Abstract
Objectives
We sought to assess the association between the presence of a septal pouch in the left atrium and ischemic stroke.
Background
Recently, a new anatomical entity, named a left septal pouch (LSP), was described in a pathology study. It was suggested that the presence of LSP may favor the stasis of blood and possibly result in thromboembolic complications. However, the embolic potential of a LSP is not known.
Methods
The association between LSP and risk of stroke was assessed using a population-based case-control study design. The presence of LSP was assessed by transesophageal echocardiography in 187 patients over age 50 with first-ever ischemic stroke (96 men, mean age 70.6 ± 9.0 years) and in 157 control subjects matched to patients by age, sex, and race/ethnicity. The association between LSP and risk of stroke was assessed after adjustment for other stroke risk factors.
Results
Patients with LSPs were younger (67.5 ± 9.1 vs. 69.6 ± 8.8; p=0.046) and had a lower proportion of hypertension (68.0% vs. 80.3%; p=0.01). There were no difference in the prevalence of LSP between stroke patients and control subjects (28.9% vs. 29.3%; p=0.93). The subgroup of 69 patients (36.9%) with crytptogenic stroke showed a similar prevalence of LSP (31.9% vs. 29.3%; p=0.70). Multivariable analysis showed that the presence of LSP was not associated with ischemic stroke (OR 1.09, 95% CI 0.64 to 1.85) or cryptogenic stroke (OR 1.41, 95% CI 0.71 to 2.78).
Conclusions
This study does not demonstrate evidence for association of the presence of LSP with ischemic stroke, or with cryptogenic stroke. The stroke risk associated with LSP may still require further evaluation in the younger stroke populations. The possibility that associated cofactors that may turn LSP from an innocent bystander into a causative mechanism for stroke remain to be elucidated.
Keywords: stroke, septal pouch, left atrium, transesophageal echocardiography
The causes of ischemic stroke remain unidentified by routine diagnostic testing in up to 40% of patients. (1) These strokes, often termed “cryptogenic” have been linked to the presence of atrial septal abnormalities. (2–6) The relationship between patent foramen ovale (PFO) and cryptogenic stroke in younger stroke patients has been confirmed in multiple studies with transthoracic or transesophageal echocardiography (TEE). (4, 7–9) The association has been more controversial in elderly subjects, in whom it has been invoked or negated. (4, 10, 11) Finally, a TEE study reaffirmed the relationship between PFO and stroke risk in an older age group after adjustment for other stroke risk factors. (12) The hypothesized stroke mechanism is paradoxical embolization, or the embolization to the systemic arterial circulation of thrombus originating in the venous circulation. The presence of atrial septal aneurysm (ASA) has also been associated with an increased risk for stroke, especially when it is associated with a PFO. (13) In a meta-analysis, the stroke risk was higher in subjects with PFO plus ASA than in those with either condition alone. (11, 14)
The diagnosis of cryptogenic stroke remains presumptive in a vast majority of patients and is based on the exclusion of other potential causes of cerebral embolism in the setting of an embolic-appearing stroke, rather than on the actual demonstration of paradoxical embolization. Recently, a new anatomical entity, the left sided pouch (LSP), has been defined in a pathology study. (15) LSP was described as an incomplete fusion in the cranial segment of the overlap between the septum primum (SP) and septum secundum (SS), resulting in a recess that opens into the left atrium, with no interatrial shunting. It was hypothesized that, with access to the systemic circulation similar to that of the left atrial appendage, LSP might serve as a nidus for thrombus formation in the presence of low flow states, and therefore predispose to embolic events. LSP has anecdotally been considered the culprit lesion for transient ischemic attack and coronary embolus. (16, 17) An association between LSP and stroke could provide novel insights on the mechanism of certain cryptogenic strokes. The aim of the present study was, therefore, to assess the existence of an association between LSP and ischemic stroke.
Methods
Study Population
As part of the National Institute of Neurological Disorders and Stroke (NINDS)-funded Aortic Plaques and Risk of Ischemic Stroke (APRIS) study, 255 patients with acute ischemic stroke and 209 stroke-free control subjects were enrolled in the study between 1997 and 2002. The details of the study population have been described before. (18) Briefly, stroke patients were consecutive patients residing in northern Manhattan and admitted to Columbia University Medical Center with a first acute ischemic stroke. All strokes were subtyped by a study neurologist on the basis of predefined criteria modeled after the NINDS Stroke Data Bank and Trial of Organon in Acute Stroke Therapy (TOAST). (19) Cryptogenic strokes typically had no definite source despite a thorough diagnostic evaluation. Control subjects were selected from the participants of the Northern Manhattan Study (NOMAS), and matched to cases by age (within 5 years), sex, and race/ethnicity. Detailed recruitment methods for NOMAS have been previously published. (20, 21) Informed consent was obtained from cases and controls, or their health proxies when necessary. The study was approved by the Institutional Review Board of Columbia University Medical Center.
Definition of Baseline Subject Characteristics
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as a systolic blood pressure recording ≥ 140 mm Hg or a diastolic blood pressure recording ≥90 mm Hg based on the mean of 2 readings, a patient's self-report of a history of hypertension, or antihypertensive medication use. Diabetes mellitus was defined by a patient's self-report of such a history, insulin use, oral hypoglycemic use, or a fasting glucose ≥ 126 mg/dl. Hypercholesterolemia was defined as total serum cholesterol > 240 mg/dl, a patient's self-report of hypercholesterolemia, or the presence of lipid-lowering treatment. Current smoking was defined by use of tobacco at the time of the interview; smoking history was defined by use of tobacco at any time. The presence of atrial fibrillation was documented based on the results of a current or past electrocardiogram. Coronary artery disease was defined as history of myocardial infarction, coronary artery bypass grafting or percutaneous coronary intervention, typical angina, and use of anti-ischemic medications. Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Race/ethnicity was determined by subject's self-report using a questionnaire modeled after the U.S. Census Bureau questionnaire.
TEE Evaluation
TEE was performed in stroke patients within 3 days of stroke onset and in control subjects upon enrollment. A predefined protocol was used to assess for potential cardiac and aortic sources of embolism, including the assessment for presence of a PFO at rest and during Valsalva maneuver using a multiplane transducer and agitated saline injection. After careful observation of the interatrial septum using 2-dimensional echocardiography and color Doppler mapping of the septum with the Valsalva maneuver, ≥2 intravenous contrast injections were administered through the right antecubital vein at rest and with the Valsalva maneuver. PFO was considered to be present if any microbubble was seen in the left atrium within 3 cardiac cycles from maximum right atrial opacification. (2–4) An ASA was defined as more than a 10-mm protrusion beyond the plane of the septum into the left or right atrium. (22) For the purpose of the present study, TEEs were systematically reviewed for LSP presence. A LSP was defined as an incomplete fusion in the cranial segment of the overlap between the SP and SS in a standard bicaval view (Figure 1A), with no evidence of right-to-left shunting on contrast injection at rest or during the Valsalva maneuver (Figure 1B). The presence of a right septal pouch (incomplete septal fusion in the caudal segment of the septum with opening into the right atrium) or of a closed pouch (incomplete fusion of the septum without opening into either atrial chamber) was recorded. TEEs were interpreted by two experienced echocardiography fellows (AT and KO) blinded to subjects' case-control status, clinical characteristics and stroke risk factors.
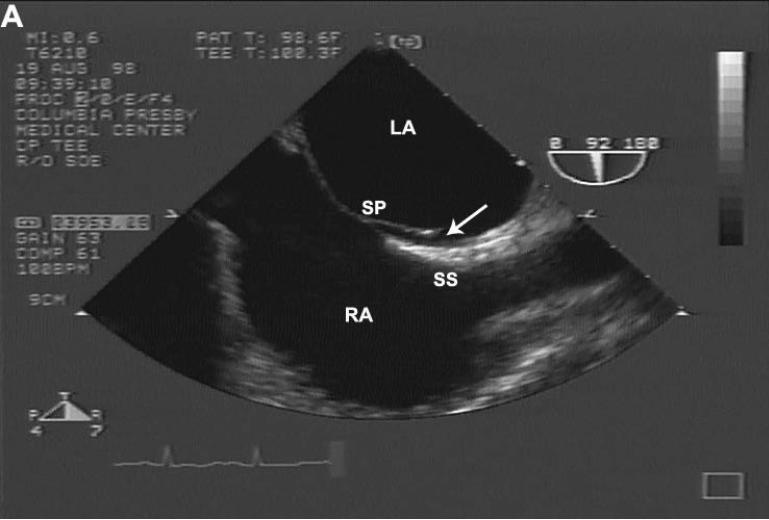
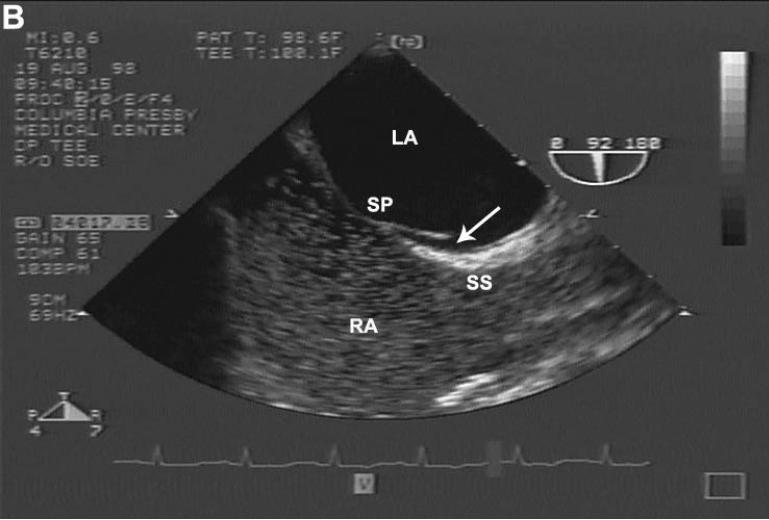
Figure 1.
(A) Example of direct visualization of LSP (arrow) by TEE. In longitudinal plane, an incomplete fusion is visible in the cranial segment of the zone of overlap between SP and SS. (B) Differentiation of LSP from PFO by contrast TEE. Injection of agitated contrast saline with a Valsalva maneuver demonstrates the absence of microbubbles crossing the interatrial septum, excluding the presence of a PFO. LSP = left sided pouch; PFO = patent foramen ovale; SP = septum primum; SS = septum secundum; TEE = transesophageal echocardiography.
Interobserver variability
Twenty randomly chosen TEE studies were read by both observers independently for the presence or absence of LSP. There was 100% agreement between the two observers (95% CI 86.7% to 100.0%).
Statistical Analysis
Data are presented as mean ± SD for continuous variables and as proportions for categorical variables. Differences between proportions were assessed by the chi-square test, replaced by the Fisher exact test when the expected cell count was <5. Differences between mean values were assessed by Student t test.
Univariable and multivariable logistic regression analysis was carried out to assess the association between LSP (independent variable) and ischemic stroke (dependent variable). Variables significantly associated with ischemic stroke at univariable analysis (arterial hypertension, diabetes mellitus, hypercholesterolemia, smoking history and atrial fibrillation) were entered as independent variables in the model, along with pertinent demographics (age, sex). The C statistic (equivalent to the area under the receiver operating characteristic curve) was calculated to assess the performance of the logistic model.
SAS (version 9.2, SAS Institute, Cary, North Carolina) was used in the analyses. A 2-tailed p value of <0.05 was considered significant for all analyses.
Results
Of the initial 464 subjects, 107 were excluded from the analysis because of the presence of a PFO (n=89), a closed pouch (n=9), a right sided pouch (n=5), or an atrial septal defect (n=4). Another 13 patients were excluded from the analyses because of technically inadequate echocardiographic contrast study. The analyses were performed in the remaining 344 subjects.
Baseline Characteristics
The final study population was comprised of 187 stroke patients and 157 stroke-free subjects. The cause of stroke could be identified by means of routine diagnostic testing in 118 patients (63.1%). The stroke was classified as cryptogenic in the remaining 69 patients (36.9%). A LSP was detected in 100 subjects (29.1 %). An ASA was found in 16 (4.7%).
Comparison of cases and controls
Demographics, cardiovascular risk factors and atrial septal abnormalities in stroke patients and in control subjects are shown in Table 1. Stroke patients were significantly older than control subjects (70.6 ± 9.0 vs. 67.0 ± 8.4; p=0.0002), and had a significantly higher prevalence of hypertension and diabetes (both p<0.0001). History of AF was also more frequent in stroke patients than in control subjects (p=0.02). The frequency of hypercholesterolemia was significantly lower in the stroke group (p=0.045). There were no differences with regard to the prevalence of LSPs and ASAs in stroke patients compared with control subjects (28.9% vs. 29.3%; p=0.93 and 5.9% vs. 3.2%; p=0.24, respectively).
Table 1.
Demographics, Risk Factors and LSP Frequency Status in Stroke and Control Subjects
| Stroke Patients (n=187) | Control Subjects (n=157) | p Value | |
|---|---|---|---|
| Age, yrs | 70.6 ± 9.0 | 67.0 ± 8.4 | 0.0002 |
| < 60 | 21 (11) | 30 (19) | |
| 60–69 | 69 (37) | 70 (45) | 0.009 |
| ≥ 70 | 97 (52) | 57 (36) | |
| Male | 96 (51.3) | 88 (56.0) | 0.38 |
| Body mass index, kg/m2 (mean ± SD) | 26.7 ± 5.1 | 27.6 ± 4.8 | 0.09 |
| Race/ethnicity | |||
| White | 28 (15.0) | 23 (14.7) | |
| Black | 52 (27.8) | 50 (31.9) | 0.71 |
| Hispanic | 107 (57.2) | 84 (53.5) | |
| Risk factors | |||
| Hypertension | 158 (85.0) | 105 (66.9) | < 0.0001 |
| Diabetes mellitus | 86 (46.5) | 36 (22.9) | < 0.0001 |
| Hypercholesterolemia | 78 (42.6) | 84 (53.5) | 0.045 |
| Current smoker | 33 (18.3) | 25 (16.2) | 0.61 |
| Smoking history | 103 (55.7) | 99 (63.1) | 0.17 |
| Coronary artery disease | 36 (19.4) | 33 (21.0) | 0.70 |
| Atrial fibrillation | 22 (11.8) | 7 (4.5) | 0.02 |
| Atrial septal abnormalities | |||
| ASA | 11 (5.9) | 5 (3.2) | 0.24 |
| LSP | 54 (28.9) | 46 (29.3) | 0.93 |
Values are given as n (%) unless otherwise indicated. ASA=atrial septal aneurysm; LSP= left sided pouch.
Comparison of patients with and without LSP
Characteristics of subjects with and without a LSP are summarized in Table 2. Patients with LSPs were younger (67.5 ± 9.1 vs. 69.6 ± 8.8; p=0.046) and had a lower frequency of hypertension (68.0% vs. 80.3%; p=0.01). The prevalence of ASA was not different between subjects with or without LSPs (4.0% vs. 5.0%; p=0.71). Other demographic features and traditional risk factors were not significantly different between the 2 groups.
Table 2.
Demographics and Risk Factors by LSP Status
| LSP (+) (n=100) | LSP (−) (n=244) | P Value | |
|---|---|---|---|
| Age, yrs (mean ± SD) | 67.5 ± 9.1 | 69.6 ± 8.8 | 0.046 |
| < 60 | 22 (22) | 29 (12) | |
| 60–69 | 39 (39) | 100 (41) | 0.050 |
| ≥ 70 | 39 (39) | 115 (47) | |
| Male | 57 (57.0) | 127 (52.0) | 0.40 |
| Body mass index, kg/m2 (mean ± SD) | 27.0 ± 4.8 | 27.1 ± 5.1 | 0.88 |
| Race/ethnicity | |||
| White | 10 (10.0) | 41 (16.8) | |
| Black | 28 (28.0) | 74 (30.3) | 0.18 |
| Hispanic | 62 (62.0) | 129 (52.9) | |
| Risk factors | |||
| Hypertension | 68 (68.0) | 195 (80.3) | 0.01 |
| Diabetes mellitus | 42 (42.0) | 80 (33.1) | 0.12 |
| Hypercholesterolemia | 45 (45.0) | 117 (48.8) | 0.53 |
| Current smoker | 12 (12.5) | 46 (19.3) | 0.14 |
| Smoking history | 63 (63.6) | 139 (57.2) | 0.27 |
| Coronary artery disease | 15 (15.0) | 54 (22.2) | 0.13 |
| Atrial fibrillation | 10 (10.0) | 19 (7.9) | 0.52 |
Values are given as n (%) unless otherwise indicated.
Abbreviations as in Table 1.
Association between LSP and ischemic stroke
The subgroup of patients with cryptogenic stroke was compared to control subjects (Table 3). Cryptogenic stroke patients were on average 2 years older and had a higher prevalence of hypertension (79.7% vs. 66.9%; p=0.05) and diabetes mellitus and (37.7% vs. 22.9%; p=0.02), and a lower prevalence of hypercholesterolemia (35.8% vs. 53.5%, p=0.02). There was no difference with regard to the prevalence of LSPs and ASAs between the two groups.
Table 3.
Demographics, Risk Factors and LSP Frequency in Cryptogenic Stroke and Control Subjects
| Cryptogenic Stroke Patients (n=69) | Control Subjects (n=157) | p Value | |
|---|---|---|---|
| Age, yrs (mean ± SD) | 69.7± 8.4 | 67.0±8.4 | 0.03 |
| < 60 | 10 (14) | 30 (19) | |
| 60–69 | 26 (38) | 70 (45) | 0.26 |
| ≥ 70 | 33 (48) | 57 (36) | |
| Male | 35 (50.7) | 88 (56.1) | 0.46 |
| Body mass index, kg/m2 (mean ± SD) | 27.2 ± 5.2 | 27.6 ± 4.8 | 0.55 |
| Race/ethnicity | |||
| White | 9 (13.0) | 23 (14.7) | |
| Black | 23 (33.3) | 50 (31.9) | 0.94 |
| Hispanic | 37 (53.6) | 84 (53.5) | |
| Risk factors | |||
| Hypertension | 55 (79.7) | 105 (66.9) | 0.05 |
| Diabetes mellitus | 26 (37.7) | 36 (22.9) | 0.02 |
| Hypercholesterolemia | 24 (35.8) | 84 (53.5) | 0.02 |
| Current smoker | 13 (20.0) | 25 (16.2) | 0.50 |
| Smoking history | 38 (55.9) | 99 (63.1) | 0.31 |
| Coronary artery disease | 10 (14.5) | 33 (21.0) | 0.25 |
| Atrial fibrillation | 3 (4.4) | 7 (4.5) | 0.96 |
| Atrial septal abnormalities | |||
| ASA | 5 (7.3) | 5 (3.2) | 0.18 |
| LSP | 22 (31.9) | 47 (29.3) | 0.70 |
Values are given as n (%) unless otherwise indicated.
Abbreviations as in Table 1.
The presence of a LSP was not found to be associated with an increased risk of ischemic stroke, in either univariable analysis (OR 0.98, 95% CI 0.61–1.56) or after adjustment for other stroke risk factors (OR 1.09, 95% CI 0.64–1.85) (Table 4). The presence of LSP was also not found to be associated with an increased risk of cryptogenic stroke (Table 5).
Table 4.
Predictors of Ischemic Stroke
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age * | 1.05 (1.02–1.08) | 0.0002 | 1.05 (1.02–1.08) | 0.0006 |
| Male | 0.83 (0.54–1.27) | 0.383 | 1.14 (0.70–1.86) | 0.60 |
| Diabetes mellitus | 2.92 (1.82–4.68) | <0.0001 | 3.47 (2.06–5.86) | < 0.0001 |
| Hypertension | 2.79 (1.66–4.71) | 0.0001 | 2.53 (1.41–4.53) | 0.002 |
| Hypercholesterolemia | 0.65 (0.42–0.99) | 0.046 | 0.60 (0.37–0.96) | 0.03 |
| Smoking history | 0.74 (0.48–1.14) | 0.17 | 0.72 (0.44–1.16) | 0.18 |
| Atrial fibrillation | 2.86 (1.19–6.88) | 0.02 | 2.18 (0.83–5.74) | 0.12 |
| LSP | 0.98 (0.61–1.56) | 0.93 | 1.09 (0.64–1.85) | 0.76 |
Age as a continuous variable
CI=confidence interval; LSP= left sided pouch; OR= odds ratio. Univariable model for LSP; chi-square=0.007, df=1, p=0.93, c-statistics=0.50. Multivariable model; chi-square=45.9, df=8, p<0.0001, c-statistics=0.73.
Table 5.
Predictors of Cryptogenic Stroke
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age * | 1.04 (1.00–1.07) | 0.03 | 1.04 (1.00–1.08) | 0.03 |
| Male | 0.81 (0.46–1.42) | 0.46 | 1.06 (0.56–2.01) | 0.86 |
| Diabetes mellitus | 2.03 (1.10–3.75) | 0.02 | 2.25 (1.14–4.44) | 0.02 |
| Hypertension | 1.95 (0.99–3.82) | 0.053 | 1.98 (0.94–4.16) | 0.07 |
| Hypercholesterolemia | 0.49 (0.27–0.88) | 0.02 | 0.50 (0.27–0.93) | 0.03 |
| Smoking history | 0.74 (0.42–1.32) | 0.31 | 0.73 (0.39–1.38) | 0.34 |
| Atrial fibrillation | 0.97 (0.24–3.86) | 0.96 | 0.48 (0.09–2.70) | 0.41 |
| LSP | 1.13 (0.61–2.08) | 0.70 | 1.41 (0.71–2.78) | 0.33 |
Age as a continuous variable
CI=confidence interval; LSP=left sided pouch; OR=odds ratio.
Univariable model for LSP; chi-square=0.15, df=1, p=0.70, c-statistics=0.51.
Multivariable model; chi-square=17.45, df=7, p=0.03, c-statistics=0.69.
Discussion
This study does not demonstrate evidence for association of the presence of LSP with ischemic stroke. The lack of association was also observed in a subgroup analysis of cryptogenic cases, in which the effect of new cardioembolic sources could be more relevant. Our results do not support the hypothesis that embolism from LSP is a cause of ischemic stroke, even among patients without another apparent mechanism of cerebral ischemia.
The left atrium is a potential source of systemic embolization. Thrombus formation in the left atrium and its appendage in patients with atrial fibrillation, and the consequent thromboembolic risk, is well known. (23–26). The pathogenesis of cryptogenic stroke in patients with interatrial septal abnormalities is not well understood. The morphologic characteristics of the interatrial septum that best predict the risk of thromboembolism remain unclear. It has long been debated whether the presence of PFO actually plays a causal role in stroke or whether there is only a noncausal statistical relationship. Although there is considerable evidence that PFO can cause ischemic stroke by means of paradoxical embolism, the difficulty of confirming the occurrence of embolism has led to the consideration of alternative explanations such as in situ thrombosis or atrial tachyarrhythmia. (22, 27) The former, however, is very rarely found by TEE or at autopsy. Although patients with interatrial abnormalities and stroke have lower thresholds for the induction of atrial fibrillation, this arrhythmia is rarely documented in patients with cryptogenic stroke and PFO. (27, 28) Moreover, in patients with a cryptogenic stroke, approximately one third of discovered PFOs are likely to be incidental. (29) Finally, the association between PFO and stroke in the general population has not been supported by two major studies. (30, 31) Therefore, there is an extensive ongoing search for other plausible mechanisms that may explain the origin of cryptogenic ischemic strokes.
LSP was first described by Krishnan et al. as a new anatomical entity with potential embolic complications. (15) It was suggested that this pouchlike structure may favor the stagnation of blood, with consequent risk of thrombus formation and thromboembolic complications. In their study, the prevalence of LSP was 39%, which is considerably higher than the prevalence of PFO in the general population (24%) (31). This observation might have great practical importance, because evidence linking such a frequent entity to ischemic stroke, could provide additional insights into the mechanisms of cryptogenic stroke.
After the exclusion of subjects with a PFO, a LSP was present in 29.1% of the subjects in our study, a prevalence that is lower compared to the previous study. Our patient population was considerably older (69 ± 9 vs. 50 ± 21) and, similar to PFO, the prevalence of LSP might decrease with advancing age. (32) In fact, subjects with LSP tended to be significantly younger than subjects without LSP, supporting this hypothesis. LSP showed an equal distribution across sex, race-ethnic subgroups and traditional risk factors analyzed, except for hypertension. Subjects without LSP had a significantly higher prevalence of hypertension. One hypothesis regarding the lower frequency of LSP in hypertensive subjects may be that an increased left atrial pressure might force the SP against the SS, and favor the fusion between these two septal components.
We observed that the prevalence of LSP was not significantly different between stroke patients and control subjects (28.9% vs. 29.3%). Given the often-discussed association of PFO and cryptogenic stroke, we also undertook a subgroup analysis for patients with cryptogenic stroke, which also showed that the prevalence of LSP did not differ between cryptogenic patients and control subjects (32.4% vs. 29.8%). Both univariable and multivariable analyses revealed that LSP did not show any impact on either ischemic (adjusted OR 1.09, 95% CI 0.64 to 1.85) or cryptogenic (adjusted OR 1.41, 95% CI 0.71 to 2.78) stroke. These findings suggest that LSP is more likely an innocent bystander than a potential thrombogenic source. However, our results may not exclude the possibility that in the presence of associated cofactors, such as hypercoagulability, LSP may favor thrombus formation and therefore cerebral embolization.
Strengths and Limitations
This case-control study is the first to date that assessed the role of LSP as a stroke risk factor. The interpretation of echocardiograms was blinded to the subjects' characteristics and risk factors, eliminating the possibility of ascertainment bias.The main limitation of our study is the relatively small sample size, which may have affected the statistical power to detect a significant risk associated with LSP. However, with the observed odds ratio of 0.98 (resulted from 28.9% pouch prevalence rate in stroke patients and 29.3% pouch prevalence rate in controls), a total of 20400 subjects (1:1 ratio for case versus control) would be needed to detect a significant difference with 80% power at the 0.1 significance level. As in other case control-studies, our study has the limitation of possibly unequal distribution of prognostic variables between cases and controls, which may have not been completely adjusted for in the analysis. Because of the relatively high mean age of the population, we could not compare different age groups (younger vs. older) Therefore, our results should not extrapolated to a younger patient population.
Conclusions
Our results show no evidence of an association between the presence of a LSP and ischemic stroke. The possibility that the stroke risk may be increased in a subgroup of younger subjects with LSP can not be excluded by this study. Also, possible associated cofactors that may turn LSP from an innocent bystander into a causative mechanism for stroke remain to be elucidated.
Supplementary Material
Acknowledgments
The Authors wish to thank Inna Titova, MPH, and Gabrielle Gaspard, MPH, for their assistance in the collection of the data.
Grant and Funding information The study was supported by R01 NS36286 from the National Institute of Neurological Disorders and Stroke (NINDS). Dr. Di Tullio was the recipient of a NINDS Mid-Career Award in Patient-Oriented Research (K24 NS02241).
Abbreviations list
- APRIS
aortic plaques and risk of ischemic stroke
- ASA
atrial septal aneurysm
- LSP
left sided pouch
- NOMAS
northern Manhattan study
- PFO
patent foramen ovale
- SP
septum primum
- SS
septum secundum
- TEE
transesophageal echocardiography
Footnotes
None of the authors have conflicts of interest to disclose
Reference List
- 1.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–90. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 2.Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–52. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 3.Webster MW, Chancellor AM, Smith HJ, et al. Patent foramen ovale in young stroke patients. Lancet. 1988;2:11–2. doi: 10.1016/s0140-6736(88)92944-3. [DOI] [PubMed] [Google Scholar]
- 4.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–5. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]
- 5.Belkin RN, Hurwitz BJ, Kisslo J. Atrial septal aneurysm: association with cerebrovascular and peripheral embolic events. Stroke. 1987;18:856–62. doi: 10.1161/01.str.18.5.856. [DOI] [PubMed] [Google Scholar]
- 6.Hausmann D, Mugge A, Daniel WG. Identification of patent foramen ovale permitting paradoxic embolism. J Am Coll Cardiol. 1995;26:1030–8. doi: 10.1016/0735-1097(95)00288-9. [DOI] [PubMed] [Google Scholar]
- 7.Cabanes L, Mas JL, Cohen A, et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–73. doi: 10.1161/01.str.24.12.1865. [DOI] [PubMed] [Google Scholar]
- 8.de Belder MA, Tourikis L, Leech G, Camm AJ. Risk of patent foramen ovale for thromboembolic events in all age groups. Am J Cardiol. 1992;69:1316–20. doi: 10.1016/0002-9149(92)91228-v. [DOI] [PubMed] [Google Scholar]
- 9.Hausmann D, Mugge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol. 1992;70:668–72. doi: 10.1016/0002-9149(92)90210-p. [DOI] [PubMed] [Google Scholar]
- 10.Jones EF, Calafiore P, Donnan GA, Tonkin AM. Evidence that patent foramen ovale is not a risk factor for cerebral ischemia in the elderly. Am J Cardiol. 1994;74:596–9. doi: 10.1016/0002-9149(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 11.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55:1172–9. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 12.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–8. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 13.Pearson AC, Nagelhout D, Castello R, Gomez CR, Labovitz AJ. Atrial septal aneurysm and stroke: a transesophageal echocardiographic study. J Am Coll Cardiol. 1991;18:1223–9. doi: 10.1016/0735-1097(91)90539-l. [DOI] [PubMed] [Google Scholar]
- 14.Bonati LH, Kessel-Schaefer A, Linka AZ, et al. Diffusion-weighted imaging in stroke attributable to patent foramen ovale: significance of concomitant atrial septum aneurysm. Stroke. 2006;37:2030–4. doi: 10.1161/01.STR.0000231655.52686.ab. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan SC, Salazar M. Septal pouch in the left atrium: a new anatomical entity with potential for embolic complications. JACC Cardiovasc Interv. 2010;3:98–104. doi: 10.1016/j.jcin.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Seyfert H, Bohlscheid V, Bauer B. Double atrial septum with persistent interatrial space and transient ischaemic attack. Eur J Echocardiogr. 2008;9:707–8. doi: 10.1093/ejechocard/jen161. [DOI] [PubMed] [Google Scholar]
- 17.Breithardt OA, Papavassiliu T, Borggrefe M. A coronary embolus originating from the interatrial septum. Eur Heart J. 2006;27:2745. doi: 10.1093/eurheartj/ehl051. [DOI] [PubMed] [Google Scholar]
- 18.Di Tullio MR, Homma S, Jin Z, Sacco RL. Aortic atherosclerosis, hypercoagulability, and stroke the APRIS (Aortic Plaque and Risk of Ischemic Stroke) study. J Am Coll Cardiol. 2008;52:855–61. doi: 10.1016/j.jacc.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Olson SH, Kelsey JL, Pearson TA, Levin B. Evaluation of random digit dialing as a method of control selection in case-control studies. Am J Epidemiol. 1992;135:210–22. doi: 10.1093/oxfordjournals.aje.a116273. [DOI] [PubMed] [Google Scholar]
- 21.Sacco RL, Roberts JK, Boden-Albala B, et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28:929–35. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 22.Mugge A, Daniel WG, Angermann C, et al. Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91:2785–92. doi: 10.1161/01.cir.91.11.2785. [DOI] [PubMed] [Google Scholar]
- 23.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–9. doi: 10.1016/0735-1097(94)00396-8. [DOI] [PubMed] [Google Scholar]
- 24.Manning WJ, Silverman DI, Waksmonski CA, Oettgen P, Douglas PS. Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation. Arch Intern Med. 1995;155:2193–8. [PubMed] [Google Scholar]
- 25.Leung DY, Davidson PM, Cranney GB, Walsh WF. Thromboembolic risks of left atrial thrombus detected by transesophageal echocardiogram. Am J Cardiol. 1997;79:626–9. doi: 10.1016/s0002-9149(96)00828-4. [DOI] [PubMed] [Google Scholar]
- 26.Heppell RM, Berkin KE, McLenachan JM, Davies JA. Haemostatic and haemodynamic abnormalities associated with left atrial thrombosis in non-rheumatic atrial fibrillation. Heart. 1997;77:407–11. doi: 10.1136/hrt.77.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthet K, Lavergne T, Cohen A, et al. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke. 2000;31:398–403. doi: 10.1161/01.str.31.2.398. [DOI] [PubMed] [Google Scholar]
- 28.Falk RH. PFO or UFO? The role of a patent foramen ovale in cryptogenic stroke. Am Heart J. 1991;121:1264–6. doi: 10.1016/0002-8703(91)90695-e. [DOI] [PubMed] [Google Scholar]
- 29.sheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40:2349–55. doi: 10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol. 2007;49:797–802. doi: 10.1016/j.jacc.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 31.Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47:440–5. doi: 10.1016/j.jacc.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 32.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.