Abstract
We tested 59 Greek patients with Behcet's Disease (BD) for serum anti-Saccharomyces cerevisiae antibodies. No increase of these antibodies was detected in the cases compared to 55 healthy unrelated blood donors from the same population. This finding is in contrast with the correlation between Saccharomyces cerevisiae antibodies and BD as reported in other populations. It seems that environmental factors may contribute to disease expression in different populations, producing different effects according to the individual's genetic predisposition. Saccharomyces cerevisiae antibodies do not seem to be of any significance in the Greek population.
Keywords: Anti-Saccharomyces cerevisiae antibodies, Behcet's disease, Greece
Behcet's Disease (BD), also called Adamantiades-BD, is an inflammatory disorder of unclear etiology that is ethnically related and more frequent among Middle Eastern and Asian populations.1,2
BD is common in populations dwelling along the path of the Old Silk Road, namely: Turks, Jews, Saudi, Iranians, Koreans, and Japanese; it is uncommon in Western populations.1,3 As the underlying condition is vasculitis, clinical manifestations may vary from case to case to a large extent.4,5 The classical clinical picture includes uveitis, aphthous stomatitis, genital ulcers, and arthropathy. It may also include skin pathergy, gastrointestinal tract disease with abdominal pain and diarrhea, and many other manifestations that include the heart, vessels, and brain.6 Brain lesions may be life threatening and they are a major cause of disease mortality.7
The aetiology of BD remains unknown; some indications for a genetic factor exist, but no clear mode of inheritance has been established.8 The association with HLA antigens is notable.9,10 This association is common to different ethnic groups, but the effect size of the HLA type is smaller in the European populations studied.11 Interestingly, some have shown a relationship between HLA phenotype and prognosis.12 Since the disease has no specific characteristic findings, diagnosis is based solely on clinical criteria such as the combination of clinical manifestations proposed in 1990 by the International Study Group for BD.13,14 Diagnosis may thus be delayed until all criteria are fulfilled. The detection of a specific laboratory marker would facilitate diagnosis.15
In searching for factor(s) causing or triggering the onset or exacerbation of the disease, several microorganisms have been incriminated, though clear indications for the direct involvement of any infectious factor do not exist. Nevertheless, it appears that saprophytes such as chlamydia trachomatis can affect the course of the disease especially if a genetic predisposition exists.16 Therefore, early detection of an infectious agent or a marker for infection may be a useful diagnostic test. In Greece, the association between anti-Saccharomyces cerevisiae antibodies (ASCA) antibodies and BD, to the best of our knowledge, has not yet been studied.
We measured the serum ASCA titers in 58 Greek patients with a diagnosis of BD (30 males, 28 females) aged between 17 and 70 years (mean age 38.5 y). All 58 BD cases fulfilled the standard International Study Group criteria for BD.13 Four cases out of the 58 complained of abdominal symptoms including recurrent diarrhea, pain, nausea, and constipation; no patient had been subjected to an endoscopy. All cases were regularly followed in the Rheumatology Clinic of our Department. Informed consent was given by all patients studied. Fifty five healthy unrelated blood donors from the same population matched for sex and age were used as controls. The ASCA serum titers were measured with a commercially available ELISA kit (ASCA IgG, ASCA IgA, QUANTA Lite, INOVA Diagnostics, San Diego, CA, USA). Both serum IgG and IgA levels of ASCA were separately titrated according to the manufacturer's protocol. The results are presented as arbitrary units.
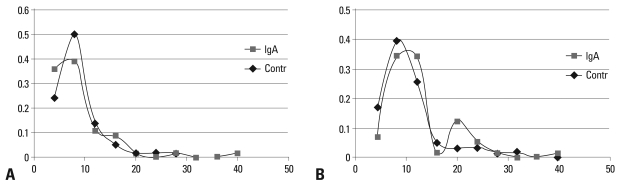
Fig. 1. depicts the distribution of IgA and IgG values of ASCA titers in patients and controls. No significant difference of IgA or IgG titers was detected in A-BD patients compared to controls, using the Student's t-test statistical analysis as presented in Table 1.
Fig. 1.
Distribution of IgA and IgG serum ASCA levels in 58 Greek BD patients and 55 healthy blood donors from the same population. ASCA, anti-Saccharomyces cerevisiae antiodies.
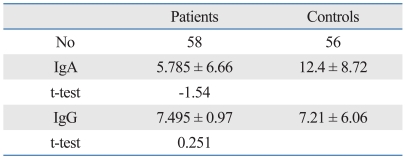
Table 1.
Statistical Analysis of the Titers of IgA and IgG Serum ASCA Levels in 58 BD Greek Patients and in 56 Healthy Blood Donors from the Same Population
ASCA, anti-Saccharomyces cerevisiae antiodies; BD, Behcet's disease.
The cause of BD remains unknown. The association with HLA types has been shown in many populations including Greeks,17 but on an individual basis, this immuno-genetic association is of no strong diagnostic value. The role of environmental factors is not clear. Markers of diagnostic value may facilitate diagnosis especially in cases not fulfilling the diagnostic criteria as frequently occurs in the early stages of the disease.15
In this context, the presence of a laboratory marker associated with the disease may be of practical value. BD shares some clinical similarities with Crohn's Disease (CD) in terms of intestinal involvement (terminal ileum), arthritis, and uveitis. These similarities raise the possibility of common causative or pathogenetic factor(s) but, as is the case in BD, the etiology of CD remains obscure.15 Recently, an increased frequency of ASCA has been reported in CD patients, including Greeks.18-20 The same antibodies were also detected in first degree relatives of patients with CD.21,22 This finding indicates either a common genetic element or similar exposure of members of a family to environmental factor(s).
BD is not very common among Greeks although epidemiological data are limited.23
Antibodies against Saccharomyces cerevisiae, a yeast applied in food technology have been reported in inflammatory diseases with intestinal involvement like CD. Interestingly, the pattern of both ASCA and anti-perinuclear cytoplasmic antibodies (p-ANCA) may differentiate CD from ulcerative colitis. Namely, ASCA are found in CD but not in ulcerative colitis, and p-ANCA are found in ulcerative colitis but not in CD.24 The prevalence of ASCA in BD has already been studied in populations with high incidence of the disease such as Israelis,15 Turks,25 and Koreans.26 All these data point to a possible association between ASCA titers and BD.
Our data failed to show that such an association in Greek BD patients exists. In addition to our findings, we note that a study in Korea was not helpful in differentiating BD from recurrent apthous ulcers.27 In another study conducted in Greece, the ASCA titers were found significantly increased among cases with CD, which alludes to a diagnostic value for ASCA in CD, as opposed to BD.19
One reason for the discrepancy of the findings may be that within the ASCA positive BD cases reported in the literature, there could in fact be some CD cases mimicking BD and expressing high titers of ASCA. This possibility has already been suggested by others.15
Another explanation may be that our patients sera were collected in disease remission during regular follow-up visits. If ASCA titers correlate with disease activity, no definite conclusion can be drawn from our data. Finally, we must take into account that ASCA antibodies in BD may simply reflect gut co-involvement widely variable in different populations. However, the relatively few patients in our study complaining of abdominal symptoms did not differ in terms of ASCA titers from the cases without gut symptoms.
In Greece, BD may be less frequent than in countries like Turkey and Israel.23 However, even within people of the same ethnic origin, prevalence differences depending on geographical area must exist as it has already been reported in Turkey, Japan, and Hawai.1,28 It is of interest that clinical expression in BD may be differentiated according to the patient's ethnicity.6,12 All these suggest that environmental factors probably influence the disease. This may be another explanation for the different pattern of ASCA titers among the several different populations studied.
In conclusion, serological testing for ASCA in Greek BD patients did not achieve any diagnostic value. ASCA testing may contribute to better diagnosis of BD in some ethnic groups but, on this topic, more data on European populations are needed.
ACKNOWLEDGEMENTS
This study was funded by Athens University Grants Authority, HERAKLEITOS Programme.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Zouboulis CC, Kötter I, Djawari D, Kirch W, Kohl PK, Ochsendorf FR, et al. Epidemiological features of Adamantiades-Behçet's disease in Germany and in Europe. Yonsei Med J. 1997;38:411–422. doi: 10.3349/ymj.1997.38.6.411. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Chetrit E, Yazici H. Thoughts on the proposed links between Behçet's disease and familial Mediterranean fever. Clin Exp Rheumatol. 2002;20:S1–S2. [PubMed] [Google Scholar]
- 3.Hamuryudan V, Yurdakul S, Yazıcı H. Morbus Behçet. Rheumatology in Europe. 1997;26:31–33. [Google Scholar]
- 4.Lakhanpal S, Tani K, Lie JT, Katoh K, Ishigatsubo Y, Ohokubo T. Pathologic features of Behçet's syndrome: a review of Japanese autopsy registry data. Hum Pathol. 1985;16:790–795. doi: 10.1016/s0046-8177(85)80250-1. [DOI] [PubMed] [Google Scholar]
- 5.Wright VA, Chamberlain MA. Behçet's syndrome. Bull Rheum Dis. 1978-1979;29:972–979. [PubMed] [Google Scholar]
- 6.Lewis KA, Graham EM, Stanford MR. Systematic review of ethnic variation in the phenotype of Behcet's disease. Scand J Rheumatol. 2007;36:1–6. doi: 10.1080/03009740600991927. [DOI] [PubMed] [Google Scholar]
- 7.Al-Araji A, Kidd DP. Neuro-Behçet's disease: epidemiology, clinical characteristics, and management. Lancet Neurol. 2009;8:192–204. doi: 10.1016/S1474-4422(09)70015-8. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz T, Langevitz P, Zemer D, Gazit E, Pras M, Livneh A. Behçet's disease in Familial Mediterranean fever: characterization of the association between the two diseases. Semin Arthritis Rheum. 2000;29:286–295. doi: 10.1016/s0049-0172(00)80015-3. [DOI] [PubMed] [Google Scholar]
- 9.O'Duffy JD. Behçet's disease. Curr Opin Rheumatol. 1994;6:39–43. [PubMed] [Google Scholar]
- 10.Brautbar C, Chajek T, Ben-Tuvia S, Lamm L, Cohen T. A genetic study of Behçet''s disease in Israel. Tissue Antigens. 1978;11:113–120. doi: 10.1111/j.1399-0039.1978.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahr A, de Menthon M, LaValley L, Guillevin L. HLA-B5(1) and risk of Bechet's Disease: A meta-analysis of genetic association studies. Clin Exp Rheumatol. 2008;26(3) Suppl 50:S-5. [Google Scholar]
- 12.Altenburg A, Papoutsis N, Krause L, Zouboulis Ch. Association between HLA Class I phenotypes and clinical manifestations in Adamantiades-Behcet disease patients in Germany. Clin Exp Rheumatol. 2008;26(3) Suppl 50:S-7. [Google Scholar]
- 13.International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 14.Kaklamani VG, Vaiopoulos G, Kaklamanis PG. Behçet's Disease. Semin Arthritis Rheum. 1998;27:197–217. doi: 10.1016/s0049-0172(98)80001-2. [DOI] [PubMed] [Google Scholar]
- 15.Krause I, Monselise Y, Milo G, Weinberger A. Anti-Saccharomyces cerevisiae antibodies--a novel serologic marker for Behçet's disease. Clin Exp Rheumatol. 2002;20(4 Suppl 26):S21–S24. [PubMed] [Google Scholar]
- 16.Myriokefalitakis I, Balaouras K, Bastakis M, Karogianni S, Bozios P, Elezoglou A, et al. Chlamidia trachomatis as trigger point to Adamantiades-Behcet's disease flare: a series of four cases. Clin Exp Rheumatol. 2008;26(3 Suppl 50):S-7. [Google Scholar]
- 17.Zervas J, Vayopoulos G, Sakellaropoulos N, Kaklamanis P, Fessas P. HLA antigens and Adamantiades-Behçet's disease (A-BD) in Greeks. Clin Exp Rheumatol. 1988;6:277–280. [PubMed] [Google Scholar]
- 18.Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt X, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- 19.Vermeire S, Joossens S, Peeters M, Monsuur F, Marien G, Bossuyt X, et al. Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology. 2001;120:827–833. doi: 10.1053/gast.2001.22546. [DOI] [PubMed] [Google Scholar]
- 20.Koutroubakis IE, Petinaki E, Mouzas IA, Vlachonikolis IG, Anagnostopoulou E, Castanas E, et al. Anti-Saccharomyces cerevisiae mannan antibodies and antineutrophil cytoplasmic autoantibodies in Greek patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:449–454. doi: 10.1111/j.1572-0241.2001.03524.x. [DOI] [PubMed] [Google Scholar]
- 21.Sendid B, Quinton JF, Charrier G, Goulet O, Cortot A, Grandbastien B, et al. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn's disease. Am J Gastroenterol. 1998;93:1306–1310. doi: 10.1111/j.1572-0241.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 22.Sutton CL, Yang H, Li Z, Rotter JI, Targan SR, Braun J. Familial expression of anti-Saccharomyces cerevisiae mannan antibodies in affected and unaffected relatives of patients with Crohn's disease. Gut. 2000;46:58–63. doi: 10.1136/gut.46.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roussou E, Efthimiades A, Settas L. Incidence of Adamantiades-Bechet's Disease in Greek prefecture inhabited by a mixed population of native Greeks and Greek refugees from Turkey. In: Bang D, Lee ES, Lee S, et al., editors. Bechet's Didease. Seoul, Korea: Design Mecca Publishing; 2000. pp. 65–67. [Google Scholar]
- 24.Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fresko I, Ugurlu S, Ozbakir F, Celik A, Yurdakul S, Hamuryudan V, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA) in Behçet's syndrome. Clin Exp Rheumatol. 2005;23(4 Suppl 38):S67–S70. [PubMed] [Google Scholar]
- 26.Choi CH, Kim TI, Kim BC, Shin SJ, Lee SK, Kim WH, et al. Anti-Saccharomyces cerevisiae antibody in intestinal Behçet's disease patients: relation to clinical course. Dis Colon Rectum. 2006;49:1849–1859. doi: 10.1007/s10350-006-0706-z. [DOI] [PubMed] [Google Scholar]
- 27.Rhee SH, Kim YB, Lee ES. Comparison of Behcet's disease and recurrent aphthous ulcer according to characteristics of gastrointestinal symptoms. J Korean Med Sci. 2005;20:971–976. doi: 10.3346/jkms.2005.20.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yurdakul S, Günaydin I, Tüzün Y, Tankurt N, Pazarli H, Ozyazgan Y, et al. The prevalence of Behçet's syndrome in a rural area in northern Turkey. J Rheumatol. 1988;15:820–822. [PubMed] [Google Scholar]