Abstract
Purpose
The aim of this study was to review our experience with splenic abscesses, with respect to the relevant aspects of splenic abscesses and treatment outcomes.
Materials and Methods
We reviewed the cases of 18 patients who had splenic abscesses and who were treated at our hospital from November 1993 to December 2008.
Results
The most common symptom at presentation was abdominal pain in 12 patients (66.7%). The median duration from symptom onset until establishment of a diagnosis was 22 days. Streptococcus viridians was the most common pathogen (27.8%), follow by Klebsiella pneumoniae (22.2%). The mortality rate during the inpatient period and the previous 90 days was 16.6%. Three of four patients with Klebsiella pneumoniae showed a single abscess pocket. Four patients (22.2%) underwent percutaneous drainage, eight (44.5%) recieved antibiotic treatment only and six (33.3%) underwent splenectomy.
Conclusion
There is no gold standard for treating splenic abscesses. Treatment should be customized for each patient.
Keywords: Abscess, infection, spleen
INTRODUCTION
Splenic abscess is an uncommon disease, and it has been noted to occur at a rate of 0.14% to 0.7% in autopy studies.1,2 Splenic abscesses generally occur in patients with neoplasia, immunodeficiency, trauma, metastatic infection, splenic infarct or diabetes.3 The incidence of splenic abscess is thought to be growing, due to the increasing number of immunocompromised patients who are particularly at risk for this disease, and also due to the widespread use of diagnostic imaging modalities such as computated tomography (CT) and ultrasonography (US).4-7 The management of splenic abscess is based on medical therapy with antibiotics and splenectomy or percutaneous drainage (PCD), with good results.8-10 However, the optimal treatment remains unclear. While it is recognized that PCD may be appropriate for some patients, a high failure rate (14.3-75%) has been observed for this procedure, and surgery remains the goldstandard treatment.3-8,11-14 The purpose of this study was to review our experience with splenic abscesses. We specifically examined the relevant aspects of splenic abscesses and the treatment outcomes.
MATERIALS AND METHODS
A retrospective review of the pathology database of Gachon University Gil Hospital, Incheon, Korea, identified 18 patients with splenic abscess over a 15 year period (1993-2008).
The medical and pathologic records of each patient were reviewed. The data extracted for analysis included age, gender, signs and symptoms, predisposing conditions, bacteriologic profile, type of treatment and the treatment outcome.
The diagnosis of splenic abscess was established with an abdominal sonogram or computed tomography (CT) of the abdomen. Solitary and multiple lesions were categorized using imaging studies (Fig. 1). The patients then underwent the necessary treatment such as surgery, antibiotics and/or PCD. The bacteriologic profile was obtained from either blood culture or drained abscess culture. In addition, for patients who underwent splenectomy, intraoperative cultures of the splenic abscess were performed.
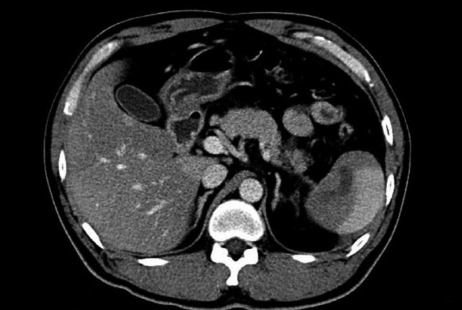
Fig. 1.
Abdominal CT showing a loculated fluid collection in the lower pole of the spleen
RESULTS
There was a total 18 patients (14 males and four females) seen during the study period. The mean patient age was 47.2 years old. The three most common symptoms at presentation were fever in five patients (27.8%), abdominal pain in 12 (66.7%) and a sensation of chills in five (27.8%). One patient (5.6%) presented with septic shock. Splenormegaly was found in 14 patients (77.8%). The duration of symptoms from onset until establishment of a diagnosis ranged from 10 to 103 days with a median of 22 days. The majority of patients (61.1%) in our series had leukocytosis (a white blood cell count > 10,000 mm3).
Three patients (16.7%) had pancreatitis. There were five patients (27.8%) with associated malignancies, including two (40%) with acute myelocytic leukemia, two (40%) with stomach neoplasm and one patient with non-Hodgkin's lymphoma (20%). Two patients' abscesses (11.1%) were secondary to endocarditis, and one (5.6%) was from a dental abscess. Three patients presented with coexisting liver abscesses (16.7%).
Chest radiography was abnormal in 16 patients (88.8%); the most frequent finding was a left pleural effusion. Of the 18 patients, 12 (66.7%) underwent an abdominal sonographic examination. Five patients (41.7%) had irregular anechoic abscesses with internal debris. The remaining seven (58.3%) showed mixed echoic patterns, which required an additional abdominal CT scan to confirm the diagnosis.
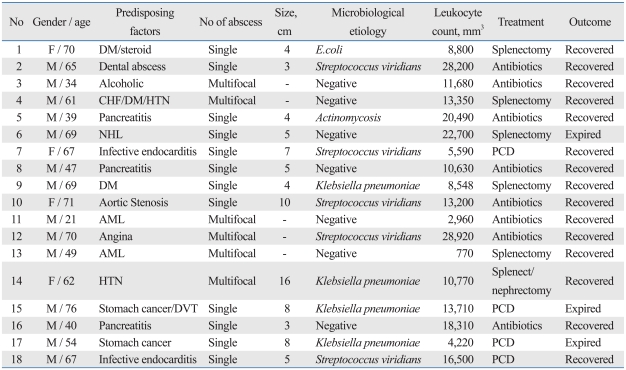
Six patients (33.3%) were diagnosed according to the CT scan alone. A solitary abscess was observed in 12 patients (66.7%), while six had multifocal splenic abscess. The cultures from 10 patients (55.6%) had a single organism whereas one patient (5.6%) grew a polymicrobial culture. Seven patients (38.8%) showed negative blood cultures. A routine sputum culture for M. Tuberculosis was done but none of the patients tested positive. On the whole, Streptococcus viridians was the most common pathogen (27.8%), follow by Klebsiella pneumoniae (22.2%). One patient was diagnosed with actinomycosis (Table 1). Three of the four patients with Klebsiella pneumoniae had a single abscess pocket. The remaining one patient had a huge single abscess with multiple satellite abscess pockets.
Table 1.
Demographic Data and Outcomes for Patients with Splenic Abscess
DM, diabetes Mellitus; CHF, congestive heart failure; HTN, hypertension; NHL, non-Hodgkin's lymphoma; AML, acute myelocytic leukemia; DVT, deep vein thrombosis; PCD, percutaneous drainage.
Eight patients (44.4%) received intravenous antibiotics as the only treatment modality for splenic abscess. The mortality rate during the inpatient period and the previous 90 days was 16.6%. Four of the deaths may have been related to splenic abscess, but underlying malignancies may also have contributed to these deaths. The range of length of in-hospital stay was 16-72 days (mean: 31 days).
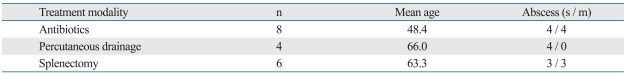
Four patients (22.2%) underwent PCD with a pigtail catheter (Table 2). The average duration of successful PCD was 27 days (range: 10-48 days). However, two patients underwent splenectomy due to inadequate drainage. None of the patients with multiple abscesses were indicated for PCD. We observed a patient with a gastrosplenic fisula, who was treated with antibiotics and EUS-guided transgastric drainage. Six patients with an average age of 63.3 underwent splenectomy. The mean age was significantly lower in the antibiotics-only group as compared to the other two groups (p < 0.05)(Table 2). The average size of the single abscesses was 12 cm (range: 3-16), while the average size of multiple abscesses was 3.7 cm (range: 2-5.5)(Fig. 2)
Table 2.
Comparison of the Three Treatment Modalities
s, single abscess; m, multiple abscesses.
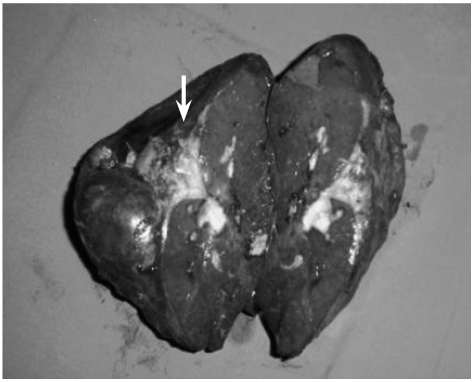
Fig. 2.
The gross specimen shows splenomegaly. There was a cystic-like structure filled with pus (arrow) on the cross-sectional surface.
DISCUSSION
Splenic abscess is an uncommon entity. The incidence of splenic absess in various autopsy series has been estimated to be between 0.2% and 0.7%.15 The rare occurrence of splenic abscess is further evidenced by the fact that no splenic abscess was reported in a review of 540 intraabdominal abscesses.5 However, this uncommon disease is recently being reported more frequently. Two main contributing factors to the apparent increase in the incidence of splenic abscess are advances in imaging studies and an increased number of immunocompromised, trauma and cancer patients.14,16 Splenic abscesses may often be misdiagnosed, because the signs and symptoms are nonspecific; nevertheless, modern imaging has improved the process of their diagnosis. The most common organisms in most reported series17 have been aerobic microbes, and particularly Streptococci and Escherichia coli. However, there are geographical variations and population differences, as Llenas-Garcia, et al.18 reported a higher percentage of M. Tuberculosis in splenic absesses. A few cases with multiple splenic abscesses caused by Salmonella typhi have also been described in the literature. Allal et al reported 400 patients with S. typhi and found splenic abscesses in 8 (2%) patients; of these only one had multiple splenic abscess.19 Torres, et al.20 reported 10 cases of typhoidal solitary splenic abscesses.
Fever was the most common symptom seen in our series, followed by abdominal pain and chills. The triad of fever, left upper quadrant pain and a tender mass was suggested by Sarr and Zuidema21 However, we did not find that a tender mass was part of the common symptom triad.
In this series, treatment was carried out by antibiotics alone, PCD or splenectomy. The mortality rate did not differ between the three groups, and it appeared that mortality was more related to the patient's general underlying condition. All the mortality in this study was related to underlying solid tumors. The remaining patients recovered from their splenic abscess regardless of the treatment modality. Thus, based on these observations, the prognosis for these patients cannot be accurately predicted by the treatment methods.
Twenty-two percent of our patients had PCD as a first line treatment. Percutaneous treatment of splenic abscess is an effective alternative to surgery; furthermore, it offers the theoretical advantages of preserving immunologic function by avoiding splenectomy in young patients. The sucess rate of PCD for splenic abscess has been reported between 67% and 100%.13,22 The procedure is most likely to be successful when the abscess collection is unilocular and when its content is liquefied enough to be drained.8 We prefer to proceed to PCD only when the above indication is met.
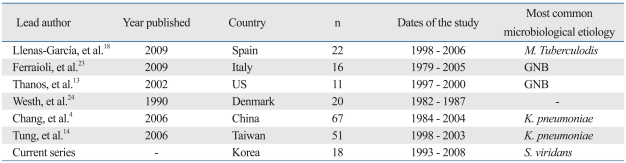
In contrast with previous reports,4,13,14,18,23,24 we found that gram-negative K. pneumonia (22.2%) was one of the most frequently encountered microbacterial pathogens. A list of countries and their most common microbiological etiologies are presented in Table 3. Primary splenic abscess due to K. pneumonia has rarely been reported. In the present study, we found that coexisting liver abscesses were found in 16.7% of patients. K. pneumonia accounted for 100% of the coexisting liver abscesses. We believe that the higher percentage of K. pneumonia in our study is most likely due to a sequela of metastatic infection from the liver to the spleen, or a coinfection of both. Three out of four patients had a single abscess pocket. The remaining one patient had huge single abscess with multiple satellite abscess pockets. We preliminarily suggest that K. pneumonia may be related to creating a large single abscess rather than developing multiple splenic abscesses. These results indicate the significance of the role of gram negative bacterial infection in splenic abscesses.
Table 3.
Results from Series Evaluating Splenic Abscess in Different Countries
n, number; GNB, gram-negative bacteria.
There is no gold standard treatment for splenic abscess. Traditional treatment includes appropriate antimicrobial therapy with or without splenectomy.15 There are a number of studies in favor of spleen preservation and management using PCD.8,13 Percutaneous aspiration or drainage may be used as a bridge to surgery, allowing nonoperative healing for splenic abscess patients who are at risk for surgery, and helps avoid the risk of a fulminant and potentially life-threatening infection. The success rate for PCD in our series was 50%.
In conclusion, the best therapeutic approach for splenic abscess is still a matter of debate. We agree with the traditional treatment of antibiotics with or without splenectomy. However, based on our experience and the current literature, percutaneous aspiration of splenic abscess can be used as a bridge to surgery for those patients who are critically ill or who have several comorbidities. Early diagnosis of splenic abscess requires a high degree of suspicion. Most of our patients presented with unexplained fever, abdominal pain and radiologic evidence of pathology in the left chest or abdomen, and these signs and symptoms should lead to suspicion of splenic abscess. Abdominal CT and ultrasonography were usually diagnostic. We suggest that treatment should be tailored and applied to splenic abscess patients on an individual basis.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Chun CH, Raff MJ, Contreras L, Varghese R, Waterman N, Daffner R, et al. Splenic abscess. Medicine (Baltimore) 1980;59:50–65. doi: 10.1097/00005792-198001000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Nelken N, Ignatius J, Skinner M, Christensen N. Changing clinical spectrum of splenic abscess. A multicenter study and review of the literature. Am J Surg. 1987;154:27–34. doi: 10.1016/0002-9610(87)90285-6. [DOI] [PubMed] [Google Scholar]
- 3.Ng KK, Lee TY, Wan YL, Tan CF, Lui KW, Cheung YC, et al. Splenic abscess: diagnosis and management. Hepatogastroenterology. 2002;49:567–571. [PubMed] [Google Scholar]
- 4.Chang KC, Chuah SK, Changchien CS, Tsai TL, Lu SN, Chiu YC, et al. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J Gastroenterol. 2006;12:460–464. doi: 10.3748/wjg.v12.i3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bree E, Tsiftsis D, Christodoulakis M, Harocopos G, Schoretsanitis G, Melissas J. Splenic abscess: a diagnostic and therapeutic challenge. Acta Chir Belg. 1998;98:199–202. [PubMed] [Google Scholar]
- 6.Ooi LL, Nambiar R, Rauff A, Mack PO, Yap TL. Splenic abscess. Aust N Z J Surg. 1992;62:780–784. doi: 10.1111/j.1445-2197.1992.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 7.Smyrniotis V, Kehagias D, Voros D, Fotopoulos A, Lambrou A, Kostopanagiotou G, et al. Splenic abscess. An old disease with new interest. Dig Surg. 2000;17:354–357. doi: 10.1159/000018878. [DOI] [PubMed] [Google Scholar]
- 8.Chou YH, Hsu CC, Tiu CM, Chang T. Splenic abscess: sonographic diagnosis and percutaneous drainage or aspiration. Gastrointest Radiol. 1992;17:262–266. doi: 10.1007/BF01888563. [DOI] [PubMed] [Google Scholar]
- 9.Green SL, Scott LK. Cryptogenic splenic abscess. Va Med. 1986;113:164–166. [PubMed] [Google Scholar]
- 10.Ralls PW, Quinn MF, Colletti P, Lapin SA, Halls J. Sonography of pyogenic splenic abscess. AJR Am J Roentgenol. 1982;138:523–525. doi: 10.2214/ajr.138.3.523. [DOI] [PubMed] [Google Scholar]
- 11.Carbonell AM, Kercher KW, Matthews BD, Joels CS, Sing RF, Heniford BT. Laparoscopic splenectomy for splenic abscess. Surg Laparosc Endosc Percutan Tech. 2004;14:289–291. doi: 10.1097/00129689-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Kang M, Saxena AK, Gulati M, Suri S. Ultrasound-guided percutaneous catheter drainage of splenic abscess. Pediatr Radiol. 2004;34:271–273. doi: 10.1007/s00247-003-1068-5. [DOI] [PubMed] [Google Scholar]
- 13.Thanos L, Dailiana T, Papaioannou G, Nikita A, Koutrouvelis H, Kelekis DA. Percutaneous CT-guided drainage of splenic abscess. AJR Am J Roentgenol. 2002;179:629–632. doi: 10.2214/ajr.179.3.1790629. [DOI] [PubMed] [Google Scholar]
- 14.Tung CC, Chen FC, Lo CJ. Splenic abscess: an easily overlooked disease? Am Surg. 2006;72:322–325. [PubMed] [Google Scholar]
- 15.Paris S, Weiss SM, Ayers WH, Jr, Clarke LE. Splenic abscess. Am Surg. 1994;60:358–361. [PubMed] [Google Scholar]
- 16.Teich S, Oliver GC, Canter JW. The early diagnosis of splenic abscess. Am Surg. 1986;52:303–307. [PubMed] [Google Scholar]
- 17.Ooi LL, Leong SS. Splenic abscesses from 1987 to 1995. Am J Surg. 1997;174:87–93. doi: 10.1016/s0002-9610(97)00030-5. [DOI] [PubMed] [Google Scholar]
- 18.Llenas-García J, Fernández-Ruiz M, Caurcel L, Enguita-Valls A, Vila-Santos J, Guerra-Vales JM. Splenic abscess: a review of 22 cases in a single institution. Eur J Intern Med. 2009;20:537–539. doi: 10.1016/j.ejim.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Allal R, Kastler B, Gangi A, Bensaid AH, Bouali O, Cherrak C, et al. Splenic abscesses in typhoid fever: US and CT studies. J Comput Assist Tomogr. 1993;17:90–93. doi: 10.1097/00004728-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Torres JR, Gotuzzo E, Istúriz R, Elster C, Wolff M, Northland R, et al. Salmonellal splenic abscess in the antibiotic era: a Latin American perspective. Clin Infect Dis. 1994;19:871–875. doi: 10.1093/clinids/19.5.871. [DOI] [PubMed] [Google Scholar]
- 21.Sarr MG, Zuidema GD. Splenic abscess--presentation, diagnosis, and treatment. Surgery. 1982;92:480–485. [PubMed] [Google Scholar]
- 22.Gleich S, Wolin DA, Herbsman H. A review of percutaneous drainage in splenic abscess. Surg Gynecol Obstet. 1988;167:211–216. [PubMed] [Google Scholar]
- 23.Ferraioli G, Brunetti E, Gulizia R, Mariani G, Marone P, Filice C. Management of splenic abscess: report on 16 cases from a single center. Int J Infect Dis. 2009;13:524–530. doi: 10.1016/j.ijid.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Westh H, Reines E, Skibsted L. Splenic abscesses: a review of 20 cases. Scand J Infect Dis. 1990;22:569–573. doi: 10.3109/00365549009027098. [DOI] [PubMed] [Google Scholar]