Abstract
Purpose
To evaluate the clinical outcomes of cantilever transforaminal lumbar interbody fusion (c-TLIF) for upper lumbar diseases.
Materials and Methods
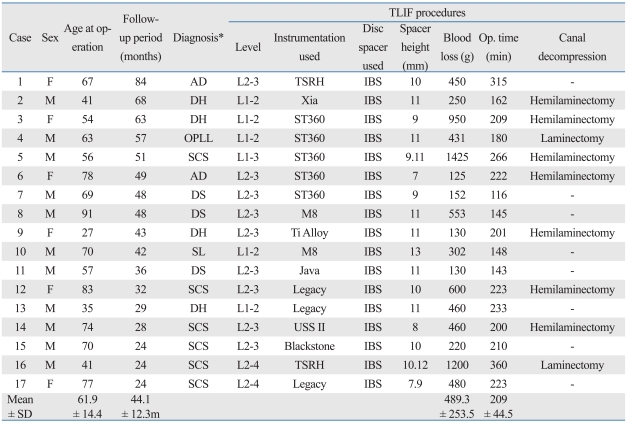
Seventeen patients (11 males, 6 females; mean ± SD age: 62 ± 14 years) who underwent c-TLIF using kidney type spacers between 2002 and 2008 were retrospectively evaluated, at a mean follow-up of 44.1 ± 12.3 months (2 year minimum). The primary diseases studied were disc herniation, ossification of posterior longitudinal ligament (OPLL), degenerative scoliosis, lumbar spinal canal stenosis, spondylolisthesis, and degeneration of adjacent disc after operation. Fusion areas were L1-L2 (5 patients), L2-L3 (9 patients), L1-L3 (1 patient), and L2-L4 (2 patients). Operation time, blood loss, complications, Japanese Orthopaedic Association (JOA) score for back pain, bone union, sagittal alignment change of fusion level, and degeneration of adjacent disc were evaluated.
Results
JOA score improved significantly after surgery, from 12 ± 2 to 23 ± 3 points (p < 0.01). We also observed significant improvement in sagittal alignment of the fusion levels, from - 1.0 ± 7.4 to 5.2 ± 6.1 degrees (p < 0.01). Bony fusion was obtained in all cases. One patient experienced a subcutaneous infection, which was cured by irrigation. At the final follow-up, three patients showed degenerative changes in adjacent discs, and one showed corrective loss of fusion level.
Conclusion
c-TLIF is a safe procedure, providing satisfactory results for patients with upper lumbar degenerative diseases.
Keywords: Transforaminal lumbar interbody fusion, upper lumbar spine, lumbar degenerative diseases, sagittal alignment, clinical outcome
INTRODUCTION
Upper lumbar disc herniation at the L1/2, L2/3, and L3/4 levels have been reported to constitute no more than 5% of all disc herniations.1-3 Although the anterior approach has been used classically for patients with upper lumbar degenerative disease, the development of spinal instrumentation has enabled effective use of the posterior approach. Posterior lumbar interbody fusion (PLIF) has become a popular surgical procedure for patients with degenerative lumbar diseases, as it enables both interbody fusion and posterior decompression.4-6 Because the PLIF procedure partially preserves the facet joint, requiring retraction of the dural sac in order to insert an interbody graft and/or spacers anteriorly, there is a possible risk for dural or nerve root injury.7 In upper lumbar lesions, the distance between the two pars interarticularis is short, the interlaminar space in all dimensions is small, and the inferior border of the lamina usually overhangs the disc space to a great extent. Therefore, PLIF carries a higher risk of nerve-related complications in upper lumbar lesions. In contrast, anterior procedures have advantages, in that a complete discectomy is possible without retracting the dura. Anterior procedures, however, also have disadvantages, including the difficulty of reconstruction of the lordosis and the risk of blood vessel injuries. Therefore, at present, the optimal surgical approach for upper lumbar lesions, anterior or posterior, is still unclear.8
A modified PLIF technique, called transforaminal lumbar interbody fusion (TLIF), was first introduced in 1982.9 Because the bone graft can be inserted far laterally, the TLIF technique can be safely indicated for interbody fusion of the upper lumbar spine. Moreover, TLIF can be performed at any lumbar level below L1, because it avoids significant retraction of the dura and conus medullaris.10,11 The cantilever TLIF procedure (c-TLIF) is similar, using a specifically designed kidney-type spacer.12 In c-TLIF, the kidney-shaped allograft/spacer/cage is inserted into the anterior column and the autologous bone in the middle column. This procedure is advantageous in that it restores sagittal alignment using only a uni-portal for spacer insertion.12
Beginning in 2002, we have been utilizing c-TLIF for upper lumbar degenerative diseases, including degenerative spondylolisthesis, spinal canal stenosis, and intervertebral disc herniation and degeneration of the adjacent disc after surgery. In this study, we evaluate the clinical outcomes of the c-TLIF procedure for patients with upper lumbar degenerative diseases.
MATERIALS AND METHODS
Operative indications
Operative indications for upper lumbar c-TLIF included: 1) Failure of conservative therapy, including physical therapy and epidural injections. 2) Back pain caused by instability of the upper lumbar lesion. 3) Sagittal imbalance (local kyphosis or scoliosis) of the thoraco-lumbar junction to the upper lumbar lesion. 4) Activity of the patient.
Patients
We retrospectively evaluated the outcomes for 17 patients (11 males, 6 females; mean ± SD age, 61.9 ± 14.4 years) with upper lumbar degenerative diseases who underwent c-TLIF from 2002 to 2008. Average follow-up was 44.1 ± 12.3 months (2 year minimum). The primary diseases we studied were disc herniation (4 patients), OPLL of lumbar (1 patient), degenerative scoliosis (3 patients), degeneration of adjacent disc after surgery (2 patients), lumbar spinal canal stenosis (6 patients), and spondylolisthesis (1 patient). The fusion areas were L1-L2 (5 patients), L2-L3 (9 patients), L1-L3 (1 patient) and L2-L4 (2 patients)(Table 1).
Table 1.
Characteristics and Clinical Courses of the Study Patients
TLIF, transforaminal lumbar interbody fusion; AD, adjacent disc degeneration after spinal operation; DH, disc herniation; OPLL, ossification of ligamentum flavum; SCS, spinal canal stenosis; DS, degenerative scoliosis; SL, spondylolisthesis.
*Diagnosis.
Operative procedures
Intervertebral fusion was performed using autogenous bone grafts from the lamina, spinosus process and iliac bone and intervertebral spacers. We used lordotic-shaped IBS® spacers (Ortho Development Company, Salt Lake City, Utah, USA), ranging in height from 7 mm to 13 mm. The spacers were inserted while the interspinous space was distracted. Distraction of disc space and regaining disc space height restores the height of the neural foramen, improves foraminal narrowing, and decreases foraminal stenosis. Indirectly, central stenosis may be relieved if it is caused by infolding of the ligamentum flavum or by annular compression.13
Nine patients underwent dural decompression by flavectomy and partial laminectomy. In 8 patients, the ligamentum flavum and lamina were preserved and the dural tube was not exposed. In all patients, the interbody spacer was first inserted and then minced bone graft impacted into the residual interbody spacer laterally while the interspinous space was spread. Thus, the dural tube was not retracted at all. Whenever a kidney-shaped IBS spacer was used, a unilateral facetectomy was performed to provide a portal for insertion, with the inserted side selected on the basis of preoperative symptoms. If a disc herniation or foraminal stenosis was present and predominantly one-sided, that side was chosen.14 If preoperative lower extremity symptoms were bilateral, the open wedge side, based on anterior-posterior plain X-ray film, was chosen for spacer insertion. In upper lumbar lesions, if adequate unilateral total facetectomy was performed followed by distraction of the intraspinosus space, sufficient exposure for an insertion portal of the kidney-shaped IBS spacer was obtained, as in lower lumbar lesions. It was important to take special care in identifying and securing exiting nerve roots, which are more likely to interfere with the spacer insertion portal of the TLIF procedure in the upper lumbar lesion. If sufficient exposure for the spacer insertion portal was not obtained by facetectomy, we performed a partial flavectomy and applied minimum medial retraction of the dural tube in a limited number of cases.
Evaluation of clinical outcomes
Operation time and blood loss
The operation time and the amount of blood loss were measured as markers of the surgical invasiveness of the TLIF procedure.
Japanese Orthopaedic Association score (JOA score) for back pain
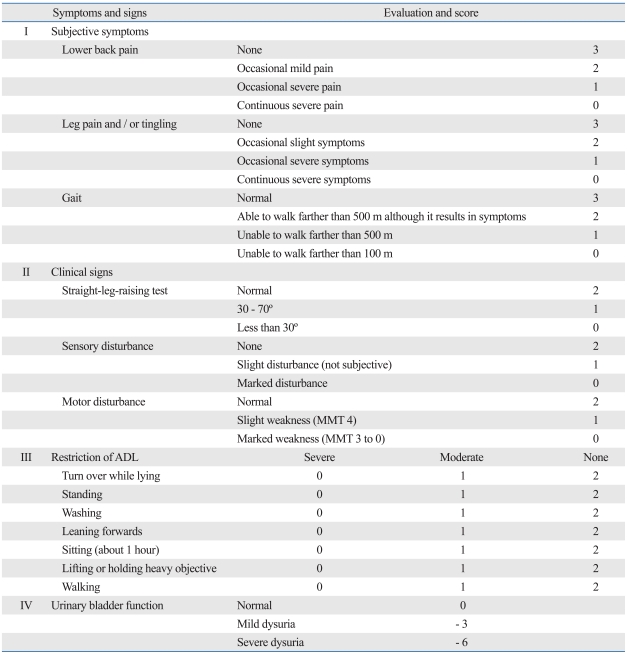
The Japanese Orthopaedic Association score (JOA score)for back pain (Table 2) was measured preoperatively, after 6 months and at final follow-up. The full score is 29 points (29 points means no physical synptoms), based on three subjective symptoms (9 points), three clinical signs (6 points), and seven activities of daily living (14 points)(Table 2). The full score indicate full activities of daily living without any symptoms.
Table 2.
The Japanese Orthopaedic Association's Evaluation System for Lower Back Pain Syndrome (JOA Score)
ADL, activities of daily living; MMT, manual muscle testing.
Radiographical evaluation
The segmental lordosis angle of the fused area and lumbar lordosis angle (L1 superior end plate to S1 superior end plate)15 were evaluated using lateral plain radiograms. Bony union was evaluated using sagittal reconstruction images of computed tomograms (CT). Degenerative change in adjacent discs was evaluated using lateral plain radiograms and magnetic resonance imaging (MRI).
Complications
Intraoperative and postoperative complications were analyzed.
Statistical analysis
Statistical comparisons of JOA score and lordotic angle of the fusion area were compared using the non-parametric Friedman test followed by the Wilcoxon signed-rank test. All data are shown as mean ± standard deviation. p values of less than 0.05 were considered significant.
RESULTS
Operation time and blood loss
The mean ± SD operation time was 209 ± 46 minutes (range: 116 to 360 minutes), and the mean ± SD intraoperative blood loss was 489 ± 254 grams (range: 125 to 1,425 grams)(Table 1). One patient experienced intraoperative bleeding from a rupture of a hypertrophied epidural varix, resulting in a relatively large blood loss of 1,425 grams (Table 1).
Japanese orthopaedic association score (JOA Score) for back pain
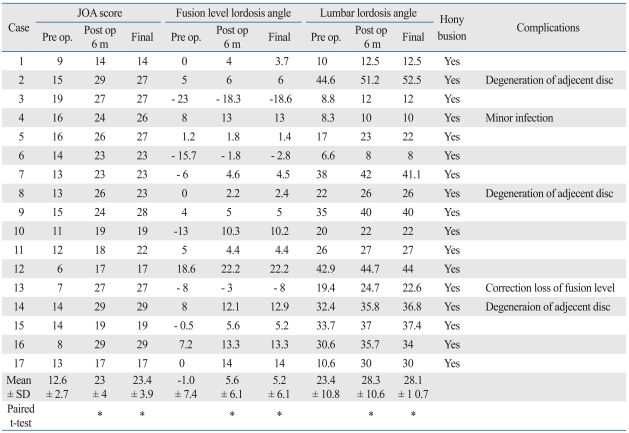
Compared with the preoperative JOA score, this parameter was significantly higher 6 months post-operation (p < 0.01). 15 patients maintained a higher JOA score until final follow-up, but two patients' scores improved for six months but had decreased at final follow-up because of adjacent disc degenerative changes (Table 3).
Table 3.
Clinical Outcomes of the Study Patients
*p < 0.01 (compared to the pre operative values).
Radiographical evaluation
Lordosis angle of the fused area
Preoperatively, most patients showed decreased lumbar lordosis and local kyphosis or flat back. Six months after surgery, the segmental lordosis angle of the fused area was significantly higher (p < 0.01). Lumbar lordosis angle also increased significantly (p < 0.01). In all patients, lordosis was maintained until the final follow-up period, except for one patient who showed correction loss at final follow-up. However, the clinical symptoms of this patient did not worsen (Table 3).
Bony union
Bony union was achieved in all patients and all disc spaces at final follow-up (Table 3).
Adjacent disc degeneration
Adjacent disc degeneration was observed in three patients. One experienced upper adjacent disc level instability, one experienced lower adjacent disc herniation, and one experienced upper adjacent disc height decrease. In two patients, the degeneration was symptomatic, resulting in decreases in total JOA score. In one patient, the degeneration was asymptomatic (Table 3).
Complications
• Intraoperative complications
Dural injury or neurological damage was not observed in any of the patients. One patient experienced a relatively large amount of bleeding due to a hypertrophied epidural varix.
• Postoperative complications
One patient experienced a subcutaneous infection, which was cured by irrigation (Table 3).
• Clinical case presentation (Case 2, a 41-year old man)
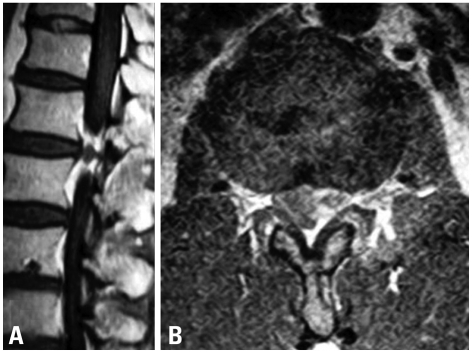
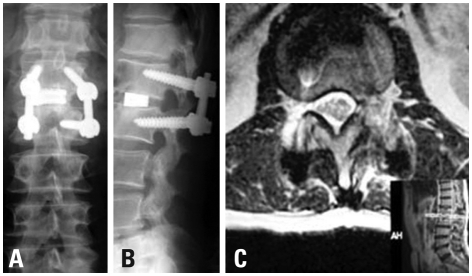
Enhanced MRI (magnetic resonance imaging) showed a ring enhanced mass (Fig. 1) an extruding herniation at L1-L2, and compressed dura on the left side. His preoperative JOA score was 15 points. At first, we considered the indications of a simple discectomy. His flexion-extension radiograph showed no remarkable instability. Retrospectively, this was partly because of his severe back pain, which restricted his lumbar segmental motion. Intraoperatively, grasping and gently lifting spinous process of L1 and L2 using Kocher klamp revealed remarkable instability at L1/2, suggesting disc fusion. In addition, removal of the disc herniation at L1/2 using curved punches required a unilateral facetectomy. Otherwise, we would have needed to retract the dural tube at the level of conus medullalis, which has the potential to cause neurological complications. Therefore, TLIF using the kidney-shaped spacer via unilateral facetectomy provided sufficient exposure for the removal of the disc herniation and good fix at the L1/2 segment. Following c-TLIF (with left side hemilaminectomy) at L1-L2 (Fig. 2A and B), his symptoms improved dramatically. One year after surgery, his complaint was completely resolved, and his JOA score was 29 points. Post-operative MRI revealed an absence of disc herniation and decompression of the dura (Fig. 2C). Three years after surgery, however, his back pain and numbness and pain of the left lower extremity reappeared. The MRI at that time revealed a lower degenerative change in the adjacent disc (L3/4 disc herniation). His JOA score at that time was 27/29.
Fig. 1.
Preoperative enhanced MRI (A: sagittal, B: axial), showing a ring enhanced mass at the L1-L2 level.
Fig. 2.
Postoperative X-P (A: anterior-posterior, B: lateral) showing good sagittal alignment. Postoperative MRI (C), showing sufficient decompression of the dural tube.
DISCUSSION
Overall results of c-TLIF for upper lumbar spinal pathologies
The results presented here show that the c-TLIF procedure using intradiscal spacers provides satisfactory clinical outcomes for patients with upper lumbar pathologies. Significant improvements in subjective and objective findings, as well as activity in daily life, were observed at final follow-up. Bony union was achieved in all patients and all disc spaces at final follow-up, and there were significant increases in the lordosis angle of the fused segment. It is important to note that, although lumbar interbody fusion with intervertebral spacers and autogenious bone graft was performed on the upper lumbar spine, no iatrogenic injury of neural elements was seen in any of these patients.
Decompression for the spinal canal
Because the c-TLIF is performed via the posterior approach, this procedure allows surgeons to perform decompression of the posterior element in any fashion. In cases of central disc herniation, the conventional PLIF procedure may not provide sufficient working portals for the insertion of punches at the upper lumbar levels. In c-TLIF, however, the unilateral facet is removed and the working portal is secured, allowing for the safe removal of centrally protruding discs with curved punches. Some of our patients were treated with hemilaminectomy or total laminectomy to achieve adequate decompression of the dura, thus ameliorating neurological symptoms in all patients.
Restoration of sagittal alignment in the upper lumbar spine
We found that c-TLIF significantly increased both the total lumbar lordosis angle and the lordosis angle of the fusion levels in all patients and maintained lordosis in 16 of 17 patients. Previous results have indicated the importance of restoring sagittal alignment in degenerative diseases of the upper lumbar spine.8 When the intervertebral disc of the upper lumbar spine is degenerated or herniated, thoracolumbar kyphosis is likely to be modulated. This pathological condition can lead to a relatively unsatisfactory outcome in posterior decompression surgery for the upper lumbar spine.8 Importantly, in c-TLIF, the removal of the unilateral facet can allow for insertion of relatively large intervertebral spacers posteriorly, without any damage to neural structures. The excellent outcomes observed in our patients may have been partly due to successful restoration of sagittal alignment of the lumbar spine. One patient (No. 13), however, showed a correction loss in the sagittal alignment of the fused area. This may have been due to the central location of the spacer, resulting in its subsidence.
Surgical invasiveness of c-TLIF for upper lumbar lesions
Earlier studies have reported that TLIF is less invasive than conventional techniques, as evidenced by shorter operating time, less blood loss, shorter hospital stay, and lower incidence of complications.10,16 We found that operation time and blood loss were slightly higher than previously reported.10 This may have been due to a learning curve difference; although TLIF can be easily mastered, there is a learning curve.14 In the present study, the operations were performed by several surgeons, who differed in their experience with the c-TLIF procedure. Second, while the box-type or cylindrically-shaped spacers used for conventional TLIF techniques are inserted in one direction, the kidney-shaped spacers in c-TLIF are inserted in a combination maneuver while striking and rotating a handle attached to the spacer.17 This suggests that c-TLIF is somewhat technically demanding. In addition, the c-TLIF procedures for upper lumbar lesions were performed more meticulously and slowly than at the cauda equine level. Therefore, advancements in learning curve should reduce the surgical invasiveness of this procedure.
Comparison with other procedures
The TLIF procedure may be a useful alternative to traditional procedures, including ALIF and PLIF, for the lumbar spine.10,16,18,19 Our results also suggest several potential advantages and disadvantages of c-TLIF procedure for pathological conditions of the upper lumbar spine. The advantages include: 1) Both posterior decompression and interbody fusion can be performed in a single stage. 2) Intervertebral spacer and graft insertion is performed by unilateral resection of the facet. 3) Since the insertion portal is wide enough, even in the upper lumbar spine, the spinal canal area need not be explored for spacer insertion.13,20 4) Using a spacer of sufficient height and pedicle screw systems, reestablishment of normal alignment is possible. Disadvantages include: 1) The procedure is technically demanding. 2) The unilateral facet has to be resected, leaving less bony contact area between two vertebral levels. This may be a fatal weakness in cases of hardware removal due to postoperative infection. 3) Similar to other spinal fusion procedures, there are risks to the adjacent disc levels.
Limitations of the present study
Our study had several limitations, including its retrospective design, the relatively small study population, and the absence of a control group. Additionally, the postoperative follow-up period may not be long enough to fully assess the risks of complications to adjacent disc levels.21,22 Future prospective trials should compare c-TLIF with conventional anterior lumbar interbody fusion for upper lumbar lesions.
In conclusion, retrospective analysis of the clinical outcomes of the c-TLIF procedure for patients with upper lumbar degenerative diseases showed that c-TLIF provided satisfactory amelioration of clinical symptoms, sagittal alignment, and solid bony union, without any neurological complications.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Albert TJ, Balderston RA, Heller JG, Herkowitz HN, Garfin SR, Tomany K, et al. Upper lumbar disc herniations. J Spinal Disord. 1993;6:351–359. doi: 10.1097/00002517-199306040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Aronson HA, Dunsmore RH. Herniated upper lumbar discs. J Bone Joint Surg Am. 1963;45:311–317. [Google Scholar]
- 3.Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar disc herniations. Case reports. Orthopedics. 1989;12:275–278. doi: 10.3928/0147-7447-19890201-11. [DOI] [PubMed] [Google Scholar]
- 4.Cloward RB. The treatment of ruptured lumber intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154–168. doi: 10.3171/jns.1953.10.2.0154. [DOI] [PubMed] [Google Scholar]
- 5.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 2000;25:1437–1446. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Shin HC, Yi S, Kim KN, Kim SH, Yoon do H. Posterior lumbar interbody fusion via a unilateral approach. Yonsei Med J. 2006;47:319–325. doi: 10.3349/ymj.2006.47.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias WJ, Simmons NE, Kaptain GJ, Chadduck JB, Whitehill R. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg. 2000;93:45–52. doi: 10.3171/spi.2000.93.1.0045. [DOI] [PubMed] [Google Scholar]
- 8.Ido K, Shimizu K, Tada H, Matsuda Y, Shikata J, Nakamura T. Considerations for surgical treatment of patients with upper lumbar disc herniations. J Spinal Disord. 1998;11:75–79. [PubMed] [Google Scholar]
- 9.Harms J, Rolinger H. [A one-stage procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author's transl)] Z Orthop Ihre Grenzgeb. 1982;120:343–347. doi: 10.1055/s-2008-1051624. [DOI] [PubMed] [Google Scholar]
- 10.Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976) 2001;26:567–571. doi: 10.1097/00007632-200103010-00023. [DOI] [PubMed] [Google Scholar]
- 11.Lowe TG, Tahernia AD. Unilateral transforaminal posterior lumbar interbody fusion. Clin Orthop Relat Res. 2002;394:64–72. doi: 10.1097/00003086-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Anand N, Hamilton JF, Perri B, Miraliakbar H, Goldstein T. Cantilever TLIF with structural allograft and RhBMP2 for correction and maintenance of segmental sagittal lordosis: long-term clinical, radiographic, and functional outcome. Spine (Phila Pa 1976) 2006;31:E748–E753. doi: 10.1097/01.brs.0000240211.23617.ae. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz A. Transforaminal lumbar interbody fusion. Orthop Clin North Am. 2002;33:359–366. doi: 10.1016/s0030-5898(01)00008-6. [DOI] [PubMed] [Google Scholar]
- 14.Lowe TG, Tahernia AD, O'Brien MF, Smith DA. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2-year results. J Spinal Disord Tech. 2002;15:31–38. doi: 10.1097/00024720-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Hioki A, Miyamoto K, Kodama H, Hosoe H, Nishimoto H, Sakaeda H, et al. Two-level posterior lumbar interbody fusion for degenerative disc disease: improved clinical outcome with restoration of lumbar lordosis. Spine J. 2005;5:600–607. doi: 10.1016/j.spinee.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Hee HT, Castro FP, Jr, Majd ME, Holt RT, Myers L. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord. 2001;14:533–540. doi: 10.1097/00002517-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Cutler AR, Siddiqui S, Mohan AL, Hillard VH, Cerabona F, Das K. Comparison of polyetheretherketone cages with femoral cortical bone allograft as a single-piece interbody spacer in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2006;5:534–539. doi: 10.3171/spi.2006.5.6.534. [DOI] [PubMed] [Google Scholar]
- 18.Whitecloud TS, 3rd, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior-posterior interbody fusion of lumbar spine: a financial analysis. J Spinal Disord. 2001;14:100–103. doi: 10.1097/00002517-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J. 2005;14:551–558. doi: 10.1007/s00586-004-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg WS, Mummaneni PV. Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery. 2001;48:569–574. doi: 10.1097/00006123-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90:163–169. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 22.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976) 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]