled phosphorylated peptide was prepared in a pCa 5 activating solution containing 0.1 mM MgATP (100 µCi/ml 32P-ATP), 4 µM calmodulin (Sigma-Aldrich), 40 µg/ml myosin light chain kinase (recombinant human MLCK residues 1425–1771, Millipore), and 50 µM peptide. The reaction was allowed to proceed for 3–5 h. The 32P labeled phosphorylated peptide was purified by liquid chromatography as described above and was used in experiments at approximately 0.1 µCi/ml.
Results
Phosphorylation of the peptide increases with stretch in the catch state
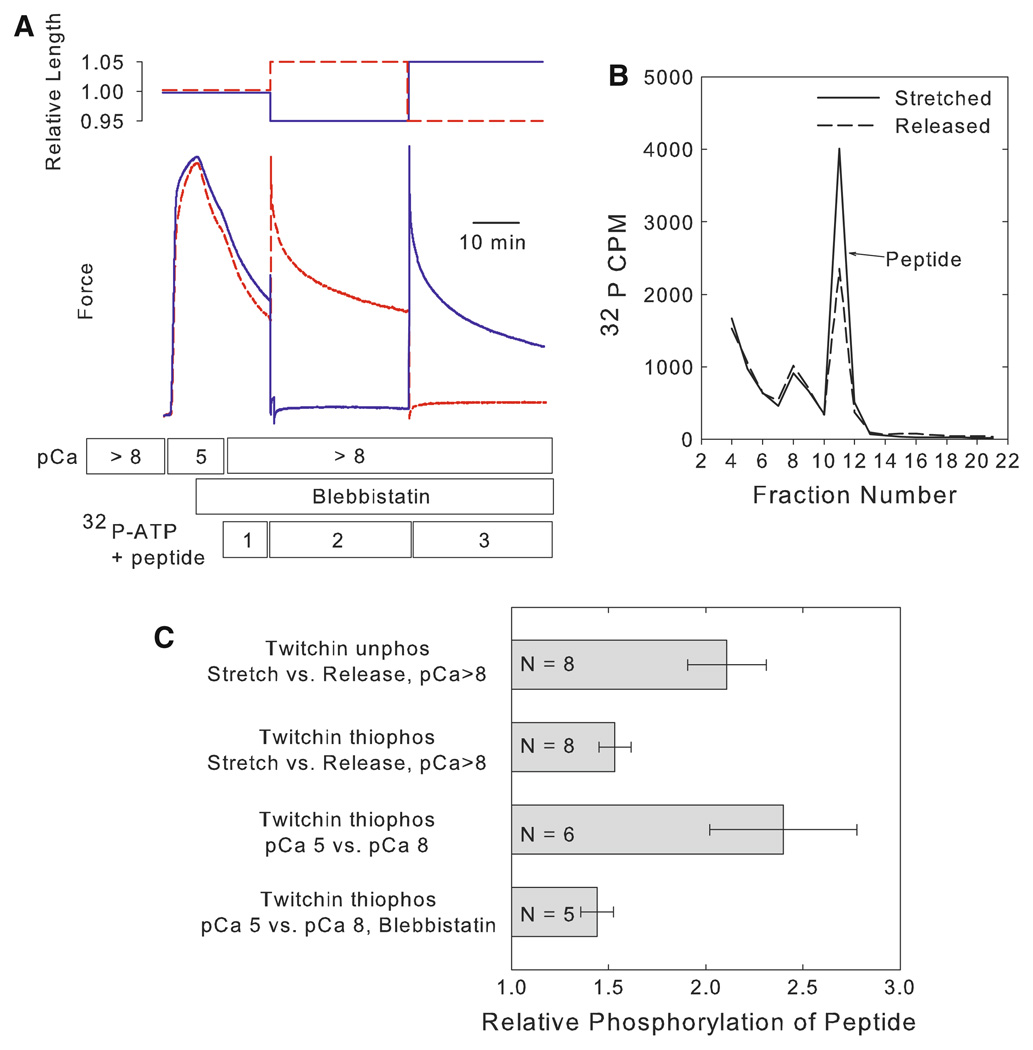
Permeabilized ABRM were put into the catch state using a procedure that is summarized in Fig. 1 Panel a (Butler et al. 2006). The muscle was incubated in pCa 5 and allowed to develop maximum active force. The myosin ATPase inhibitor blebbistatin (50 µM) was then added, and it remained in the solutions for the duration of the experiment. The [Ca+2] was then decreased to pCa > 8. These manipulations decreased force output to about 30–40% of maximum. Importantly, all of this force was catch force as indicated by separate experiments that showed that ATPase is very low, and that the force is relaxed by addition of cAMP and phosphorylation of twitchin (Butler et al. 2006). The muscle was then incubated in peptide (200 µM) and γ32P-ATP for 10 min. This was followed by transfer to an identical solution, and the muscle length was increased by 5%. The muscle was incubated in this solution for 30 min, and was then transferred to a new solution immediately followed by a 10% decrease in muscle length. The incubation continued for an additional 30 min. The incorporation of 32P into the peptide was determined for each solution. In other muscles, the procedure was identical except that a decrease in muscle length preceded the increase in length. One half of the muscles were subjected to the stretch-release protocol, and the others were subjected to the release-stretch protocol. This allowed correction for any time-dependent change in phosphorylation of the peptide. The average force during the period when the muscle was stretched was 0.51 ± 0.06 P/Po versus 0.03 ± 0.03 P/Po during the time the muscle was released. Figure 1, Panel b shows a typical chromatogram of the radioactivity associated with the peptide in the solutions bathing the muscle under stretched and released conditions. The ratio of the phosphorylation of the peptide during stretched versus released conditions was determined for each muscle. There was a near doubling of the phosphorylation of the peptide during the time when stretched compared to when released (Fig. 1, Panel c). There is, therefore, either activation of a kinase or kinases and/or inhibition of a phosphatase or phosphatases resulting from stretch of the muscle. Additionally, there is no significant difference in the increase in peptide phosphorylation with stretch in the stretch then release (2.08 ± 0.26, N = 4) versus the release then stretch (2.14 ± 0.35, N = 4) protocols.
Fig. 1.
Dependence of peptide phosphorylation on muscle stretch and force output. Panel a Experimental design showing typical force responses in stretch and release protocols. Permeabilized muscles were activated in pCa 5 followed by addition of blebbistatin (50 µM). The [Ca+2] was then decreased to pCa > 8. One muscle (red dashed line) was stretched by 5% as indicated and then released by 10%. The other muscle (blue line) was released by 5% followed by a stretch by 10%. The solutions containing 32P-ATP and peptide are identified as 1, 2, and 3. Panel b Typical curves showing the radioactivity following chromatographic separation of the peptide in solutions 2 and 3. Results are shown for the muscle while released (dashed line) and while stretched (solid line). Panel c Relative phosphorylation of the peptide in different experimental designs. In the stretch versus release protocols, the phosphorylation of the peptide when the muscle was stretched is reported relative to when released. In the pCa 5 versus pCa 8 protocols, the phosphorylation of the peptide when the muscle was in pCa 5 is reported relative to that in pCa 8. See text for details of experimental designs. Data are shown as mean ± SEM
We next tested whether A-kinase mediated phosphorylation of twitchin and the loss of catch force altered the extent of stretch-induced phosphorylation of the peptide. When twitchin was pre-thiophosphorylated, the average force maintained by the stretched muscle (P/Po = 0.21 ± 0.11, N = 8) was significantly less than when twitchin was unphosphorylated. The extent of stretch-induced phosphorylation of the peptide was also significantly smaller than that when twitchin is unphosphorylated (Fig. 1, Panel c). This means that the extent of peptide phosphorylation decreased in the muscles that have a small force response (twitchin prethiophosphorylated) even though the extent of the stretch is unchanged. This strongly suggests that the mechanism responsible for peptide phosphorylation responds to the force change as a result of the stretch, at least in the case when the magnitude of force response to stretch is modulated by the presence or absence of the catch state.
At first glance, these results seem consistent with the idea that stretch of the twitchin tether may activate twitchin kinase, and that detachment of the tether by A-kinase mediated thiophosphorylation of twitchin decreases the extent of activation. However, the extent of phosphorylation of the peptide when twitchin is thiophosphorylated is still about 50% of that when the muscle is in catch. This raised the possibility that the mechanism of increased peptide phosphorylation has little to do with the catch state. Rather, it may simply depend on the force output from the muscle.
Phosphorylation of the peptide increases with activation in pCa 5
In order to address the possibility that peptide phosphorylation is related to force output in general, we measured the change in phosphorylation of the peptide when force output increases as a result of an increase in calcium concentration (pCa 5). The reference condition in this experiment is the peptide phosphorylation that occurs when the muscle is fully relaxed (P/Po = 0) at pCa > 8. Twitchin was also pre-thiophosphorylated by treatment with ATPγS and cAMP to remove any possibility of involvement of the catch state in these studies. In this experiment there is no stretch of the muscle, and the force increase is strictly due to activation of myosin crossbridge cycling by calcium. The results (Fig. 1, Panel c) show a large increase in phosphorylation of the peptide during the time that the muscle is activated. As expected, the average force is high (0.88 ± 0.03 P/Po). In order to test whether the large increase in peptide phosphorylation resulted from the increase in [Ca+2] itself or from the myosin crossbridge cycling and associated force output, blebbistatin (50 µM) was used to inhibit crossbridge cycling (Butler et al. 2006). Only 2.5 ± 0.5% (N = 4) of pCa 5 force is developed in the presence of 50 µM blebbistatin. There is a significantly smaller increase in peptide phosphorylation in pCa 5 in the presence compared to the absence of blebbistatin (Fig. 1, Panel c). We conclude that the extent of phosphorylation of the peptide is regulated by force output. This force-dependent phosphorylation is also likely to be independent of the catch state since there is no evidence of the catch state being present in the fully activated muscle with twitchin thiophosphorylated. Rather, the kinase(s) and/or phosphatase(s) responsible for the change in extent of phosphorylation of the peptide respond directly to changes in force output from the muscle. The small, but significant, increase in phosphorylation in pCa 5 with blebbistatin (Fig. 1, Panel c), suggests that a small increase in force as seen in the presence of blebbistatin may be sufficient to activate the phosphorylation mechanism to some extent.
Increase in peptide phosphorylation results from activation of one or more kinases
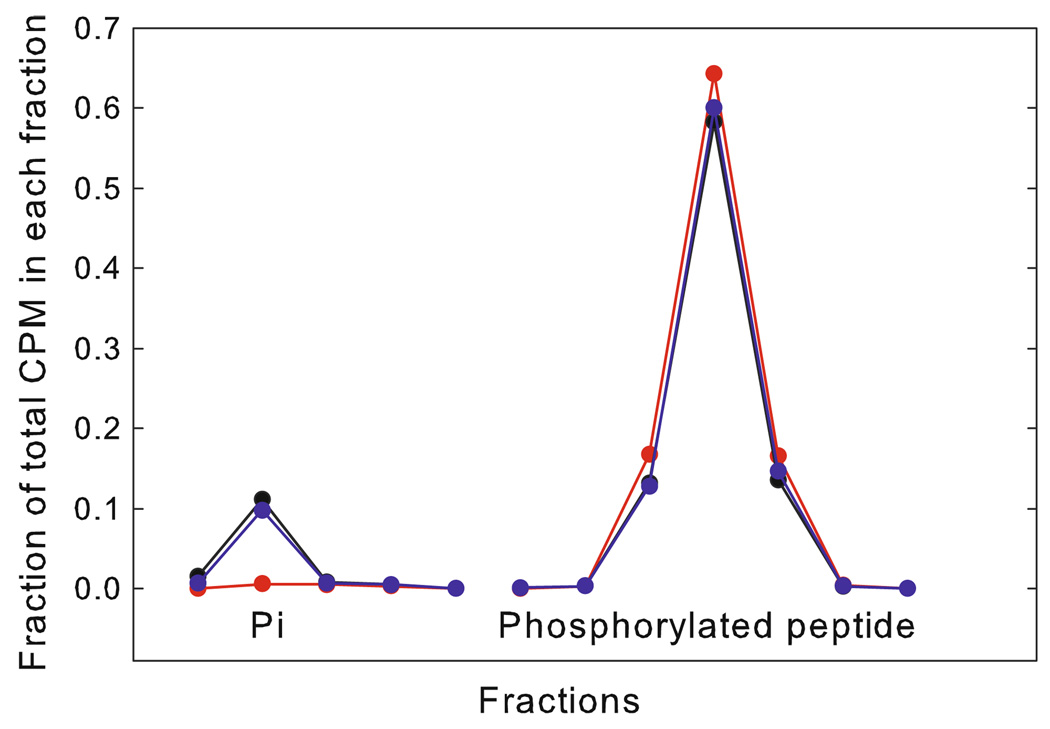
The increase in phosphorylation of the peptide could result from an increase in kinase activity and/or a decrease in phosphatase activity with stretch. The possibility of a change in phosphatase activity was tested by measuring the rate of dephosphorylation of 32P-labeled phosphorylated peptide as a function of stretch. The muscle was subjected to a procedure similar to that shown in Fig. 1 except that instead of using 32P-ATP, the muscle was incubated with 32P-labeled phosphorylated peptide. The appearance of 32P-Pi in solution as well as any change in 32P in the peptide was determined by liquid chromatography. Typical results are shown in Fig. 2. The amount of 32P-Pi in the solution containing the 32P-labeled peptide before incubation with the muscle was extremely small, and after 30 min incubation, the 32P-Pi was 10 ± 2% of the total label in both the stretched and released condition. This increase in 32P-Pi indicated phosphatase activity. In paired measurements on the same muscles the difference in the fraction of total label in inorganic phosphate in released versus stretched conditions is not significant (−0.003 ± 0.015, N = 7). Therefore, there was no measurable difference in phosphatase activity in released versus stretched muscles. These data suggest that the force-dependent phosphorylation results from force-induced activation of kinase(s) rather than inhibition of a phosphatase.
Fig. 2.
Typical liquid chromatographic runs on muscle incubation solutions containing 32P-labeled phosphorylated peptide. Fractions containing 32P-Pi and 32P-labeled phosphorylated peptide are shown. The muscle was incubated in a solution containing 32P-labeled phosphorylated peptide under either stretched (blue) or released (black) conditions. Also shown is a chromatogram of a solution containing the 32P-labeled phosphorylated peptide, but not used for muscle incubation (red)
ML-9 and ML-7 inhibit force-dependent phosphorylation
ML-9 and ML-7 are effective inhibitors of molluscan twitchin kinase activity (Heierhorst et al. 1996), and we tested whether these compounds inhibited the force-sensitive kinase. In these experiments, the muscle with unphosphorylated twitchin was put into the catch state as shown in Fig. 1, and the peptide phosphorylation was measured for 30 min following a 5% decrease in muscle length. At the reduced muscle length, the muscle was incubated for an additional 30 min in the presence of the inhibitor (ML-9, 300 µM; ML-7, 20 µM). This allowed determination of the effect of the inhibitor on peptide phosphorylation when muscle force was low. The muscle was then subjected to a 10% increase in muscle length, and a final 30 min measurement of peptide phosphorylation was made in the presence of the kinase inhibitor. This procedure allowed determination of the effect of the inhibitor on peptide phosphorylation at low force as well as any effect on the response to stretch. The results (Fig. 3) show that ML-9 is an effective inhibitor of the force sensitive phosphorylation. The phosphorylation under released conditions with ML-9 was only 26% of that in the same muscle in the absence of ML-9. When the muscle was stretched, phosphorylation increased about two fold, but the absolute increase was only about a quarter of that in the absence of the inhibitor. The average force during the measurement with ML-9 was not different than that in its absence (0.58 ± 0.04 P/Po and 0.51 ± 0.06 P/Po, respectively).
Fig. 3.
Effect of kinase inhibitors ML-9 (300 µM), ML-7 (20 µM), and Y-27632 (20 µM) on peptide phosphorylation response to stretch. Muscles were put into the catch state as shown in Fig. 1, Panel a, and then subjected to a 5% decrease in muscle length followed by a 30 min incubation and determination of peptide phosphorylation. The inhibitor was then added and measurement of peptide phosphorylation made at the released length and following stretch by 10%. The phosphorylation of the peptide in the presence of the inhibitor is shown relative to that at the released length without the inhibitor. Open bar released conditions; Shaded bar stretched conditions; Hatched bar difference between stretched and released conditions. Also shown are similar data with muscles not subjected to inhibitor treatment. *Released muscle with inhibitor significantly lower than no inhibitor. #Difference between stretched and released muscle with inhibitor significantly lower than no inhibitor. + Difference between stretched and released muscle with inhibitor significantly higher than no inhibitor. Data are mean ± SEM
There was also a large effect of ML-7 on peptide phosphorylation (Fig. 3). When the muscle was released, the phosphorylation in the presence of ML-7 (20 µM) was 38% of that in the same muscle in the absence of ML-7. The phosphorylation still increased by about 2-fold when the muscles were stretched in the presence of the inhibitor, but the overall extent of phosphorylation compared to that in the absence of the inhibitor was decreased by about 50%. The fact that both ML-9 and ML-7 are effective inhibitors of the kinase responsible for force-dependent peptide phosphorylation supports the idea that a myosin light chain kinase-like enzyme such as twitchin kinase is involved in the phosphorylation. It is interesting that phosphorylation increased by about 2-fold with stretch compared to the released state in the presence of both ML-9 and ML-7. This suggests that the kinase responsible for the majority of the peptide phosphorylation in the released low force condition is the same kinase responsible for the large increase in phosphorylation in response to an increase in force output.
The rho-kinase inhibitor Y-27632 does not inhibit force-dependent phosphorylation
Rho activated kinase has been implicated in the response to force sensing (for review see Mammoto and Ingber 2009) and has also been shown to phosphorylate the regulatory light chain of smooth muscle (Amano et al. 1996). We tested the possible involvement of ROCK in the force sensitive kinase activity by use of the Rho-kinase inhibitor Y-27632 (Uehata et al. 1997). In experiments similar to those using ML-9 and ML-7, Y-27632 (20 µM) caused a small decrease (~ 20%) in peptide phosphorylation when the muscle was under low force conditions (Fig. 3). When the muscle was stretched in the presence of Y-27632, the phosphorylation increased dramatically. Indeed, the increase was significantly larger than that seen in the absence of the rho kinase inhibitor. The increase in force-sensitive kinase activity in the presence of the rho kinase inhibitor shows that ROCK activity is not necessary for the force dependent phosphorylation of the peptide; rather decreasing ROCK activity seems to enhance the activation of the force sensitive kinase.
Force-sensitive phosphorylation of twitchin, but not myosin light chains
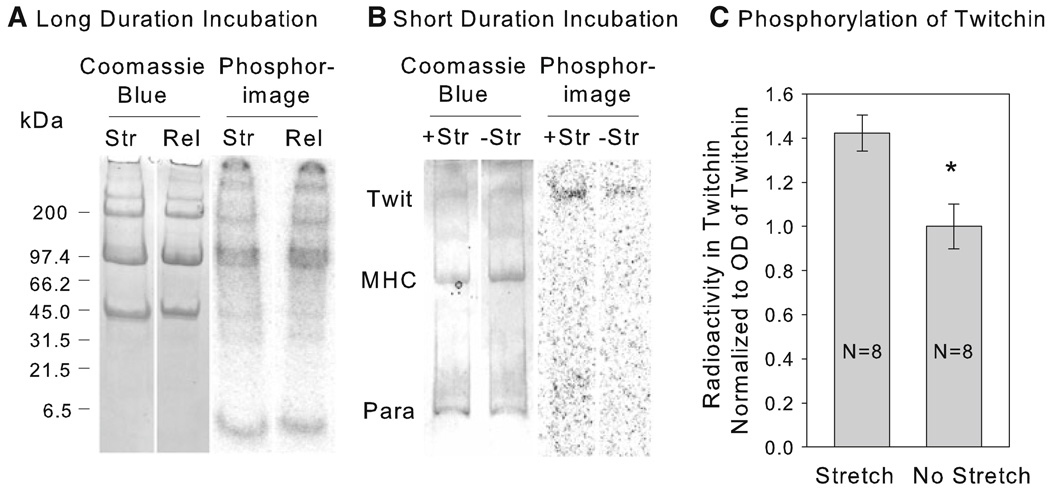
An experiment similar to that described in Fig. 1 was used to try to identify whether 32P incorporation into a specific protein resulted from stretch of the muscle. No peptide was added in these experiments. The muscles were put into catch and then incubated in 32P-ATP for 10 min. They were transferred to a new 32P-ATP solution, and experimental muscles were stretched by 5%, whereas control muscles were released by 5%. After 30 min, the muscles were frozen and proteins were run on gradient gels whose radioactivity was subsequently determined. Typical Coomassie Blue stained gels and autoradiograms are shown in Fig. 4, Panel a. There was significant incorporation of 32P into several proteins during the 40 min incubation with 32P-ATP. The proteins had molecular masses consistent with twitchin (527 kDa), myosin heavy chain (200 kDa), myorod (113 kDa), paramyosin (100 kDa) and others. There was, however, no obvious increase in 32P labeling of any protein associated with stretch. It is interesting that there is no detectable 32P incorporation into the region of the gel (~ 18 kDa) that contains the myosin light chains in either stretched or released muscles. Morita and co-workers (Miyanishi et al. 1985) have shown that the regulatory light chain a (RLCa) in scallop catch muscle can be phosphorylated by added myosin light chain kinase at a site that shares some sequence homology with the added peptide that is phosphorylated during stretch. In order to further investigate whether there might be phosphorylation of an RLCa equivalent in the ABRM as a result of stretch, an antibody raised against the phosphorylated form of the peptide A-T-SP-N-V was used. This sequence is present in RCLa in scallop catch muscle (Miyanishi et al. 1985). There was no detectable signal from the light chain region of blots containing muscles that were either released (N = 4) or stretched (N = 4). This supports the results from our 32P labeling experiments and suggests that in this permeabilized muscle there is no phosphorylation of the light chains under the conditions used.
Fig. 4.
32P incorporation into proteins as a result of stretch. Panel a shows typical Coomassie Blue stained gels and autoradiograms of proteins from muscles put into catch and then incubated in 32P-ATP for 30 min under either stretched (Str) or released (Rel) conditions as shown in Fig. 1, Panel a. Gradient gels of 4–15% acrylamide were used. Panel b shows similar data for muscles in a solution with pCa > 8 and 32P-ATP either kept at slack length (−Str) or stretched (+Str) by 10%. The muscles were frozen after 8 min. 5% acrylamide gels were used. The regions containing twitchin (Twit), myosin heavy chain (MHC), and paramyosin (Para) are identified. Panel c Quantification of the effect of stretch on radioactivity in twitchin in the design described in Panel b. The radioactivity in twitchin is normalized to the Coomassie Blue staining of twitchin. Data are reported relative to the mean ratio in the non-stretched muscles. Data are mean ± SEM. *P < 0.01
The possible force dependence of phosphorylation of twitchin, myosin heavy chain, myorod and paramyosin was further studied in a simplified experiment in which the total duration of exposure to 32P-ATP was shortened to 11 min to decrease the extent of background 32P incorporation into the proteins. The muscles were incubated for 3 min at slack length in a solution with pCa > 8 and 32P-ATP, and then experimental muscles were stretched by 10% whereas control muscles remained at the original length. The muscles were frozen after an additional 8 min. Previous experiments have shown that most of the force response to such a stretch is due to catch since it is removed with addition of cAMP and phosphorylation of twitchin (data not shown). Typical gels containing proteins from stretched and non-stretched muscles are shown in Fig. 4, Panel b. 32P labeling of myosin heavy chain and paramyosin is often not detectable, but there is a small amount of labeling of twitchin. Quantification of the radioactivity in twitchin normalized to the Coomassie Blue staining of the protein is shown in Fig. 4, Panel c. There is a small (~ 40%), but statistically significant higher 32P incorporation into twitchin in the stretched compared to the non-stretched muscle. This suggests that twitchin is a substrate for the force-sensitive kinase, and the observation that molluscan twitchin is able to autophosphorylate (Heierhorst et al. 1994) is consistent with the kinase being twitchin itself.
Discussion
The force-dependent kinase activity described here occurs in triton permeabilized muscles where most small molecules and soluble proteins are absent from the cells. This means that the force sensitive kinase is bound in the muscle. The kinase is activated by an increase in force with stretch and subsequently decreases in activity when the muscle is shortened and force decreases. Therefore, there must be a reversal of the activation of the mechanism responsible for the phosphorylation. If the activation of the kinase involves a multi-step signaling mechanism, then not only must the mechanism sense the decrease in force, but there must also be a means of shutting down the signaling mechanism.
An increase in force could result in an increase in phosphorylation by (1) direct activation of a kinase, (2) inhibition of a phosphatase, (3) increasing availability of the substrate to the kinase, or (4) changing the substrate and making it more susceptible to phosphorylation by a kinase (Sawada et al. 2006). When considered in its entirety, the data shown here are only consistent with the possibility of a direct activation of a kinase. Inhibition of a phosphatase has been ruled out by experiments that show no effect of stretch and increased force on dephosphorylation of prephosphorylated peptide. Since the substrate is the added peptide, an increase in availability of the soluble peptide to the kinase is unlikely when force is increased. Finally, the “substrate priming” mechanism for force sensing that has been described for p130Cas (Sawada et al. 2006) does not apply here since the small peptide substrate does not change its conformation with stretch or force output from the muscle; rather, there appears to be a change in the intrinsic activity of a kinase. The question becomes what is the identity of the force-sensitive kinase?
The activation of the kinase occurs as a result of an increase in force resulting from either stretching muscles in catch or activation of the muscles via an increase in [Ca+2]. This suggests that the mechanism responsible for kinase activation responds to increases in force in structures in the pathway of force transmission involving the contractile filaments. This places the force sensitive structure somewhere within the thick or thin filaments, the cytoplasmic dense bodies, or the membrane bound dense bodies similar to focal adhesions. The complexity of mechanotransduction at the focal adhesion complex is well known and summarized in the review by Geiger et al. (2009). Focal adhesion complexes contain several kinases whose activities could increase as a result of stretch. Many of these are tyrosine kinases that could not directly phosphorylate the peptide which contains no tyrosine residues. It was especially important to test the possible involvement of Rho kinase since it has been implicated as one of the factors leading to feedback from changes in the focal adhesion complex to myosin light chain phosphorylation in vertebrate cells. Indeed, Rho kinase has been shown to directly phosphorylate the regulatory myosin light chain at the site mimicked by the peptide used in these studies (Amano et al. 1996). The persistence of the force-dependent kinase in the presence of the Rho kinase inhibitor shows that Rho kinase is not required for activity, but the increase in the force-dependent kinase activity when Rho kinase is inhibited suggests that Rho kinase can modulate the force dependent kinase activity.
While it is not possible to conclusively identify twitchin kinase as the force-activated kinase, there are several findings that support the idea. These are the following: (1) twitchin is localized to the thick filament (Vibert et al. 1993) and thus in close association with the contractile filament force transmission pathway; (2) the peptide that was used as a measure of force-sensitive phosphorylation is a good substrate of molluscan twitchin kinase (Heierhorst et al. 1996); (3) ML-7 and ML-9 which inhibit the force sensitive kinase are both twitchin kinase inhibitors (Heierhorst et al. 1996). Whereas ML9 inhibits several different kinases, ML7 is quite specific for smooth muscle MLCK (Bain et al. 2003) and related enzymes (Heierhorst et al. 1996). This strongly argues that the force sensitive kinase is a myosin light chain kinase-related enzyme. There is no evidence that a typical myosin light chain kinase enzyme exists in Mytilus ABRM smooth muscle. A kinase that phosphorylates the regulatory light chain of scallop smooth muscle has been identified by Morita and co-workers (Sohma and Morita 1986). Its molecular weight was about 40 kDa, it does not require Ca+2-calmodulin for activity, and is actually inhibited by Ca+2 (Sohma and Morita 1987). Subsequent studies showed that the enzyme interacts with a cAMP dependent regulatory protein which modulates its kinase activity (Sohma et al. 1988). The fact that there is no 32P incorporation into the myosin light chain as a result of stretch makes it unlikely that this enzyme is responsible for force-activated kinase activity. Added vertebrate myosin light chain kinase phosphorylates myorod in catch muscle (Sobieszek et al. 2006), but it has been suggested that that this phosphorylation is mediated by twitchin rather than by a myosin light chain kinase (Matusovsky et al. 2010). (4) Molluscan twitchin kinase has been shown to autophosphorylate (Heierhorst et al. 1994). (5) Molluscan twitchin kinase is autoinhibited (Kobe et al. 1996) and shows a similar structure to nematode twitchin kinase whose activity is thought to be activated by stretch (Greene et al. 2008). The force-sensitive kinase activity increases when the muscle is activated and there is no twitchin mediated tether between thick and thin filaments. This suggests that the activation of the twitchin kinase involves more than a simple mechanism involving an extension of the molecule while one end is attached to thick filaments and the other end attached to thin filaments. Twitchin from Mytilus has been shown to interact with the head region of myosin (Funabara et al. 2009), and this interaction could be part of the mechanism by which the molecule senses active force.
In 32P-labeling studies on intact muscles we found that there is significant phosphorylation of twitchin in the catch state which then doubles with addition of serotonin and relaxation of catch (Siegman et al. 1997). The phosphorylation present in catch is likely at least in part due to the force-activated kinase activity that is described here, and is not on the sites that result in relaxation of catch force because catch force is well-maintained. Therefore, the site(s) on twitchin that are phosphorylated by the force-activated kinase are probably different from those that are phosphorylated by A-kinase and cause relaxation of catch force. Additionally, the sites that control catch are all consensus sequences for substrates of protein kinase A (Funabara et al. 2001; Butler and Siegman 2010) while the peptide used in these studies is not. Although there is a significant increase in 32P incorporation into twitchin with stretch, the overall amount of incorporation is small as shown in Fig. 4, Panels b and c. The physiological effect of such an autophosphorylation of twitchin is not known, but might involve a change in the molecule’s mechanical properties as is the case for phosphorylation of cardiac titin (Yamasaki et al. 2002; Fukuda et al. 2005; Hidalgo et al. 2009).
These results on a force-activated kinase were obtained on molluscan catch muscles, in which there is substantial evidence for the thick and thin filament tethering properties of twitchin. There is some evidence that twitchin from molluscan striated muscles that are not catch muscles also mediates binding of thick and thin filaments in vitro (Tsutsui et al. 2007). In addition, a tethering function of twitchin could be responsible for its phosphorylation-mediated regulation of relaxation rate in Aplysia accessory radula closer muscles (Probst et al. 1994), and could play a role in the constant twitching of body wall muscles associated with its mutation in C. elegans (Moerman et al. 1988). Given such limited information, however, it is not known to what extent the twitchin molecules share functions in different muscles and organisms, and it is possible that any force-mediated activation of twitchin kinase occurs via a mechanism only present in catch muscles.
In conclusion, the force-activated kinase in catch smooth muscle is likely to be twitchin, which, when force increases, is autophosphorylated on site(s) other than those responsible for relaxation of catch.
Acknowledgments
This work was supported by grant RO1 AR042758 from the National Institutes of Health. The authors thank Susan U. Mooers and Srinivasa R. Narayan for their expert technical assistance.
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Nagy A, Kengyel A, Szatmari D, Martonfalvi Z, Huber T, Kellermayer MS. Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J. 2007;93:2102–2109. doi: 10.1529/biophysj.107.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Siegman MJ. Mechanism of catch force: tethering of thick and thin filaments by twitchin. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/725207. Article ID 725207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Mooers SU, Li C, Narayan S, Siegman MJ. Regulation of catch muscle by twitchin phosphorylation: effects on force, ATPase, and shortening. Biophys J. 1998;75:1904–1914. doi: 10.1016/S0006-3495(98)77631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Narayan SR, Mooers SU, Hartshorne DJ, Siegman MJ. The myosin cross-bridge cycle and its control by twitchin phosphorylation in catch muscle. Biophys J. 2001;80:415–426. doi: 10.1016/S0006-3495(01)76024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Mooers SU, Siegman MJ. Catch force links and the low to high force transition of myosin. Biophys J. 2006;90:3193–3202. doi: 10.1529/biophysj.105.077453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Mooers SU, Narayan SR, Siegman MJ. The N-terminal region of twitchin binds thick and thin contractile filaments: redundant mechanisms of catch force maintenance. J Biol Chem. 2010;285:40654–40665. doi: 10.1074/jbc.M110.166041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke AS, Mooers SU, Narayan SR, Siegman MJ, Butler TM. Myosin cross-bridge kinetics and the mechanism of catch. Biophys J. 2007;93:554–565. doi: 10.1529/biophysj.107.105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125:257–271. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabara D, Kinoshita S, Watabe S, Siegman MJ, Butler TM, Hartshorne DJ. Phosphorylation of Molluscan Twitchin by the cAMP-dependent protein kinase. Biochemistry. 2001;40:2087–2095. doi: 10.1021/bi0022691. [DOI] [PubMed] [Google Scholar]
- Funabara D, Hamamoto C, Yamamoto K, Inoue A, Ueda M, Osawa R, Kanoh S, Hartshorne DJ, Suzuki S, Watabe S. Unphosphorylated twitchin forms a complex with actin and myosin that may contribute to tension maintenance in catch. J Exp Biol. 2007;210:4399–4410. doi: 10.1242/jeb.008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabara D, Osawa R, Ueda M, Kanoh S, Hartshorne DJ, Watabe S. Myosin loop 2 is involved in the formation of a trimeric complex of twitchin, actin and myosin. J Biol Chem. 2009;284:18015–18020. doi: 10.1074/jbc.M109.016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Grater F, Shen J, Jiang H, Gautel M, Grubmuller H. Mechanically induced titin kinase activation studied by force-probe molecular dynamics simulations. Biophys J. 2005;88:790–804. doi: 10.1529/biophysj.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DN, Garcia T, Sutton RB, Gernert KM, Benian GM, Oberhauser AF. Single-molecule force spectroscopy reveals a stepwise unfolding of Caenorhabditis elegans giant protein kinase domains. Biophys J. 2008;95:1360–1370. doi: 10.1529/biophysj.108.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heierhorst J, Probst WC, Vilim FS, Buku A, Weiss KR. Autophosphorylation of molluscan twitchin and interaction of its kinase domain with calcium/calmodulin. J Biol Chem. 1994;269:21086–21093. [PubMed] [Google Scholar]
- Heierhorst J, Tang X, Lei J, Probst WC, Weiss KR, Kemp BE, Benian GM. Substrate specificity and inhibitor sensitivity of Ca2+/S100-dependent twitchin kinases. Eur J Biochem. 1996;242:454–459. doi: 10.1111/j.1432-1033.1996.454rr.x. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105:631–638. doi: 10.1161/CIRCRESAHA.109.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SH, Parker MW, Lei JY, Wilce MC, Benian GM, Kemp BE. Insights into autoregulation from the crystal structure of twitchin kinase. Nature. 1994;369:581–584. doi: 10.1038/369581a0. [DOI] [PubMed] [Google Scholar]
- Kobe B, Heierhorst J, Feil SC, Parker MW, Benian GM, Weiss KR, Kemp BE. Giant protein kinases: domain interactions and structural basis of autoregulation. EMBO J. 1996;15:6810–6821. [PMC free article] [PubMed] [Google Scholar]
- Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res. 2001;89:874–881. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- Leonard TR, Herzog W. Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am J Physiol Cell Physiol. 2010;299:C14–C20. doi: 10.1152/ajpcell.00049.2010. [DOI] [PubMed] [Google Scholar]
- Linke WA, Kulke M, Li H, Fujita-Becker S, Neagoe C, Manstein DJ, Gautel M, Fernandez JM. PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol. 2002;137:194–205. doi: 10.1006/jsbi.2002.4468. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Matusovsky OS, Shelud’ko NS, Permyakova TV, Zukowska M, Sobieszek A. Catch muscle of bivalve molluscs contains myosin- and twitchin-associated protein kinase phosphorylating myorod. Biochim Biophys Acta. 2010;1804:884–890. doi: 10.1016/j.bbapap.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Mayans O, van der Ven PF, Wilm M, Mues A, Young P, Furst DO, Wilmanns M, Gautel M. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–869. doi: 10.1038/27603. [DOI] [PubMed] [Google Scholar]
- Miyanishi T, Maita T, Morita F, Kondo S, Matsuda G. Amino acid sequences of the two kinds of regulatory light chains of adductor smooth muscle myosin from Patinopecten yessoensis. J Biochem (Tokyo) 1985;97:541–551. doi: 10.1093/oxfordjournals.jbchem.a135089. [DOI] [PubMed] [Google Scholar]
- Moerman DG, Benian GM, Barstead RJ, Schriefer A, Waterston RH. Identification and intracellular localization of the unc-22 gene product of Caenorhabditis elegans. Genes Dev. 1988;2:93–105. doi: 10.1101/gad.2.1.93. [DOI] [PubMed] [Google Scholar]
- Nagy A, Cacciafesta P, Grama L, Kengyel A, Malnasi-Csizmadia A, Kellermayer MS. Differential actin binding along the PEVK domain of skeletal muscle titin. J Cell Sci. 2004;117:5781–5789. doi: 10.1242/jcs.01501. [DOI] [PubMed] [Google Scholar]
- Olson NJ, Pearson RB, Needleman DS, Hurwitz MY, Kemp BE, Means AR. Regulatory and structural motifs of chicken gizzard myosin light chain kinase. Proc Natl Acad Sci USA. 1990;87:2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst WC, Cropper EC, Heierhorst J, Hooper SL, Jaffe H, Vilim F, Beushausen S, Kupfermann I, Weiss KR. cAMP-dependent phosphorylation of Aplysia twitchin may mediate modulation of muscle contractions by neuropeptide cotransmitters. Proc Natl Acad Sci USA. 1994;91:8487–8491. doi: 10.1073/pnas.91.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchner EM, Gaub HE. Exploring the conformation-regulated function of titin kinase by mechanical pump and probe experiments with single molecules. Angew Chem Int Ed Engl. 2010;49:1147–1150. doi: 10.1002/anie.200905956. [DOI] [PubMed] [Google Scholar]
- Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci USA. 2008;105:13385–13390. doi: 10.1073/pnas.0805034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelud’ko NS, Matusovsky OS, Permyakova TV, Matusovskaya GG. “Twitchin-actin linkage hypothesis” for the catch mechanism in molluscan muscles: evidence that twitchin interacts with myosin, myorod, and paramyosin core and affects properties of actomyosin. Arch Biochem Biophys. 2007;466:125–135. doi: 10.1016/j.abb.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Siegman MJ, Mooers SU, Li C, Narayan S, Trinkle-Mulcahy L, Watabe S, Hartshorne DJ, Butler TM. Phosphorylation of a high molecular weight (approximately 600 kDa) protein regulates catch in invertebrate smooth muscle. J Muscle Res Cell Motil. 1997;18:655–670. doi: 10.1023/a:1018683823020. [DOI] [PubMed] [Google Scholar]
- Siegman MJ, Funabara D, Kinoshita S, Watabe S, Hartshorne DJ, Butler TM. Phosphorylation of a twitchin-related protein controls catch and calcium sensitivity of force production in invertebrate smooth muscle. Proc Natl Acad Sci USA. 1998;95:5383–5388. doi: 10.1073/pnas.95.9.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieszek A, Matusovsky OS, Permyakova TV, Sarg B, Lindner H, Shelud’ko NS. Phosphorylation of myorod (catchin) by kinases tightly associated to molluscan and vertebrate smooth muscle myosins. Arch Biochem Biophys. 2006;454:197–205. doi: 10.1016/j.abb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sohma H, Morita F. Purification of a protein kinase phosphorylating myosin regulatory light chain-a (RLC-a) from smooth muscle of scallop, Patinopecten yessoensis. J Biochem. 1986;100:1155–1163. doi: 10.1093/oxfordjournals.jbchem.a121819. [DOI] [PubMed] [Google Scholar]
- Sohma H, Morita F. Characterization of regulatory light chain-a myosin kinase from smooth muscle of scallop, Patinopecten yessoensis. J Biochem. 1987;101:497–502. [PubMed] [Google Scholar]
- Sohma H, Inoue K, Morita F. A cAMP-dependent regulatory protein for RLC-a myosin kinase catalyzing the phosphorylation of scallop smooth muscle myosin light chain. J Biochem (Tokyo) 1988;103:431–435. doi: 10.1093/oxfordjournals.jbchem.a122287. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J. Roles of titin in the structure and elasticity of the sarcomere. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/612482. Article ID 612482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui Y, Yoshio M, Oiwa K, Yamada A. Striated muscle twitchin of bivalves has “catchability”, the ability to bind thick filaments tightly to thin filaments, representing the catch state. J Mol Biol. 2007;365:325–332. doi: 10.1016/j.jmb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Vibert P, Edelstein SM, Castellani L, Elliott BW. Mini-titins in striated and smooth molluscan muscles: structure, location and immunological crossreactivity. J Muscle Res Cell Motil. 1993;14:598–607. doi: 10.1007/BF00141557. [DOI] [PubMed] [Google Scholar]
- Yamasaki R, Berri M, Wu Y, Trombitas K, McNabb M, Kellermayer MS, Witt C, Labeit D, Labeit S, Greaser M, Granzier H. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/s100a1. Biophys J. 2001;81:2297–2313. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]