Mineral elements are often preferentially stored in vacuoles of specific leaf cell types, but the mechanism and physiological role for this phenomenon is poorly understood. We use single-cell analysis to reveal the genetic basis underpinning mesophyll-specific calcium storage in Arabidopsis leaves and a variety of physiological assays to uncover its fundamental importance to plant productivity.
Abstract
The physiological role and mechanism of nutrient storage within vacuoles of specific cell types is poorly understood. Transcript profiles from Arabidopsis thaliana leaf cells differing in calcium concentration ([Ca], epidermis <10 mM versus mesophyll >60 mM) were compared using a microarray screen and single-cell quantitative PCR. Three tonoplast-localized Ca2+ transporters, CAX1 (Ca2+/H+-antiporter), ACA4, and ACA11 (Ca2+-ATPases), were identified as preferentially expressed in Ca-rich mesophyll. Analysis of respective loss-of-function mutants demonstrated that only a mutant that lacked expression of both CAX1 and CAX3, a gene ectopically expressed in leaves upon knockout of CAX1, had reduced mesophyll [Ca]. Reduced capacity for mesophyll Ca accumulation resulted in reduced cell wall extensibility, stomatal aperture, transpiration, CO2 assimilation, and leaf growth rate; increased transcript abundance of other Ca2+ transporter genes; altered expression of cell wall–modifying proteins, including members of the pectinmethylesterase, expansin, cellulose synthase, and polygalacturonase families; and higher pectin concentrations and thicker cell walls. We demonstrate that these phenotypes result from altered apoplastic free [Ca2+], which is threefold greater in cax1/cax3 than in wild-type plants. We establish CAX1 as a key regulator of apoplastic [Ca2+] through compartmentation into mesophyll vacuoles, a mechanism essential for optimal plant function and productivity.
INTRODUCTION
Calcium (Ca) is an essential plant macronutrient with unique structural and signaling roles (White and Broadley, 2003). Tight spatio-temporal control of Ca ion concentration ([Ca2+]) in the cytosol is crucial for cell and whole-plant function and responses to environmental stress (McAinsh and Pittman, 2009; Dodd et al., 2010). Therefore, to fulfill a multitude of signaling roles within plant tissues, the regulation of Ca2+ nutritional flow and storage is critical (Hirschi, 2004; Dayod et al., 2010).
Within plants, the majority of Ca2+ transport occurs via apoplastic pathways (Clarkson, 1984; White, 2001). The transpiration stream carries Ca2+ from root to shoot, via the xylem, from where it is unloaded into the leaf and is distributed apoplastically within the cell wall. Within the apoplast, the majority of Ca2+ binds to negatively charged carboxylic groups of galacturonic acids (pectin) and oxalates (Sattelmacher, 2001), with the residual Ca2+ remaining free in the apoplast for signaling functions (Hirschi, 2004). Pectate cross-linking by Ca2+ within the cell wall affords greater strength, but little is known about the role of Ca2+ signaling in regulating cell wall extensibility (Hepler and Winship, 2010). Apoplastic Ca2+ is also taken up by cells where it fulfills roles in intracellular signaling, but as Ca2+ is relatively immobile in the cell and is not transported in the phloem, it is not normally redistributed following deposition in leaf vacuoles (Clarkson, 1984; Leigh, 1997; White and Broadley, 2003).
Calcium is differentially accumulated between organs, being abundant in transpiring leaves and less so in tissues with low transpiration rates (White and Broadley, 2003; Dayod et al., 2010). Ca2+ storage across cell types is also heterogeneous (Karley et al., 2000a; Conn and Gilliham, 2010). Within most dicots so far examined, Ca2+ appears to preferentially accumulate within mesophyll cell vacuoles (Conn and Gilliham, 2010). Significant Ca2+ accumulation has also been observed in trichomes (De Silva et al., 1996a; Ager et al., 2003). De Silva et al. (1996a) hypothesized that accumulation of Ca2+ in trichomes, and to a lesser extent in the mesophyll, facilitates normal stomatal function by removing Ca2+ from the apoplastic fluid around the guard cells. It has also been suggested that Ca2+ is stored in specific cells to prevent precipitation with inorganic phosphate (Pi) stored in vacuoles of different leaf cells (Dietz et al., 1992; Leigh and Storey, 1993; Karley et al., 2000a, 2000b). However, the role of cell-specific Ca2+ accumulation is yet to be interrogated experimentally, and mechanisms by which cell-specific nutrient accumulation occur are unknown (Karley et al., 2000b; Conn and Gilliham, 2010).
There are two distinct ways in which cell-specific Ca2+ storage could arise. At one extreme, only certain cells may have the ability to accumulate Ca2+ from the transpiration stream. While at the other, low vacuolar Ca concentration ([Ca]vac) may result from reduced exposure to apoplastic Ca2+. Studies of rough lemon (Citrus jambhiri) in which all leaf cells were exposed to strontium ions (as a tracer for Ca2+) or high apoplastic [Ca2+] ([Ca2+]apo) demonstrated that only certain cells had the ability to accumulate significant [Ca]vac (Storey and Leigh, 2004). This suggests transport capabilities of different cell types determine the ability to store Ca2+. However, Karley et al. (2000b) found no difference in 45Ca2+ unidirectional influx rate into protoplasts isolated from cell types that differentially accumulate Ca2+ in barley (Hordeum vulgare) leaves. This indicates that differential Ca2+ storage in leaf cells is either (1) not active or distinguishable in isolated protoplasts; (2) not conferred by transport characteristics of the plasma membrane; and/or (3) mediated by tonoplast–transport characteristics, differences that may be underpinned by unique transcriptional profiles within different cell types (Conn and Gilliham, 2010).
The vacuole is the major Ca2+ store in plant cells. The best characterized Ca2+ transporters present in leaves of Arabidopsis thaliana capable of catalyzing Ca2+ influx into vacuoles are tonoplast-localized ACA and CAX proteins (Geisler et al., 2000a; McAinsh and Pittman, 2009; Dodd et al., 2010). Members of the (autoinhibited) type-P2A Ca2+-ATPases (ACA) family are high-affinity Ca2+ pumps that are stimulated by calmodulin and are implicated in adjusting cytosolic [Ca2+] ([Ca2+]cyt) within the nanomolar range (Harper et al., 1998). Of the 10 members in Arabidopsis, only ACA4 and ACA11 have been shown to be both expressed in the leaf and localized on the tonoplast; however, the specific roles of these proteins and the regulation of their expression or activity are not currently well defined (Geisler et al., 2000b; Baxter et al., 2003; Bolte et al., 2004; Lee et al., 2007; see Discussion). The Ca2+/H+ antiporters (CAX) have lower transport affinities for Ca2+ than ACA proteins (CAX1, Km= 10 to 15 μM; Hirschi et al., 1996), possess an N-terminal autoinhibitory domain, and use the proton-motive force across the tonoplast to remove Ca2+ from the cytosol when free [Ca2+]cyt is significantly above resting levels. In Arabidopsis, there are six CAX genes belonging to two clades, CAX1, 3, and 4 within clade I-A and CAX2, 5, and 6 within clade I-B (Shigaki and Hirschi, 2006). Overexpression of modified CAX proteins, some with the autoinhibitory domain removed (sCAX), result in significant increases in Ca content in a variety of crops (Park et al., 2005a, 2005b; Morris et al., 2008) but in some cases also poor growth phenotypes (reviewed in Dayod et al., 2010). Knockout of CAX1 by insertional mutagenesis results in no significant change in leaf [Ca] compared with wild-type plants, whereas the simultaneous knockout of both CAX1 and CAX3 causes a 17% lower leaf [Ca] and slower plant growth phenotype than the Columbia-0 (Col-0) parent (Cheng et al., 2005). The mechanism by which abolition of CAX expression could result in this growth phenotype is not known.
To elucidate the basis and role of cell type–specific Ca2+ accumulation in Arabidopsis, we examined the distribution of Ca within leaves. Transcript profiles within RNA extracted from small populations of representative cells with low Ca storage (adaxial epidermis) and high Ca storage (palisade mesophyll) were compared using a microarray screen and single-cell quantitative PCR (qPCR). Numerous transcripts were identified that were disproportionately expressed between these cell types, but only disruption of CAX expression specifically reduced the ability of mesophyll cells to accumulate Ca2+ within the vacuole. This resulted in an increase in apoplastic free [Ca2+] and reductions in transpiration, CO2 assimilation, cell wall extensibility, and growth. The genetic basis for these phenotypes was explored, and the role of apoplastic free [Ca2+] in controlling the expression of Ca2+ transporters, cell wall–modifying proteins, and cell wall carbohydrate composition and thickness was tested. We summarize our findings in a model that highlights the mechanisms underpinning cell-specific vacuolar Ca2+ storage as an essential physiological process in maintaining optimal transpiration and CO2 assimilation, cell wall extensibility, and plant productivity.
RESULTS
Ca Is Preferentially Stored within Mesophyll Cell Vacuoles of Arabidopsis Leaves
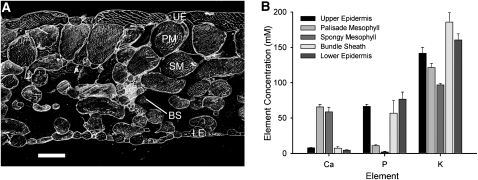
Total [Ca] was measured within vacuoles of different cells of 6-week-old frozen-hydrated Arabidopsis leaves using x-ray microanalysis (XRMA) and compared with potassium (K) and phosphorus (P) concentrations ([K] and [P]) (Figures 1A and 1B). In both epidermal layers and bundle sheath cells, [Ca] was below the reliable detection limit (~12 mM), but in palisade and spongy mesophyll cells, [Ca] was 65.7 ± 3.5 mM and 58.6 ± 6.6 mM, respectively (Figure 1B). By contrast, [K] was largest (≥140 mM) in the abaxial (lower) epidermis, bundle sheath, and adaxial (upper) epidermis and was significantly smaller in the palisade and spongy mesophyll (range 70 to 131 mM) (P < 0.05, Student’s t test). A similar pattern was observed for [P]. No statistically significant change in elemental accumulation occurred within different cell types of leaf 8 between 3.5 to 8 weeks. Over this period, whole-leaf [Ca] of wild-type plants did not vary significantly, ranging between 50.2 ± 1.9 mM (mean ± se) and 52.2 ± 5.5 mM, while the adaxial epidermis [Ca]vac was 4.0 ± 0.3 mM to 4.2 ± 1.3 mM and the palisade mesophyll was 58.3 ± 0.9 mM to 62.5 ± 3.4 mM. Similar stability of concentrations was also observed for K and P. Thus, 6 weeks was deemed a representative sampling time point (see Supplemental Table 1 online).
Figure 1.
Vacuolar Concentrations of K, P, and Ca Vary between Cell Types within Leaves of Arabidopsis.
(A) Scanning electron micrograph of a cryosectioned, 6-week-old Arabidopsis leaf, ecotype Col-0. UE, upper epidermis; PM, palisade mesophyll; SM, spongy mesophyll; BS, bundle sheath; LE, lower epidermis. Bar = 50 μm.
(B) Concentrations of Ca, P, and K in vacuoles of different cell types in leaf 8 of 6-week-old Arabidopsis ecotype Col-0, grown in BNS. Mean + se; n = 4 cells per cell type per leaf, across 12 plants. For calibration curves, see Supplemental Figure 1 online.
Ca2+ Transporters Are Differentially Expressed between Cells That Differ in Their Ability to Accumulate Ca
Gene transcripts from Arabidopsis adaxial epidermis (low vacuolar [Ca]) and palisade mesophyll cells (high vacuolar [Ca]) were extracted using a microcapillary, amplified and hybridized to a custom microarray to screen for differential expression of Ca2+ transporters (see Supplemental Figure 2 online). Line KC464 was used for these experiments because it expresses green fluorescent protein (GFP) in adaxial epidermis, providing a means to detect contamination of palisade mesophyll extracts with RNA from the overlying adaxial epidermal cells. Line KC464 also had the same [Ca]vac in adaxial epidermal and palisade mesophyll cells as the Col-0 wild type (see Supplemental Table 1 online). However, variation in individual transcripts within the same tissue type was observed across biological (but not technical) repeats, consistent with biological stochastic gene transcription events in small cell population sizes (Zhong et al., 2008) or small pools of cells (Kralj et al., 2009). Intrinsic noise (cell-specific temporal variation) and extrinsic noise (cell-to-cell variation between cells) are believed to contribute to commonly detected variation in gene expression when sampling from small numbers of cells (Swain et al., 2002; Raser and O’Shea, 2004). To overcome this, a transcript was only deemed present in a particular cell type if it was detected above the minimum expression threshold (log2 > 8.0) in at least two biological replicates. Results of the single-cell sampling and analysis (SiCSA) microarray were treated as a screen only.
However, a rudimentary analysis revealed that RNA from each cell type was not contaminated with the other cell type; 72% of all annotated Arabidopsis gene transcripts were expressed in the combined epidermal and mesophyll transcriptomes (see Supplemental Figure 3A online); and over 2200 cell-specific transcripts (9.2%) were consistently identified as present in one cell type and absent in the other, with 90% of these shown to be mesophyll specific (see Supplemental Figure 3A online). These included nuclear-encoded transcripts for chloroplast-targeted proteins that were enriched in palisade mesophyll cell samples (4.0%) compared with those extracted from epidermis (0.1%) or the whole transcriptome (2.2%) (see Supplemental Figures 3B to 3D online).
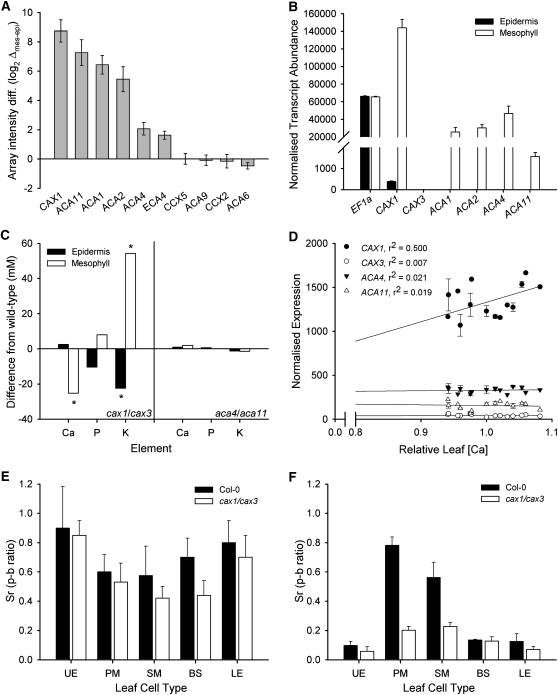
The SiCSA microarray screen also indicated that there were differences in the expression of known and putative Ca2+ transporters between the adaxial epidermal and palisade mesophyll transcriptomes. Genes encoding CAX, CCX, ACA, and ECA gene families are shown in Figure 2A (for extended data set, see Supplemental Table 3 online). Key data were further examined using independent biological material with SiCSA qPCR and laser capture microdissection (LCM) (Figure 2B; see Supplemental Figure 4 online). CAX1 was the only member of the CAX family with higher transcript abundance in palisade mesophyll than adaxial epidermis (Figure 2A) and was the most highly abundant Ca2+ transporter within the palisade mesophyll as confirmed by qPCR on biologically independent samples (~375-fold more abundant than in adaxial epidermis) (Figure 2B) and LCM (see Supplemental Figure 4 online). Four members of the ACA/ECA gene family, ACA1, 2, 4, and 11, were also more highly expressed in palisade mesophyll compared with the adaxial epidermis (Figures 2A and 2B; see Supplemental Figure 4 online). The differential abundance of Pi and K+ transporter gene transcripts was also screened for, but no enrichment in the (P and K accumulating) epidermis was observed (see Supplemental Tables 4 and 5 online). Given that ACA1 is localized to the chloroplast envelope or plastids/endoplasmic reticulum (ER) (Huang et al., 1993; J. Harper, personal communication) and ACA2 to the ER (Hong et al., 1999), ACA4, ACA11, and CAX1 were prioritized for further investigation as candidates that could catalyze Ca2+ accumulation preferentially into mesophyll vacuoles.
Figure 2.
Identification of Candidate Transporters Underscoring Preferential Accumulation of Ca in the Mesophyll by Comparing Arabidopsis Epidermal and Mesophyll Transcriptomes Using SiCSA/Microarray and qPCR.
Mesophyll accumulation of Ca (Sr) is a dominant factor in total leaf Ca accumulation, and CAX transporters play the key role in both mesophyll preferential and total leaf Ca accumulation. Sr accumulates in the apoplast of all cell types and yet preferentially in the mesophyll of Col-0 and cax1/cax3 plants.
(A) Microarray-based screen of comparative expression for Ca2+-ATPase (ACA/ECA) and Ca2+/H+ exchanger (CAX and CCX) families within palisade mesophyll and adaxial epidermal cells of 5-week-old Arabidopsis KC464 line grown hydroponically in BNS. Data are presented as the log2 difference between the mean intensity ± se for each gene in the palisade mesophyll minus that for the adaxial epidermis. Data are derived from three epidermal and three mesophyll samples taken from three plants (refer to Methods for array details and labeling). For extended data set, see Supplemental Table 3 online.
(B) qPCR confirmation of preferential expression of Ca2+ transporters within palisade mesophyll cells. aRNA from Arabidopsis KC464 line isolated by SiCSA was normalized using Elongation Factor 1α (EF1α) and β-tubulin 5 (see Supplemental Figure 2C online), while for EF1α transcript abundance, normalization was performed against β-tubulin 5. Three amplifications were performed on three independent plants to those used in Figure 2A, with data presented as mean normalized expression levels + se. qPCR was performed in triplicate for each biological replicate.
(C) Difference in Ca, P, and K concentration in adaxial epidermal and palisade mesophyll cells between the Col-0 parent and T-DNA insertion lines aca4/aca11 and cax1/cax3. Six-week-old plants grown in BNS (1 mM aCa) were analyzed by SiCSA/XRMA analysis, with data presented as mean change in [Ca]vac compared with Col-0 (n = 25 cells across five plants). Asterisk indicates significant difference from Col-0; Student’s t test (P < 0.05).
(D) Correlation of ICP-MS and microarray data across 15 Arabidopsis ecotypes, reinforcing the importance of CAX1 in Ca accumulation. Normalized microarray data obtained from Lempe et al. (2005) and ICP data obtained from Purdue ionomics information management system database (Baxter et al., 2007) (www.ionomicshub.org), normalized using REML (Broadley et al., 2010) on leaves of plants grown in soil with the same fertilization and light regimens. Weight-normalized values (in ppm) were used and presented relative to Col-0 to facilitate interexperimental normalization of ICP data (mean ± se).
(E) and (F) Sr content was measured within excised leaf 8 from BNS-grown plants sampled at 6 weeks of age, fed with 50 mM SrCl2 through the petiole for 16 h in an artificial sap background (AS). p-b, peak over background ratio as defined by Storey and Leigh (2004). n = 4 cells per leaf 8, four separate plants (mean + se). For cell-type abbreviations, see Figure 1A. Despite no significant difference in apoplastic Ca surrounding all cells (E), mesophyll vacuoles (F) of cax1/cax3 leaves are inhibited in their ability to accumulate Sr (Ca) compared with Col-0.
Ca2+/H+ Antiporters Have a Key Role in Mesophyll Ca2+ Accumulation
Adaxial epidermal and palisade mesophyll cell vacuolar [Ca] was examined in T-DNA insertion lines of ACA4, ACA11, CAX1, and CAX3 (which has 77% amino acid sequence identity to CAX1); no line exhibited significant alteration in cell-specific [Ca], with all lines having ~4 and 50 mM in adaxial epidermal and palisade mesophyll cell vacuoles, respectively. In cax1-1, it was previously observed that functional compensation for loss of CAX1 occurs through a transcriptional upregulation in other transporters (CAX3, CAX4, and ACA4), which results in no significant change in total leaf [Ca] (Cheng et al., 2003, 2005). We found no alteration in total leaf [Ca], epidermal, or mesophyll [Ca]vac in all lines and no change in Ca2+ transporter transcript abundance within aca4-3 and aca11-5 (see Supplemental Figures 5A and 5B online).
Leaf 8 [Ca] from 5-week-old Col-0, aca4-3/aca11-5 (aca4/aca11), and cax1-1/cax3-1 (cax1/cax3) lines grown in hydroponic basal nutrient solution (BNS) containing a Ca2+ activity (aCa) of 1 mM was measured by inductively coupled plasma–mass spectroscopy (ICP-MS). Total leaf [Ca] was 50.4 ± 2.0 mM and 49.9 ± 1.7 mM (mean ± se; n = 6 plants per line) in Col-0 and aca4/aca11, respectively. By contrast, leaf [Ca] in cax1/cax3 was significantly lower (40.8 ± 1.5 mM; P < 0.05; Student’s t test), which confirmed the result obtained in cax1/cax3 soil-grown plants (Cheng et al., 2005; see Supplemental Table 1 online). In cax1/cax3, palisade mesophyll [Ca]vac was 42% lower (P < 0.002, Student’s t test) compared with the Col-0 wild type, but no significant alteration was observed in adaxial epidermal [Ca] or in either cell types of aca4/aca11 (Figure 2C). A meta-analysis from publically available ionomic and transcriptional data for 15 Arabidopsis ecotypes uncovered a strong correlation between leaf [Ca] and transcript abundance of CAX1, but not ACA4, ACA11, or CAX3 (Figure 2D). A similar meta-analysis was performed on accessions that vary in trichome density because it was suggested that trichomes are important in Ca storage in leaves (De Silva et al., 1996a); there was no significant correlation between trichome density and total leaf [Ca] (see Supplemental Figure 6 online).
Strontium chloride (SrCl2) was fed through petioles of excised cax1/cax3 and Col-0 leaves to trace Ca2+ distribution and cellular uptake. Apoplastic Sr surrounded all cell types, with no significant difference in [Sr]apo detected between corresponding cell types of Col-0 and cax1/cax3 (Figure 2E). Sr was selectively accumulated within mesophyll cell vacuoles, while in cax1/cax3, mesophyll accumulation of Sr was significantly lower compared with Col-0 (Figure 2F). Qualitatively similar results were observed in leaves vacuum infiltrated with 50 mM SrCl2 after 4.5 h (data not shown).
The Physiological Role of Ca Compartmentation within the Leaf: Regulation of Stomatal Aperture
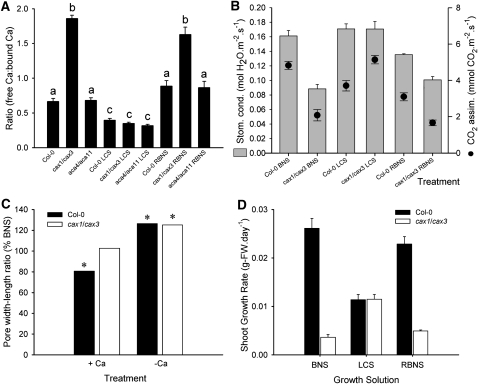
Apoplastic [Ca] was assayed in leaf 8 of Col-0, cax1/cax3, and aca4/aca11 plants. Specifically, sorbitol-extractable [Ca] (SeCa), which gives an indication of apoplastic free [Ca], and barium-exchangeable [Ca] (BeCa), which gives an indication of a less readily available Ca fraction bound to cell walls, was measured. Apoplastic BeCa was not significantly different between all lines (at ~0.5 mM), whereas apoplastic SeCa was 3 times greater in cax1/cax3 (at 0.97 ± 0.05 mM; mean ± se) compared with Col-0 and aca4/11 (~0.33 ± 0.03 mM) when grown in BNS. Therefore, only in cax1/cax3 leaves did SeCa exceed BeCa (Figure 3A). Hypocotyl xylem [Ca] was not statistically different between cax1/cax3 (3.27 ± 0.25 mM, n = 6) and Col-0 plants (3.10 ± 0.22 mM, n = 6), suggesting that the effect of cax1/cax3 was due to altered sequestration of Ca2+ from the apoplastic fluid following xylem unloading in the leaf, not an altered supply of Ca from the roots.
Figure 3.
Slow Growth, Low Transpiration, Reduced Photosynthesis, and Guard Cell Aperture Phenotypes Can Be Conditionally Suppressed by Ca Starvation in the cax1/cax3 T-DNA Insertion Mutant.
(A) Apoplastic calcium concentrations of Col-0, aca4/aca11, and cax1/cax3 plants presented as the ratio of free Ca to bound Ca (SeCa:BeCa, sorbitiol-extractible Ca:barium-extractible Ca). Measurements performed on 6-week-old Arabidopsis plants grown for 7 d prior in BNS or LCS (50 μM aCa) and subsequently returned to BNS (RBNS) for an additional 7 d. Data presented as mean ratio + se (n = 6 leaves per genotype per condition). Three replicate samples each comprising leaves from two plants performed across three experiments. a, b, and c represent no statistical difference between each treatment (ratio calculated between leaves of the same plant, permitting statistical analysis on biological replicates by two-way ANOVA, P < 0.05).
(B) Stomatal conductance (gS; mean + se) and CO2 assimilation rates (A; mean ± se) of leaf 8 of 7-week-old Col-0 and cax1/cax3 plants in BNS, LCS (from week 6), and RBNS (LCS from week 5, BNS from week 6). n = 5 plants per genotype per treatment, with experiment repeated five times with identical trends.
(C) Guard cell aperture of epidermal strips from Col-0 and cax1/cax3 plants floated on DS. Data presented as the aperture ratios on high calcium solution (+Ca; 1 mM) or in the presence of EGTA to chelate calcium (−Ca) as a percentage of apertures of each respective phenotype incubated solely on DS. Student’s t test was performed on raw data; asterisk indicates statistical significance from treatment compared with Col-0 in BNS (P < 0.05). For replicate number, refer to the main text.
(D) Changes in shoot biomass of Col-0 and cax1/cax3 plants during 7 d growth in BNS followed by transfer and a further 7 d growth in LCS and then return to BNS for a further 7 d (RBNS). Data presented as mean growth rate + se (n = 6 plants per treatment).
By transferring plants to a solution with low aCa (0.025 mM low calcium solution [LCS]), SeCa was reduced to the same level in all lines (0.13 ± 0.01 mM); importantly, this manipulation reduced SeCa of cax1/cax3 to below that of BeCa (0.36 ± 0.04 mM) (Figure 3A). When all lines were returned from LCS to BNS (RBNS), only in cax1/cax3 did SeCa again exceed BeCa (Figure 3A). These treatments appear consistent with an ability to manipulate [Ca2+]apo in cax1/cax3 experimentally using LCS to obtain similar SeCa:BeCa (free:bound Ca) ratios as those observed in wild-type plants. This treatment was used in all future experiments to observe the effects of altering [Ca2+]apo.
Since high [Ca2+]apo can close stomata of wild-type plants (Webb et al., 2001; De Silva et al., 1996b) measurements of photosynthetic CO2 assimilation (A) and leaf conductance, to which stomatal conductance (gs) is the major contributor, were made on cax1/cax3 and Col-0 plants grown in BNS (Figure 3B). Both these parameters were lower, A by 57 and gs by 45% in cax1/cax3, when compared with Col-0. However, by equalizing [Ca]apo in cax1/cax3 and Col-0 using LCS, these phenotypes were recovered to Col-0 wild-type levels, but again reappeared when plants were returned to BNS (RBNS) (Figure 3B). This conditional suppression of apoplasmic [Ca] on leaf gas exchange parameters was not observed in aca4/aca11 (see Supplemental Figure 7A online).
The mean pore width:length ratio of Col-0 stomata in epidermal fragments incubated in depolarizing solution (DS) was significantly larger at 0.58 ± 0.018 (P < 0.0001) (mean ± se, mean length 7.9 μm, width 4.3 μM, n = 173) than that of cax1/cax3 stomata at 0.51 ± 0.018 (mean ± se; average length 6.8 μm, width 3.2 μM, n = 164), indicating that cax1/cax3 stomata were more closed in DS. Increasing [Ca2+]apo to 1 mM in DS decreased the pore width:length ratio of Col-0 to 80.7% of the value in DS alone (n = 175, P < 0.001), whereas cax1/cax3 stomatal apertures were unchanged (n = 183) (Figure 3C). However, both Col-0 and cax1/cax3 stomatal pores were responsive to removal of Ca (incubation with 2 mM EGTA), which increased pore sizes to 126.5% (n = 67) and 125.2% (n = 78) of the respective DS-incubated genotype controls (n = 73 and 67) (P < 0.0001) (Figure 3C).
Compared with Col-0, both aca4/aca11 (see Supplemental Figure 7B online) and cax1/cax3 (Figure 3D) plants grew significantly slower in BNS. Growth rate of cax1/cax3 plants, but not Col-0 or aca4/aca11, increased when transferred to LCS equivalent to that of Col-0 in LCS after 7 d (Figure 3D; see Supplemental Figure 7B online). When plants were returned to BNS (RBNS), growth rates of all lines were restored over the following 7 d to those originally observed in BNS. Reductions in mean palisade mesophyll [Ca]vac were equivalent between Col-0 and cax1/cax3 when transferred from BNS to LCS at 43% (60.5 to 34.2 mM, n = 6) and 42% (35.5 to 20.3 mM, n = 6), respectively, indicating differences between responses in [Ca]vac are not responsible for the observed changes in growth. Instead, the response of cax1/cax3 to LCS is consistent with an apoplastic Ca2+-dependent conditional suppression of the growth phenotype.
The Physiological Role of Ca Compartmentation within the Leaf: Regulation of Cell Wall Extensibility
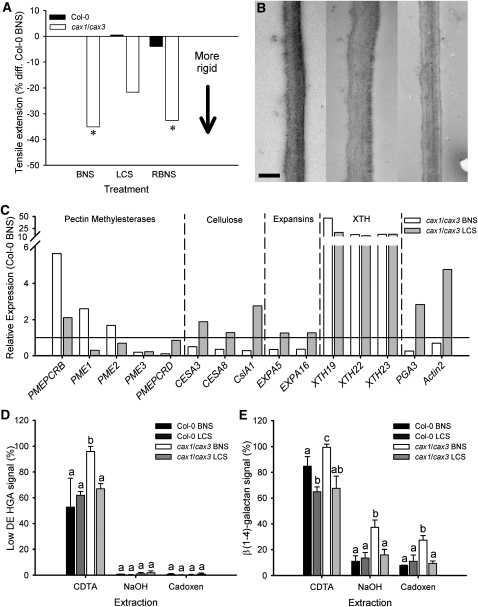
Leaf growth can also be regulated by cell wall strength (Lu and Neumann, 1999; Cosgrove, 2005). Figure 4A shows the extensibility of leaves from Col-0 and cax1/cax3 grown in the same conditions as for Figure 3B (BNS, LCS, and RBNS). Tensile extension at automatic break of cax1/cax3 leaves in BNS was 0.75 ± 0.09 mm (mean ± se; n = 52), a value that was 65% of that in equivalent Col-0 leaves (1.16 ± 0.13 mm, n = 32). This difference was statistically significant (P < 0.05) and indicates that cax1/cax3 leaves are more rigid than Col-0 leaves in BNS. When cax1/cax3 plants were transferred to LCS for 7 d, the extensibility of leaves increased to 0.91 ± 0.06 mm (n = 35), which was not significantly different to Col-0 in BNS or LCS (n = 28). In addition, when cax1/cax3 plants were returned to BNS (RBNS), the extensibility of their leaves was again significantly decreased, to 67% of Col-0 in BNS (P < 0.05) (Figure 4A). Extensibility of leaves from aca4/aca11 and Col-0 were not significantly different in any condition tested (see Supplemental Figure 8 online). As a result of the insensitivity of aca4/aca11 to changes in [Ca]apo, this mutant line was excluded from further analyses of cell wall properties.
Figure 4.
Cell Wall Rigidity and Architecture of cax1/cax3 Loss-of-Function Mutant Leaves Recoverable by Low Calcium Treatment and Correlated with Gene Expression.
(A) Tensile extension at maximum break for Col-0 (black bars) and cax1/cax3 (white bars) of leaves 7 to 11 from plants grown in BNS (Col-0, cax1/cax3) (n = 52 and 32), LCS (n = 35 and 28), or RBNS (n = 28 and 30) over four experimental runs. Student’s t test was performed on raw data comparing Col-0 and genotype/treatment; asterisk indicates significance at P < 0.05. Data shown as normalized to the percentage of difference from Col-0 in BNS.
(B) Increase in cell wall thickness of cax1/cax3 plants ameliorated by reducing apoplastic Ca. Representative single spongy mesophyll cell wall from BNS-grown Col-0 (left panel), cax1/cax3 (middle panel), and LCS-grown cax1/cax3 (right panel) leaf sections. Images captured on a Philips CM100 transmission electron microscope equipped with Megaview II Image capture running iTEM software (SIS Image Analysis) (n = 160 from 40 spongy mesophyll cell wall segments, four readings per segment from three independent leaf specimens). Values for Col-0 BNS, 185 ± 11 nm; Col-0 LCS, 170 ± 11 nm; cax1/cax3 BNS, 250 ± 16 nm; cax1/cax3 LCS, 209 ± 10 nm (mean ± se). Bar = 200 nm.
(C) qPCR on cell wall biosynthesis genes performed on RNA from 6-week old Col-0 and cax1/cax3 plants grown in BNS and cax1/cax3 plants grown for 5 weeks in BNS and 7 d in LCS (0.025 μM Ca). Line at y = 1 represents expression level in leaves of Col-0 (n = 3 independent plant samples per treatment, with qPCR performed in triplicate). In this experiment, Actin2 is excluded as a normalization gene, instead using EF-1α, β-tubulin5, and GAPDH-A.
(D) and (E) Analysis of cell wall glycans from Col-0 and cax1/cax3 plants grown under both BNS and LCS treatments by CoMPP assay (Moller et al., 2007). Results of hybridization with JIM5 antibody (D) (low methyl-esterified HGA) and LM5 antibody (E) [for β(1→4)-linked galactan]. Presented as signal intensity with the strongest signal given the value of 100%; mean + se. Three biological replicate samples per genotype per condition, each comprised of material pooled from three independent plants, were analyzed in triplicate. a, b, and c represent data groups that are not statistically different, with each extraction treated independently, as determined by one-way ANOVA and Tukey’s HSD posthoc test.
Abundance of Cell Wall Genes Is Correlated with Cell Growth, Cell Wall Thickness, and Glycan Levels
Microscopy observations of leaf cross sections showed that mesophyll cells of cax1/cax3 grown in BNS were smaller, resulting in 42% higher cell density compared with Col-0 (see Supplemental Figures 9A to 9D online). Furthermore, transmission electron microscopy (TEM) on these leaves found spongy mesophyll cells of BNS-grown cax1/cax3 plants had cell walls that were 35% thicker (250 ± 16 nm; mean ± se) than those of equivalent Col-0 leaves (185 ± 11 nm) (Figure 4B). However, both cell density and cell wall thickness differences between cax1/cax3 and Col-0 leaves were reduced by growing cax1/cax3 in LCS (Figure 4B; see Supplemental Figure 8D online).
Microarray analysis indicated that numerous leaf-expressed transcripts encoding proteins that modify cell wall structure were significantly changed in expression between Col-0 and cax1/cax3 plants (see Supplemental Table 6 online). qPCR analysis of candidates from representative gene families was performed to validate the microarray data and to determine if there were any transcriptional responses to reduced apoplastic [Ca] in cax1/cax3 leaves grown in LCS (Figure 4C). For all candidate genes analyzed by qPCR, transcript abundance correlated with microarray results from BNS-grown Col-0 and cax1/cax3 plants (see Supplemental Table 6 online). For all candidates examined from the cellulose synthase (CESA), cellulose synthase-like (Csl), expansin (EXP), and polygalacturonase (PGA) families, gene expression in cax1/cax3 plants grown in BNS was significantly below that in BNS-grown Col-0 leaves (Figure 4C; see Supplemental Table 7 online). Whereas growth of cax1/cax3 plants in LCS resulted in greater transcript abundance of these genes, similar to the level observed from Col-0 grown in BNS (Figure 4C). By contrast, three xyloglucan endotransglucosylase/hydrolase (XTH) genes (XTH19, XTH22, and XTH23) were significantly more abundant in BNS-grown cax1/cax3 leaves compared with equivalently grown Col-0 leaves and were still significantly higher in LCS-grown cax1/cax3 leaves (Figure 4B). Only XTH19, a transcript with minimal abundance in BNS-grown Col-0 (see Supplemental Table 7 online), was significantly downregulated by LCS treatment. Pectin methylesterase (PME) genes PMEPCRB, PME1, and PME2 were also more highly expressed in BNS-grown cax1/cax3 leaves than Col-0 leaves but were significantly reduced by LCS treatment (Figure 4C). However, PME3 and PMEPCRD were more lowly expressed in BNS-grown cax1/cax3 leaves than in Col-0, and only the latter was found to recover to near wild-type levels following LCS treatment (Figure 4C).
Cell wall glycan composition of leaves of Col-0 and cax1/cax3 plants grown in BNS and LCS was compared using comprehensive microarray polymer profiling (CoMPP), which identifies the occurrence of epitopes belonging to major polysaccharides present in cell walls (including pectin, hemicelluloses, and cellulose families of macromolecules) (Moller et al., 2007). A higher amount of pectin components, specifically, low demethylesterifed homogalacturonan (HGA; 44% higher than Col-0), was detected in CDTA extracts from cax1/cax3 leaves grown in BNS (Figure 4D). Accordingly, there were lower amounts of low methyl-esterified HGA in cax1/cax3 compared with Col-0 in BNS (see Supplemental Figure 10C online). There was also a higher accumulation of the rhamnogalacturnonan-I pectin branching molecule, β(1→4)-linked galactan (35% higher than Col-0), in BNS-grown cax1/cax3 leaves (Figure 4E). While the majority of β(1→4)-linked galactan was solubilized by CDTA extraction (known to solubilize pectin-associated glycans) from Col-0 (BNS and LCS) and cax1/cax3 LCS leaf cell walls, there was greater residual β(1→4)-linked galactan within the cax1/cax3 BNS plants (Figure 4E). This was observed in both subsequent fractionation steps performed (16 to 26% higher) to isolate noncellulosic polysaccharides (NaOH extraction) and cellulose (cadoxen extraction) (Figure 4E). Furthermore, as occurred with cell wall transcript profiles and growth, these differences in glycan composition were abolished in cax1/cax3 plants by growth in LCS for 7 d (Figures 4D and 4E). No line or treatment-dependent differences were found in either cellulose, xyloglucans, or linearized (1→5)-linked l-arabinans (see Supplemental Figure 10 online).
Exploring the Mechanism of Ionic Homeostasis in Leaves
In cax1/cax3 in BNS, there was higher [K]vac in mesophyll cells when compared with Col-0, which was coincident with a decrease in mesophyll [Ca]vac (Figure 2C); this could also be seen in whole leaves (see Supplemental Table 1 online). Concurrently, there was a decrease in epidermal [K]vac (Figure 2C). This suggests interdependence between the two elements. By contrast, total leaf [P] was higher by 53% in cax1/cax3 than Col-0 in BNS (44.6 ± 2.6 compared with 28.9 ± 1.0 mM; mean ± se, n = 6 independent plants), but this was not reflected in [P]vac, which was not significantly different from control values (Figure 2C). Interestingly, total leaf [P] decreased to control values when cax1/cax3 was grown in LCS (53.2 ± 4.6 mM, n = 6). In addition to [Ca]vac, there was no significant difference of total leaf, and [K]vac and [P]vac, of aca4/aca11 and Col-0 in all conditions tested (see Supplemental Table 1 online).
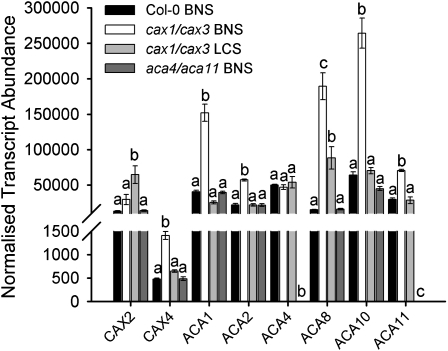
In mesophyll that lack expression of CAX1 and CAX3, Ca was still selectively accumulated into mesophyll cells over other leaf cell types, albeit in a reduced amount (Figures 2C and 2F). Accordingly, potential Ca2+ transporter genes that may facilitate selective accumulation into cax1/cax3 and aca4/aca11 mesophyll were examined by qPCR. Compared with Col-0 in BNS, no significant difference in gene expression was observed in aca4/aca11 (Figure 5). However, in cax1/cax3 plants grown in BNS, expression of Ca2+ transporters targeted to the vacuole (CAX4 and ACA11), plasma membrane (ACA8 and ACA10), chloroplast/plastid/ER (ACA1), and ER (ACA2) were all significantly greater than in Col-0 leaves (Figure 5). For cax1/cax3 plants grown in LCS, all Ca2+ transporter transcripts examined, except CAX2, which significantly increased, were in similar abundance to Col-0 leaves grown in BNS (Figure 5).
Figure 5.
Expression of Ca2+ Transporters in Col-0 and cax1/cax3 in BNS and LCS.
Transcript abundance of Ca2+ transporters in leaves of Col-0 and aca4/aca11 and cax1/cax3 T-DNA insertion lines. Transcripts were normalized against EF1α (At1g07940), β-tubulin5 (At1g20010), and Actin2 (At3g18780). Mean ± se, n = 3 plants. qPCR was performed in triplicate. a, b, and c represent data groups that are not statistically different, with each extraction treated independently, Student’s t test (P < 0.05).
DISCUSSION
Cell-Specific Ca Compartmentation Involves Cell-Specific, Selective Transport Processes
Metabolic reactions and nutrient storage are compartmentalized into specific cell types, but the mechanisms and reasons underpinning this compartmentation are not fully understood (Leegood, 2008; Conn and Gilliham, 2010). Arabidopsis preferentially accumulates Ca in the vacuoles of mesophyll cells and K and P within vacuoles of the epidermis and bundle sheath (Figure 1B). This pattern has been consistently observed across all eudicots so far examined; conversely, the cereal monocots barley, wheat (Triticum aestivum), and Sorghum bicolor accumulate Ca in epidermal cells and not within mesophyll or bundle sheath cells (the two latter cell types have greater [K] and [P] than in epidermal cells) (reviewed in Conn and Gilliham, 2010). Therefore, cell-specific Ca and P and cell-type preferential K accumulation in leaves have been observed in different plant species and as such are likely to be conserved mechanisms that fulfill an important role in plant function.
We tested the hypothesis that the cell-specific complement of vacuolar ion transporters could be responsible for the preferential accumulation of Ca within that cell type (Conn and Gilliham, 2010). A cell-specific microarray comparing Arabidopsis epidermal (Ca-poor) and mesophyll (Ca-rich) transcriptomes uncovered three candidate vacuolar Ca transporters with preferentially expression in the mesophyll: CAX1, ACA4, and ACA11. A previous study that compared epidermal and mesophyll transcriptomes from Arabidopsis using SiCSA and an EST-based microarray only detected 5% of the genes we detected as differentially expressed (Brandt et al., 2002). Of the 120 genes described by Brandt et al. (2002), the chloroplastic ACA1 was the only differentially expressed Ca2+ transporter. However, two studies using Arabidopsis mesophyll protoplast preparations, a microarray by Leonhardt et al. (2004), and proteomic analyses of vacuolar preparations (Carter et al., 2004) detected the three vacuolar Ca2+ transporters we isolated. Although Leonhardt et al. (2004) compared transcript abundance between guard cells and mesophyll cells, the transcript abundance of these transporters between the leaf epidermis and mesophyll has not been compared previously. Significant advances yielded by our approach include increased sensitivity and specificity over Brandt et al. (2002) through use of linear RNA amplification and oligonucleotide arrays and elimination of nonuniform transcriptional changes induced by protoplasting and transcriptional inhibitors (Yang et al., 2008).
We also focused our bioinformatic analyses on the transcript abundance of known and putative Pi and K+ transporters. We found two candidates that differed in transcript abundance for Pi transporters (PHT4.4 and PHO1-H8) and one misexpressed K+ efflux transporter (KEA1) (see Supplemental Tables 4 and 5 online). All three transcripts were more highly expressed in the mesophyll; however, PHT4.4 has previously been localized to the chloroplast (Roth et al., 2004), and PHO1-H8 and KEA1 are predicted to be targeted to the mitochondria and chloroplast, respectively (SUBA database) (Heazlewood et al., 2007). Further support for the role of KEA1 in chloroplasts comes from its closest two homologs in barley also being highly expressed in the mesophyll (Richardson et al., 2007). Therefore, none of these candidates are likely to encode a transporter that directly facilitates epidermal enrichment of vacuolar [P] or [K]. Accordingly, we concentrated on the mechanisms that underpin Ca storage.
CAX1 Has a Primary Role in Ca Accumulation in the Leaf Mesophyll
CAX1 was the most abundant and differentially expressed Ca2+ transporter between epidermal and mesophyll cells (Figures 2A and 2B). A role for the tonoplast localized CAX1 in Ca2+ transport has been demonstrated by heterologous expression in yeast and other plant species (Hirschi et al., 1996; Hirschi, 1999; Cheng et al., 2003, 2005; Zhao et al., 2009). However, its role in cell-specific Ca storage has not been examined until now. In addition to CAX1, other vacuolar Ca2+ transporters, notably, ACA4 and ACA11, were preferentially expressed in the mesophyll of Arabidopsis leaves (Figure 2A), but a metadata analysis of publicly available ionomic and expression data from various Arabidopsis ecotypes revealed a positive correlation for Ca accumulation with only CAX1 expression (Figure 2D). Furthermore, when compared with other plant tissues, CAX1 has greatest expression in the leaf, whereas CAX3 is more abundant in roots, and both ACA4 and ACA11 have comparable expression elsewhere in the plant (Geisler et al., 2000a, Cheng et al., 2005; Lee et al., 2007). Taken together, these observations highlight the importance of CAX1 as the dominant mechanism in Ca accumulation in the leaf. However, disruption of CAX transporter expression revealed considerable complexity in the relationship between these and other Ca2+ transporters (Figures 5 and 6).
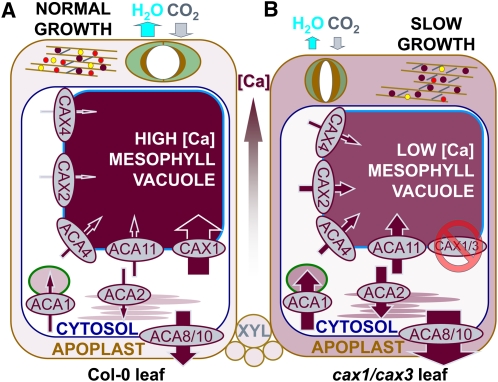
Figure 6.
Proposed Model for Control of [Ca2+]apo in Col-0 and Consequences of a Perturbed Ability to Secrete Ca2+ from the Apoplast into Mesophyll Vacuoles in cax1/cax3.
Size of arrows indicates proposed flux through transporters as extrapolated from transcript abundance and phenotypes of plants.
(A) In Col-0, free [Ca2+]apo is maintained at a low enough concentration so as not to close stomata; gas exchange rates are high, and growth rate is normal.
(B) When CAX1 and CAX3 are not present on the tonoplast and mesophyll cells, the transcription of other tonoplast transporters (ACA11, CAX2, and CAX4) increases, but the ability to accumulate as high [Ca]vac is compromised. As a result, (1) stomatal apertures and gas exchange decrease; (2) transcription of nontonoplast transporters increases, potentially increasing the Ca2+ flux into, and [Ca] within, the chloroplast/plastid (ACA1), ER (ACA2), and apoplast (ACA8 and ACA10); (3) cell wall components and xyloglucan (gray lines) increase between cellulose microfibrils, resulting in a thicker cell walls. In addition, Ca-pectate (purple circles) and PME increase and PGA (yellow circles) and expansins (red circles) decrease, resulting in less extensible cell walls and smaller cells. [K] increases in cax1/cax3 vacuoles to compensate for the reduction in [Ca2+] at the expense of epidermal [K+]vac through an unknown mechanism.
CAX1 Is Important in Controlling Apoplastic [Ca2+], Stomatal Aperture, and Growth
Increases in [Ca2+]apo regulate stomatal aperture (De Silva et al., 1996b; Webb et al., 2001); therefore, it is possible that the growth limitation in cax1/cax3 (Figure 3D) could be due to excessive [Ca2+]apo limiting CO2 uptake through the stomatal pores. Consistent with this hypothesis, leaf conductance, CO2 assimilation, stomatal apertures, and growth rate were lower in cax1/cax3 grown in BNS when compared with the Col-0 wild-type backgrounds (Figures 3B to 3D). Furthermore, gas exchange rates and growth could be brought to nearer wild-type levels when plants were grown in a solution with low Ca activity (LCS) (Figures 3B and 3D), a treatment that equalized [Ca]apo between Col-0 and cax1/cax3 (Figure 3A). In both the mutant and wild type, decreasing [Ca]apo by treatment with EGTA resulted in stomatal opening matching the recovery of gas exchange and growth in the mutant. This result indicates that cax1/cax3 stomata are capable of responding to decreases in [Ca2+]apo, but it is the elevated [Ca2+]apo in cax1/cax3 that reduces stomatal conductance, photosynthetic capacity, and growth rate.
Control of Apoplastic Ca Is Important in Determining Cell Wall Architecture
Modifications in cell wall architecture are linked to alterations in vegetative growth of plants with stronger and thicker walls predominantly resulting in reduced cell expansion (Cosgrove, 2005; Derbyshire et al., 2007a, 2007b; Douchiche et al., 2010); this can be seen in cax1/cax3 (Figures 4A and 4B; see Supplemental Figure 9 online). These characteristics may also contribute to the perturbed stomatal phenotype, as guard cell movements are influenced by variations in mechanical advantage of adjoining cells, which would result from changes in thickness and composition of their own and adjoining cell walls (DeMichele and Sharpe, 1973; Thompson, 2008).
The stronger, thicker cell walls of cax1/cax3 are associated with higher free [Ca]apo. In LCS, cax1/cax3 demonstrate a conditional suppression of the slower growth phenotype seen in BNS, concomitant with a lower free [Ca]apo, greater leaf extensibility, and reduced wall thickness (Figures 3A, 3D, and 4). Furthermore, a suite of cell wall–modifying proteins (including CESA, Csl, EXP, and PGA) have altered transcript abundance in cax1/cax3 plants in BNS, but most transcripts are similar to Col-0 in BNS when cax1/cax3 plants are moved to LCS. These results in general agree with those of the CoMPP assay to detect abundance of pectin, cellulose, and xyloglucan in both conditions.
HGA has a conformational flexibility that responds to growth, development, and environmental cues (Willats et al., 2001). Demethylesterifed HGA through the action of PMEs binds Ca2+ to form Ca pectate that increases the rigidity of the cell wall and its resistance to degradation (van Cutsem and Messiaen, 1996). Those PMEs in Arabidopsis with greatest expression in the leaf, PMEPCRB, PME1, and PME2 (Pelloux et al., 2007), were all more abundant in cax1/cax3 grown in BNS than in Col-0 (Figure 4C). Higher expression of these PME genes correlated with an increase in low methyl-esterified HGA, a greater amount of Ca2+ in the cell wall available to bind pectate, thicker cell walls, lower leaf extensibility, and lower growth rate (Figures 3D and 4A to 4C). Increases in HGA have previously been observed within the hypocotyls of mung bean (Vigna radiata) associated with high [Ca2+]apo and flax (Linum usitatissimum) in response to high concentrations of cadmium (0.5 mM) (Liberman et al., 1999; Douchiche et al., 2010) but not previously in leaves. The increased growth of cax1/cax3 increased in LCS correlated with lower transcript abundance of PMEPCRB, PME1, and PME2, along with reduced wall thickness, a decrease in low methyl-esterified HGA, lower [Ca]apo, and increased leaf extensibility (Figure 4C). The other two PMEs analyzed by qPCR, PME3 and PMEPCRD, had lower expression in cax1/cax3 grown in BNS and did not recover to Col-0 levels with LCS treatment (Figure 4C). While these two PMEs are expressed in the leaf, they are expressed more highly in the petal and silique, respectively (Pelloux et al., 2007), which may contribute to the lower fertility and seed yield cax1/cax3 (Cheng et al., 2005) and not leaf extensibility.
Numerous proteins are known to interact with PMEs, or modify their action, including PGAs, PME inhibitors, pectin acetylesterases, and pectate lyases (Cosgrove, 2005). PMEs and PGAs are known to act in concert to weaken cell walls as low methyl-esterified HGA is highly susceptible to PGA-mediated degradation (Micheli, 2001). In support of the greater proportion of low methyl-esterified HGA in the cax1/cax3 plant, we see 10 of the 11 leaf-expressed Arabidopsis PGAs are lower in abundance than in Col-0 (see Supplemental Table 6 online). PGA3, analyzed by qPCR, increased in abundance (above that of Col-0) when cax1/cax3 plants were grown in LCS (Figure 4C), which correlates with the reduction in low methyl-esterified HGA and greater cell wall extensibility in LCS-treated cax1/cax3 plants (Figures 4A, 4C, and 4D). In cax1/cax3 plants grown in BNS, there was a lower abundance of three putative PME inhibitor proteins (see Supplemental Table 6 online) (Lionetti et al., 2007). Of the pectin acetylesterases expressed in the leaf, which are predicted to soften cell walls (Vercauteren et al., 2002), 4 of 10 are lower in expression in cax1/cax3 in BNS. Of the putative pectate lyase transcripts known to catalyze cleavage of deesterified pectin, 9 of 20 were also reduced (Marín-Rodríguez et al., 2002) in the cax1/cax3 leaf (see Supplemental Table 6 online). This could signify a higher degree of cross-linking of the pectic network and hence result in cell wall strengthening (Marín-Rodríguez et al., 2002).
Despite changes in the transcript abundance for members of both the XTH and CESA/Csl families, there was no significant change in the cell wall composition directly linked with these biosynthetic processes. However, XTH22, a transcript rapidly induced by mechanical stimulation or wounding, is thought to play a role in cell wall strengthening or stiffening as opposed to cell expansion (Braam, 2005); thus, its expression being inversely correlated with cell growth and leaf extensibility in cax1/cax3 reinforces this predicted role. β-(1→4)-Linked galactan, the branching molecule of RG-1, has been predicted to aid hydration and elongation of cell walls (McCartney et al., 2003), but its unique enrichment in cellulosic fractions in cax1/cax3 grown in BNS but not LCS suggests otherwise. Endogenous EXPAs have also been strongly implicated in regulation of vegetative growth (McQueen-Mason et al., 1992; Cho and Cosgrove, 2000; Pien et al., 2001). Redundancy within this family is thought to result in most single T-DNA insertion lines for expansins not displaying an obvious growth phenotype (Cosgrove et al., 2002). However, the antisense regulation of EXPA10 resulted in the alteration of leaf growth and pedicel abscission (Cho and Cosgrove, 2000). Within the cax1/cax3 plant, multiple EXPAs are downregulated, potentially overcoming any familial degeneracy and limiting flexibility (see Supplemental Table 6 online). When cax1/cax3 is grown in LCS, the expression of two members within this family returns to that of wild-type Col-0 along with the growth rate (Figures 3D and 4F). The rapid increase in growth of cax1/cax3 in LCS also correlates with large increases in expression of key cytoskeletal genes, including Actin2 (Figure 4C).
The Requirement for Ca Compartmentation away from the Apoplast Has Consequences on Where Other Nutrients Are Stored
In cax1/cax3 mesophyll, [Ca]vac was lower compared with Col-0, but [K]vac was greater than the Col-0 wild type (Figure 2D). Such a reciprocal and inverse relationship between K and Ca accumulation has also been observed in barley when K-depleted, Ca accumulates to much greater levels in the epidermis than under K-replete conditions (Leigh et al., 1986; Karley et al., 2000a). We interpret the reciprocal K and Ca storage within particular cell types to have a significant role in osmotic/ionic/charge balance and will be important if cells continue to increase in size during fluctuations in Ca supply.
No such reciprocal relationship exists for Ca and P; in fact, the opposite is true where Ca and P are usually stored in different cell types to prevent precipitation (Dietz et al., 1992; Karley et al., 2000b; Conn and Gilliham, 2010). While we found a similar increase in [P] of cax1/cax3 leaves as per Cheng et al. (2005), we showed this was not a result of vacuolar accumulation. Furthermore, whole leaf [P] was returned to Col-0 levels under LCS treatment (see Supplemental Table 1 online), which may indicate that cytosolic P sequestration may be higher in cax1/cax3 plants, perhaps as a result of the need to separate apoplastic Ca and P.
Model for Cellular and Apoplastic Ca Homeostasis
While the hypothesis by De Silva et al. (1996a) implicated trichomes as important drivers for buffering fluctuations in [Ca]apo, a meta-analysis across various Arabidopsis ecotypes showed no correlation between trichome density and leaf [Ca] (see Supplemental Figure 6A online). The role of the mesophyll, at least in Arabidopsis, therefore appears greater in buffering fluctuations in [Ca]apo than that of trichomes under these conditions.
Despite a reduction in the amount of Ca stored in cax1/cax3 mesophyll, there is still a preferential accumulation of Ca2+ into mesophyll vacuoles (Figures 2D to 2F). The activity of other Ca2+ transporters appears to functionally compensate for absence of CAX1, as was previously perceived in cax1-1 where residual (~40%) root tonoplast Ca2+/H+ antiporter activity was detected and attributed in the main to the increase in CAX3 transcript abundance (Cheng et al., 2003, 2005). In cax1/cax3, two other tonoplast-localized Ca2+/H+ antiporters, CAX2 and CAX4 (Cheng et al., 2002; Pittman et al., 2005), may contribute to mesophyll Ca2+ accumulation, with an increase in CAX4 mRNA previously detected through RNA gel blot analysis in cax1/cax3 (Cheng et al., 2005). While in cax1/cax3, CAX4 expression was higher than in Col-0 plants, CAX2 may play a more significant role, as it is 20-fold more abundant than CAX4 (Figure 5). Intriguingly, both CAX2 and CAX4 have a higher transport affinity for Mn than both CAX1 and CAX3 (Schaaf et al., 2002; Korenkov et al., 2007), and the Mn content of cax1/cax3 was 50% higher than in Col-0 (Cheng et al., 2005), suggesting that CAX2 and/or CAX4 activity are greater in cax1/cax3 than Col-0.
In the face of a greater [Ca]apo and the reduced ability of the mesophyll to secrete Ca2+, the transcript abundance of leaf-expressed ACA genes also alters in cax1/cax3. In cax1-1, a 36% increase in vacuolar Ca2+-ATPases activity compared with wild-type Col-0 (Cheng et al., 2003) correlates with the greater abundance of the tonoplast-localized ACA4 and ACA11. Interestingly, ACA4 is not more abundant in cax1/cax3 leaf in BNS compared with Col-0; by contrast, ACA11 is threefold more abundant than in Col-0 (Figure 5). ACA4 is localized on the prevacuolar compartment and so is unlikely to have a significant role in Ca2+ secretion to the main storage vacuole unlike ACA11 (Geisler et al., 2000b; Lee et al., 2007). Compared with cax1/cax3, simultaneous knockout of ACA4 and ACA11 does not appear to reveal a phenotype associated with perturbed apoplastic Ca2+ homeostasis under the conditions tested; however, this does not rule out a signaling role for these Ca2+-ATPases during stress events. In fact, the role of ACA4 and ACA11 is the focus of a recent study indicating a role in salicylic acid signaling (Boursiac et al., 2010). Further to that study, we can independently confirm that there is no transpiration or mesophyll [Ca] phenotype in aca4/aca11 mutants despite both transporters usually being highly expressed in the mesophyll.
Expression of other ACA genes is also altered in cax1/cax3 plants, suggesting excess Ca2+ may be sequestered into the ER (ACA2) or the chloroplast/plastid/ER (ACA1) (Figure 5; see Supplemental Figure 5C online). Therefore, it is possible the reduction in photosynthesis in cax1/cax3 may result from increased chloroplast [Ca2+] through increased activity of ACA1. In addition, there is an increase in transcript abundance of the plasma membrane–localized Ca2+ transporters ACA8 and ACA10 (Bonza et al., 2000; George et al., 2008), which may result in greater Ca2+ efflux into the apoplast and a greater [Ca2+]apo, as was observed in cax1/cax3. These changes in transcript profile within cax1/cax3 leaves, and the phenotypes observed, are consistent with a dominant [Ca2+]cyt homeostasis mechanism within the leaf that diverts Ca2+ into other compartments when capacity for secretion into the vacuole is compromised. A model of how this could be achieved and the downstream consequences are summarized in Figure 6.
Previous studies have highlighted CAX1 as a driver of Ca2+ accumulation into leaf tissue (Cheng et al., 2003); CAX1 has also been implicated in increased freezing tolerance after cold acclimation (Catala et al., 2003), delayed germination on sucrose, increased sensitivity of germination to abscisic acid, tolerance to ethylene with respect to germination, inhibition of hypocotyl elongation, and delayed flowering (Cheng et al., 2003, 2005). Some of these phenotypes may be related to an altered [Ca2+]apo profile or Ca2+ homeostasis in different compartments of the leaf, and through our study, we are able to build up an integrative Ca2+ transport model for the leaf that can provide a basis for further interrogation. Cell-specific storage of Ca2+ is essential in plants to regulate [Ca2+]apo to optimize cell wall expansion, photosynthesis, transpiration, and plant productivity.
METHODS
General Methods, Plant Materials, and Growth Conditions
Experiments were conducted over a 4-year period and replicated multiple times. Refer to appropriate figure legends or the main text for the number of replicates for each experiment. When statistical tests were performed, significance was determined according to Student’s t test or analysis of variance (ANOVA) using Excel software (Microsoft) and is indicated in the figure legends or main text. All chemicals were obtained from Sigma-Aldrich unless stated.
Arabidopsis thaliana T-DNA insertional loss-of-function mutants cax1-1, cax3-1, cax1-1/cax3-1 (cax1/cax3) (Cheng et al., 2005), aca4-3, aca11-5, aca4-3/aca11-5 (Boursiac et al., 2010), GAL4-VP16UAS-GFP enhancer trap line KC464 with epidermal-specific GAL/GFP expression (Gardner et al., 2009), and the wild type, all of ecotype background Col-0, when grown were arranged randomly in a 6 × 8 format hydroponic tank in constantly aerated BNS [in mM, aCa = 1; 2 NH4NO3, 3 KNO3, 0.1 CaCl2, 2 KCl, 2 Ca(NO3)2, 2 MgSO4, 0.6 KH2PO4, and 1.5 NaCl, with micronutrients; in μM, 50 NaFe(III)EDTA, 50 H3BO3, 5 MnCl2, 10 ZnSO4, 0.5 CuSO4, and 0.1 Na2MoO3, pH 5.6, NaOH]. When stated, plants were transferred to a low Ca nutrient solution with other nutrients adjusted to keep the same activity except Cl− [LCS, aCa = 25 μM; in mM unless stated, 2 NH4NO3, 5 KNO3, 50 μM CaCl2, 1.8 MgSO4, 0.6 KH2PO4, 0.4 NaCl, 1.2 NaNO3, and 0.2 Mg(NO3)2, with micronutrients (as above), pH 5.6, NaOH]. Ion activities in solutions were calculated with Visual Minteq software, version 2.52 (KTH). All plants were grown under a 9-h-light/15-h-dark photoperiod at an irradiance of 150 μmol photons m−2 s−1 and at 22°C.
Single-Cell Sampling and Cryogenic Scanning Electron Microscopy/XRMA
SiCSA and single-cell RNA extraction using micropipettes have been described elsewhere (Tomos et al., 1994; Brandt et al., 2002; Roy et al., 2008). All samples were taken from cells situated in the middle of leaf 8 of 3.5- to 8-week-old Arabidopsis plants. Elemental concentration was measured using XRMA as described by Tomos et al. (1994) with sample grids receiving an additional 1-nm Cr plasma coating to prevent damage to the 1% Pioloform membrane. Analysis was performed with an EDAX Genesis XM4 (EDAX International) attached to a Philips XL30 field emission scanning electron microscope (FEI Company). Accelerating voltage was 14 keV, with beam spot size 4 (current 0.61 nA), and data acquisition was continued until 5000 Rb counts were collected. Peak heights for individual elements were normalized against the Rb peak and concentration calculated by interpolation from a simultaneously collected standard curve for each element (see Supplemental Figure 1 and Supplemental Table 7 online; Tomos et al., 1994).
For XRMA of frozen-hydrated leaf samples, a 4 × 10-mm section from the mid-region of leaf 8 of 5- to 8-week-old Arabidopsis was dissected, mounted in deionized water on a brass stub in under 10 s, and then snap frozen in a liquid N2 slush. The stub was transferred under vacuum to a CT1500 HF cryotransfer stage (Oxford Instruments) attached to the above scanning electron microscope, where samples were cryoplaned flat using a microtome blade within the stage housing. The samples were etched for 45 s at −92°C. Samples were recooled to –120°C and sputter coated with Ni for 2.5 min at 10 mV, giving a thickness of 4 nm. XRMA spectra were recorded at a voltage of 15 keV, spot size 4 (current 0.64 nA) for 180 live seconds (800 to 2000 cps) at a working distance of 10 mm. All XRMA spectra were analyzed with EDAX eDXi software and converted from peak over background ratios to concentration values using calibration standards (see Supplemental Figure 1 online). All XRMA data were processed using an automated algorithm, XRMAplot (Australian Centre for Plant Functional Genomics). Total leaf elemental content was measured by ICP as per Cheng et al. (2005).
Strontium Feeding Assays
Leaves 8- of 6-week-old plants were excised under deionized water and the petioles inserted into an artificial sap solution (AS) within a 0.5-mL microcentrifuge tube and each petiole recut using small dissecting scissors to avoid cavitation within the xylem. AS contained (in mM) 1 K2HPO4, 1 KH2PO4, 1 CaCl2, 0.1 MgSO4, 1 KNO3, and 0.1 MnSO4 plus 50 mM SrCl2. Parafilm (Pechiney Plastic Packing Company) was used to seal the leaf in the tube and prevent evaporation. Leaf discs were also taken from 5-week-old leaves of Col-0 and cax1/cax3 plants, vacuum infiltrated with AS + SrCl2 for 30 min, and incubated under light for 4 h.
SiCSA RNA Isolation and Amplification for Microarrays
To compare epidermal and mesophyll transcriptomes and differentiate between the homologous members of ion transporter families, we extracted RNA from individual epidermal and mesophyll cells of unfixed Arabidopsis plants using a modification of the SiCSA technique (Roy et al. 2008), with variations as described below. Prior to comparing the transcriptomes of single-cell types, we verified that amplification did not introduce intolerable bias and that it was possible to sample free of contamination from other cell types (see Supplemental Figure 2A online). The use of plants with cell-specific expression of GAL4 and mGFP5-ER within the epidermis (enhancer trap line KC464) was an important part of this strategy (see Supplemental Figure 2B online; Haseloff, 1999; Roy et al., 2008; Gardner et al., 2009). As such, we also verified that there was no difference in the elemental accumulation pattern within KC464 compared with its parental background Col-0 (see Supplemental Table 1 online).
To confirm that very small amounts of RNA, typical of the amount extracted from individual cells, could be reliably amplified, total leaf RNA was diluted and 10 pg of this dilution was used as the template for test amplification. Assuming 5% poly(A) mRNA content per cell extract, the amplification starting material was 0.2 pg mRNA (Livesey, 2003). The expression of a number of genes in the amplified RNA (aRNA) was compared with that from undiluted, unamplified RNA. The results showed that the amplification method gave comparable results between the two samples except that genes with an expression level below 1 copy pg−1 RNA (or ~10 copies per cell) were underrepresented in the aRNA sample (see Supplemental Figure 2A online). Similar trends have been previously reported (Schneider et al., 2004). In an attempt to overcome this, subsequent experiments using amplification were performed on pooled samples from 30 epidermal or three mesophyll cells from each plant. However, it is acknowledged that lowly abundant, but key, transcripts may still be missed, and the subsequent microarray analysis can only be used as a screen for transcripts above this detection limit.
To control against contamination, certain transcripts were monitored in all amplified samples using RT-PCR and qPCR (see Supplemental Table 2 online). Mesophyll cell samples were deemed free of contamination from overlying epidermal cells when transcripts for GFP, GAL4, LTP1, and CUT1 were absent. Epidermal cell samples were used if CA1 and RBCS-3b were absent and all epidermal-specific transcripts present. Additionally, it was a requirement that housekeeping genes β-tubulin-5, EF1α, and Actin2 were detected and showed similar expression levels in both cell types if samples were to be further analyzed. Samples, such as those shown in Supplemental Figure 2C online, passing these quality control tests were further processed for microarray analysis.
RNA was isolated from specific cell types of Arabidopsis enhancer trap line KC464 as per Roy et al. (2008) with the following modifications. Cellular contents used in the microarray screen were extracted from the mesophyll or epidermis of three plants each grown in separate hydroponic tanks. Mesophyll samples consisted of the contents from three mesophyll cells, sampled using three different capillaries, and then pooled into one sample, while 30 epidermal cells were sampled with a single capillary to constitute an epidermal sample. Mesophyll or epidermal samples were expelled into 10 units of RNaseOUT (Invitrogen) in 3 μL of diethylpyrocarbonate-treated water and amplified using the TargetAMP 2-round aRNA amplification kit (EpiCentre Biotechnologies). In addition, another three mesophyll and epidermal samples were taken and amplified as stated above from a plant grown in the same conditions to allow for an independent verification of transcript abundance by qPCR. RNA was quantified with a nanodrop ND-1000 (Thermo Scientific), and 100 ng was reverse transcribed with random hexamers using Superscript II reverse transcriptase (Invitrogen). RT-PCR was used to screen for genomic DNA and cellular contamination: epidermal contamination was screened by the use of mGFP5, GAL4, CUT1, and LTP1, and mesophyll contamination was screened using RBCS-3b and CA1 (see Supplemental Table 2 online for list of primers). RNA size and quality was assessed after RNA amplification using an Agilent Bioanalyzer 2100 RNA picochip (Agilent Technologies), with only samples possessing an average cRNA size >500 nucleotides passing to the next round of analysis. aRNA was labeled with either Cy3 or Cy5 using the Kreatech ULS aRNA labeling kit according to the manufacturer’s instructions for Agilent arrays (Kreatech Diagnostics). Four arrays were performed with array #1 from plant 1 [labeled: epidermis (E)-Cy3 and mesophyll (M)-Cy5); array #2 from plant 2 (E-Cy5 and M-Cy3); array #3 from plant 3 (E-Cy3 and M-Cy5), and array #4 plant 2 and plant 1 (M-Cy3 and M-Cy5)] as a technical replicate. The degree of labeling was calculated using a Nanodrop 1000 prior to fragmentation. Bioanalyzer analysis was performed on pre- and postfragmented labeled aRNA to confirm correct size profiles.
Custom 4 × 44 k Agilent DNA microarrays designed using eArray v.5.3.1 (Agilent Technologies) incorporated the entire Arabidopsis 3 oligo gene list. In addition, user-defined probes for both mGFP5 and GAL4-VP16 and duplicates of known leaf-expressed ion transporters (Design ID: 017700) were used in this study. Cy3- and Cy5-labeled aRNA (825 ng) was hybridized to each array, washed, and scanned as per the manufacturer’s instructions for two-color arrays and imaged on an Agilent Microarray Scanner in XDR mode. Image files were processed, and within-slide normalization was performed using Agilent’s feature extraction software (version 9.5.3.1; Agilent Technologies). Additional between-slide normalization was not undertaken because this would be compromised by the expected stochastic related fluctuations in gene expression within individual cells (Elowitz et al., 2002). Instead, a list of putatively differently expressed genes was prepared by splitting transcripts into groups that were consistently expressed at very high levels [log2(expression) > 13.3] in one cell type while being consistently expressed at below background levels [log2(expression) < 8.0] in the other cell type. Hybridized arrays were omitted from analysis if they contained control genes from both an epidermal and mesophyll origin (see Supplemental Table 2 online for list of control genes).
Laser Microdissection
Arabidopsis plants (Col-0) were grown in BNS hydroponics for 5 weeks and then transferred to a high calcium solution (aCa = 5 mM) for 18 h. Three-millimeter transverse sections of leaf number 8 from three independent plants were prepared for laser microdissection as per Inada and Wildermuth (2005). After allowing paraffinized leaf samples to solidify at room temperature, 7-μm transverse sections were cut on a Leica RM2265 microtome (Leica) and deparaffinized by two 10-min xylene incubations and dried on a 42°C heating block to remove residual xylene. Epidermal and mesophyll sections were isolated using a Leica AS LMD microscope and sections captured within PCR tube caps. Approximately 800 epidermal and 3000 mesophyll cells were sampled using three representative cross sections from three different plants. RNA was extracted using the RNAqueous-Micro kit (Ambion) and DNase treated with Turbo DNA-free (Ambion) and underwent two rounds of Eberwine RNA amplification using the MessageAmp II aRNA amplification kit (Ambion) according to the manufacturer’s instructions. The size, integrity, and concentration of the RNA was analyzed using Bioanalyzer (Agilent) with the Total RNA pico chip. aRNA was reverse transcribed using SuperScript II reverse transcriptase (Life Technologies) and 50 ng random hexamers according to the manufacturer’s instructions and cDNA interrogated by qPCR analysis as detailed below.
Whole-Plant Microarray Analysis
Wild-type (Col-0 ecotype) and cax1/cax3 seeds were surfaced sterilized, germinated on 0.5× Murashige and Skoog media supplemented with 0.5% sucrose, and grown for 10 d (16 h day/8 h night) at an irradiance of 150 μmol photons m−2 s−1 and at 22°C. Eighteen 10-d-old seedlings from each genotype were then transferred into soil (Sunshine Mix) with one plant from each genotype grown in the same pot. In total, 18 pots were grown for 3 weeks under the same conditions as described above until the cax1/cax3 plants started to display necrotic lesions (Cheng et al., 2005). Plants were collected and soil removed with care to maintain root integrity. Six whole plants from each genotype were pooled as one sample in triplicate for each genotype and were frozen in liquid N2 and stored at −80°C before use. Total RNA was isolated using an RNeasy mini kit (Qiagen) following the manufacturer’s instructions. RNA sample quality was monitored using an Agilent 2100 bioanalyzer. Three microarrays for each genotype were performed at Baylor College of Medicine Microarray Core Facility using the GeneChip Arabidopsis ATH1 genome array (Affymetrix).
Signal intensities were imported from the CEL files into R using Bioconductor packages (http://www.bioconductor.org). The Robust Multichip Average method was used for background correction, quantile normalization, and summarization of probe signal intensities (Irizarry et al., 2003). Differentially expressed genes were identified at P < 0.05 with an empirical Bayes t test (Smyth, 2004) using false discovery rate for multiple testing correction (Benjamini and Hochberg, 1995). Functional annotation of the genes represented by the probe sets was obtained from the Affymetrix website (https://www.affymetrix.com/support/technical/annotationfilesmain.affx).
Real-Time qPCR
RNA was extracted from leaves of 5- or 6-week-old Arabidopsis plants using the SV Total RNA extraction kit with on-column DNase treatment (Promega). Reverse transcription and qPCR were performed using IQ SYBR Green PCR reagent (Bio-Rad Laboratories) and an RG 6000 Rotor-Gene real-time thermal cycler (Corbett Research) essentially as per Burton et al. (2008), with the following modifications. Normalization was performed using control genes Actin2, β-Tubulin-5, EF1α, and GAPDH-A for each experiment (see Supplemental Table 2 online) and the final concentrations of mRNAs of genes of interest expressed as arbitrary units that represent the numbers of copies per 30 ng of cDNA from total RNA (or amplified equivalent assuming 5% mRNA component), normalized against the geometric means of three control genes that vary the least with respect to each other, using geNORM software, version 3.5 (Vandesompele et al., 2002).
Apoplastic Washes
Apoplastic washes on Col-0, aca4/aca11, and cax1/cax3 were performed as per Lohaus et al. (2001) using 250 mM sorbitol and 100 mM BaCl2. Known volumes of apoplastic wash fluid (neat and 10× concentrated by vacuum concentration) was placed onto pioloform-coated microscope grids using a micropipette and analyzed by XRMA as described above. Leaf air volume (Vair) was calculated for all plants by infiltration of high-viscosity silicon oil and used to correct for apoplast concentrations according to Lohaus et al. (2001). Calculation of bound Ca (barium extracted Ca; BeCa) was obtained from subtracting the [Ca] in the sorbitol extract (SeCa) from the BaCl2 exudate (a bound apoplastic Ca fraction).
Xylem Sap Analysis
Arabidopsis Col-0 and cax1/cax3 plants were placed into a vacuum chamber with roots immersed in BNS. Shoots were excised by cutting the hypocotyl immediately below the cotyledons and a pressure of ~15 mm Hg applied to encourage xylem flow. Initial xylem sap was absorbed for 2 min using Whatmann filter paper (No. 1), and samples (2 to 5 μL volume) were subsequently collected from the excised face after 5 min of flow. A subsample underwent vacuum concentration with subsequent analysis of samples by XRMA. Analysis was performed on three plants per line, with the experiment run in duplicate.
Gas Exchange Measurements
Gas exchange measurements were performed on leaf 8 of 5- to 8-week-old plants using a LI-6400 infrared gas exchange analyzer (LiCOR), fitted with a 6400-15 extended reach 1-cm chamber or Arabidopsis whole-plant chamber (as stated), according to the manufacturer’s instructions. Chamber reference CO2 was set at 400 ppm, relative humidity at 50 to 56%, leaf temperature was ~21°C, light intensity was 350 μmol·m−2·s−1, and flow rate was 100 μmol s−1. Leaf area was calculated by analysis of digital photographs of leaves held within the leaf chamber using ImageJ (National Institutes of Health).
Stomatal Aperture Measurements
Col-0 and cax1/cax3 seedlings were grown on 0.8% agar (Bacto) plates with 0.5× Murashige and Skoog medium (Sigma-Aldrich) for 23 d, and epidermal fragments were obtained as described by Dodd et al. (2006). Epidermal fragments were transferred to 10 mM MES-KOH buffer, pH 6.2, in the dark for 1 h at 20°C to close the stomata. At staggered 10-min intervals, with one interval per treatment to allow for time to complete measurements, homogenate from each line was transferred into deep 5-cm Petri dishes in 10 mL of DS, 5 mM KCl, 10 mM MES-KOH, pH 6.2, or DS plus Ca2+ or EGTA (±1 mM CaCl2 or 2 mM EGTA, as stated) into an inverted light box at 20°C and aerated with CO2 free air for 3 h. Afterwards, a drop of solution was placed on a microscope slide and covered with a cover slip, and the width and length of stomatal pores were measured as described by Dodd et al. (2006). Stomatal apertures on one to three epidermal strips were measured per interval.
Cell Wall Strength Assay
Extensibility of Col-0, cax1/cax3, and aca4-aca11 leaves (number 7-11) grown in BNS, LCS, or RBNS (see above) was tested using a 5543 Materials Testing Instrument (Instron) following the manufacturer’s instructions. Replicate leaves from each line were held in pneumatic grips set at a distance of 5 mm and pulled apart at a constant anvil rate of 60 mm·min−1.The mean tensile extension at automatic break (in millimeters) and the distance the anvil travels before a significant drop in maximum load for each line was compared and normalized against the value for Col-0 in BNS.
Cell Density and Cell Wall Thickness Measurements
Sections were cut from leaf number 8 of 6-week-old Col-0 and cax1/cax3 plants fixed in 4% paraformaldehyde and 0.25% glutaraldehyde, dehydrated by graduated ethanol (30, 50, 70, 90, 95, and 100%), and embedded in LR White. One-micrometer-thick transverse sections were stained with toluidine blue and observed on a Zeiss AxioPhot D-7082 light microscope with images captured by a Nikon DXM1200F digital camera with ACT1 imaging software (Nikon). Cell density was measured as the number of whole mesophyll cells within a single field of view at ×20 magnification, encompassing a frame of 400 × 200 μm (length × height). Twenty frames per leaf (encompassing ~50 to 100 cells per frame) were enumerated from three independent replicate leaves (n = 60 frames). For TEM, ultrathin sections (90 nm) were poststained in uranyl acetate for 10 min and observed under a Philips CM100 TEM equipped with SIS MegaView II Image Capture running iTEM software (SIS Analysis). Cell wall thicknesses were measured by TEM at ×13,500 magnification by imaging sections between the junctions of three spongy mesophyll cells to be confident of sampling single cell walls. Average widths were obtained for 40 to 60 cell walls per treatment, with four measurements per wall section (n = 160 to 240).
Cell Wall Glycan Profiling
Leaves from Arabidopsis Col-0 and cax1/cax3 plants grown in either BNS or LCS were collected, snap-frozen, and freeze-dried. Cell wall components were sequentially extracted from this material and relatively quantified using CoMPP according to Moller et al. (2007). Samples consisted of three technical repeats for each genotype under each condition; a biological replicate was represented by pooled material from three plants. Molecular probes used for this analysis were CBM3a along with antibodies JIM5, JIM7, LM5, LM6, LM7, LM13, and LM15.
Accession Numbers
Sequence data for the major genes examined in this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: CAX1 (At2g38170), CAX3 (At3g51860), ACA4 (At2g41560), and ACA11 (At3g57330). Also see Supplemental Tables 2 to 6 online for accession numbers of all genes examined in this study. The raw data for the whole-plant microarray are available at the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE23820.
Author Contributions
B.N.K., M.G., R.A.L., and S.D.T. conceived the project and helped design experiments. S.J.C. performed all experiments except that M.G. performed cryoscanning electron microscopy and infrared gas exchange analyzer measurements, I.M. performed CoMPP, A.W.S. and U.B. analyzed microarray data, A.A.R.W. and M.A.S. provided guard cell aperture measurements, K.D.H. and N.-H.C. performed cax1/cax3 microarrays, A.A. performed LCM qPCR, R.B. advised on cell wall experiments, and M.G. and S.J.C. performed cell wall strength measurements and drafted the manuscript. All authors discussed results and made comments. S.D.T. and R.A.L. contributed equally to the supervision of this work.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. In Vitro Calibration Standard Curves for XRMA.
Supplemental Figure 2. Validation of SiCSA-Based RNA Sampling and Amplification for qPCR and Microarray Study Using the Enhancer Trap Line KC464.
Supplemental Figure 3. Summarization of Microarray Data, Including Gene Ontological Classification.
Supplemental Figure 4. Cell-Specific Transcript Abundance of Calcium Transporters in Laser Microdissected Epidermal and Mesophyll Leaf Cells of Arabidopsis.
Supplemental Figure 5. Calcium Accumulation and Transcript Abundance of Selected Ca2+ Transporters within Single T-DNA Insertion Lines of CAX1, CAX3, ACA4, and ACA11.
Supplemental Figure 6. Trichome Density in Arabidopsis Lines.
Supplemental Figure 7. Stomatal and Growth Phenotypes of aca4/aca11 Are Not Regulated by Apoplastic [Ca].
Supplemental Figure 8. Leaf Extensibility of aca4/aca11 and Col-0.
Supplemental Figure 9. Cell Density Measurements on cax1/cax3 Loss-of-Function Mutants.
Supplemental Figure 10. Analysis of Cell Wall Glycans from Col-0 and cax1/cax3 Plants.
Supplemental Table 1. Temporal Stability of Ion Concentrations in Epidermis, Mesophyll, and Whole Arabidopsis Leaves.
Supplemental Table 2. List of Primers Used in This Study.
Supplemental Table 3. Microarray Intensities from Epidermal/Mesophyll SiCSA Array for ACA, ECA, and CAX/CCX Gene Families.
Supplemental Table 4. Microarray Intensities from Epidermal/Mesophyll SiCSA Array for Potassium Transporter Gene Families.
Supplemental Table 5. Microarray Intensities from Epidermal/Mesophyll SiCSA Array for Phosphate Transporter Gene Families.
Supplemental Table 6. Microarray Intensities from Col-0 and cax1/cax3 T-DNA Insertion Line Array for Cell Wall–Related Transcripts.
Supplemental Table 7. Normalized Transcript Abundance for Cell Wall–Related Transcripts Analyzed by qPCR.
Supplementary Material
Acknowledgments
We thank the following (from the University of Adelaide unless stated): (for seeds) Jim Haseloff and Julian Hibberd (University of Cambridge, UK) for enhancer trap lines and Jeff Harper (University of Nevada) for aca T-DNA knockout lines; (for technical assistance), Daniele Belluoccio (Pacific Laboratory Products, SiCSA microarray); microscopy, John Terlet, Lyn Waterhouse, Peter Self, and Ken Neubauer (Adelaide Microscopy); SiCSA and confocal microscopy, Stuart Roy; Marilyn Henderson (TEM); qPCR analysis, Neil Shirley; LCM, Gwenda Mayo; infrared gas exchange analysis, Maclin Dayod; ICP, Waite Analytical Service; Jill Taylor, Instron; general, Sam Henderson. This work was supported by an Australian Research Council grant awarded to R.A.L., B.N.K., and S.D.T., an Australian Professorial Fellowship awarded to S.D.T., and University of Adelaide, Faculty of Science grants awarded to R.A.L. and M.G.
References
- Ager F.J., Ynsa M.D., Dominguez-Solis J.R., Lopez-Martin M.C., Gotor C., Romero L.C. (2003). Nuclear micro-probe analysis of Arabidopsis thaliana leaves. Nucl. Instrum. Methods Phys. Res. B 210: 401–406 [Google Scholar]
- Baxter I., Ouzzani M., Orcun S., Kennedy B., Jandhyala S.S., Salt D.E. (2007). Purdue ionomics information management system. An integrated functional genomics platform. Plant Physiol. 143: 600–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter I., Tchieu J., Sussman M.R., Boutry M., Palmgren M.G., Gribskov M., Harper J.F., Axelsen K.B. (2003). Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 132: 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - A practical and powerful approach to multiple testing. J. R. Stat. Soc., B 57: 289–300 [Google Scholar]
- Bolte S., Brown S., Satiat-Jeunemaitre B. (2004). The N-myristoylated Rab-GTPase m-Rabmc is involved in post-Golgi trafficking events to the lytic vacuole in plant cells. J. Cell Sci. 117: 943–954 [DOI] [PubMed] [Google Scholar]
- Bonza M.C., Morandini P., Luoni L., Geisler M., Palmgren M.G., De Michelis M.I. (2000). At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol. 123: 1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y., Lee S.M., Romanowsky S., Blank R., Sladek C., Chung W.S., Harper J.F. (2010). Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol. 154: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J. (2005). In touch: Plant responses to mechanical stimuli. New Phytol. 165: 373–389 [DOI] [PubMed] [Google Scholar]
- Brandt S., Kloska S., Altmann T., Kehr J. (2002). Using array hybridization to monitor gene expression at the single cell level. J. Exp. Bot. 53: 2315–2323 [DOI] [PubMed] [Google Scholar]
- Broadley M.R., Hammond J.P., White P.J., Salt D.E. (2010). An efficient procedure for normalizing ionomics data for Arabidopsis thaliana. New Phytol. 186: 270–274 [DOI] [PubMed] [Google Scholar]
- Burton R.A., Jobling S.A., Harvey A.J., Shirley N.J., Mather D.E., Bacic A., Fincher G.B. (2008). The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiol. 146: 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C., Pan S.Q., Zouhar J., Avila E.L., Girke T., Raikhel N.V. (2004). The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16: 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala R., Santos E., Alonso J.M., Ecker J.R., Martinez-Zapater J.M., Salinas J. (2003). Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 15: 2940–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N.-H., Pittman J.K., Barkla B.J., Shigaki T., Hirschi K.D. (2003). The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15: 347–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N.-H., Pittman J.K., Shigaki T., Hirschi K.D. (2002). Characterization of CAX4, an Arabidopsis H(+)/cation antiporter. Plant Physiol. 128: 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N.-H., Pittman J.K., Shigaki T., Lachmansingh J., LeClere S., Lahner B., Salt D.E., Hirschi K.D. (2005). Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 138: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.-T., Cosgrove D.J. (2000). Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 97: 9783–9788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson D.T. (1984). Calcium-transport between tissues and its distribution in the plant. Plant Cell Environ. 7: 449–456 [Google Scholar]
- Conn S., Gilliham M. (2010). Comparative physiology of elemental distributions in plants. Ann. Bot. (Lond.) 105: 1081–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. (2005). Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J., Li L.C., Cho H.-T., Hoffmann-Benning S., Moore R.C., Blecker D. (2002). The growing world of expansins. Plant Cell Physiol. 43: 1436–1444 [DOI] [PubMed] [Google Scholar]
- Dayod M., Tyerman S.D., Leigh R.A., Gilliham M. (2010). Calcium storage in plants and the implications for calcium biofortification. Protoplasma 247: 215–231 [DOI] [PubMed] [Google Scholar]
- DeMichele D.W., Sharpe P.J.H. (1973). An analysis of the mechanics of guard cell motion. J. Theor. Biol. 41: 77–96 [DOI] [PubMed] [Google Scholar]
- Derbyshire P., Findlay K., McCann M.C., Roberts K. (2007a). Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. J. Exp. Bot. 58: 2079–2089 [DOI] [PubMed] [Google Scholar]
- Derbyshire P., McCann M.C., Roberts K. (2007b). Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol. 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva D.L.R., Hetherington A.M., Mansfield T.A. (1996a). Where does all the calcium go? Evidence of an important regulatory role for trichomes in two calcicoles. Plant Cell Environ. 19: 880–886 [Google Scholar]
- De Silva D.L.R., Honour S.J., Mansfield T.A. (1996b). Estimations of apoplastic concentrations of K+ and Ca2+ in the vicinity of stomatal guard cells. New Phytol. 134: 463–469 [Google Scholar]
- Dietz K.J., Schramm M., Lang B., Lanzlschramm A., Durr C., Martinoia E. (1992). Characterization of the epidermis from barley primary leaves. 2. The role of the epidermis in ion compartmentation. Planta 187: 431–437 [DOI] [PubMed] [Google Scholar]
- Dodd A.N., Jakobsen M.K., Baker A.J., Telzerow A., Hou S.W., Laplaze L., Barrot L., Poethig R.S., Haseloff J., Webb A.A. (2006). Time of day modulates low-temperature Ca signals in Arabidopsis. Plant J. 48: 962–973 [DOI] [PubMed] [Google Scholar]
- Dodd A.N., Kudla J., Sanders D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Douchiche O., Driouich A., Morvan C. (2010). Spatial regulation of cell-wall structure in response to heavy metal stress: Cadmium-induced alteration of the methyl-esterification pattern of homogalacturonans. Ann. Bot. (Lond.) 105: 481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz M.B., Levine A.J., Siggia E.D., Swain P.S. (2002). Stochastic gene expression in a single cell. Science 297: 1183–1186 [DOI] [PubMed] [Google Scholar]
- Gardner M.J., Baker A.J., Assie J.-M., Poethig R.S., Haseloff J.P., Webb A.A.R. (2009). GAL4 GFP enhancer trap lines for analysis of stomatal guard cell development and gene expression. J. Exp. Bot. 60: 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M., Axelsen K.B., Harper J.F., Palmgren M.G. (2000a). Molecular aspects of higher plant P-type Ca(2+)-ATPases. Biochim. Biophys. Acta 1465: 52–78 [DOI] [PubMed] [Google Scholar]
- Geisler M., Frangne N., Gomès E., Martinoia E., Palmgren M.G. (2000b). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 124: 1814–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George L., Romanowsky S.M., Harper J.F., Sharrock R.A. (2008). The ACA10 Ca2+-ATPase regulates adult vegetative development and inflorescence architecture in Arabidopsis. Plant Physiol. 146: 716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.F., Hong B.M., Hwang I.D., Guo H.Q., Stoddard R., Huang J.F., Palmgren M.G., Sze H. (1998). A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J. Biol. Chem. 273: 1099–1106 [DOI] [PubMed] [Google Scholar]
- Haseloff J. (1999). GFP variants for multispectral imaging of living cells. Methods Cell Biol. 58: 139–151 [DOI] [PubMed] [Google Scholar]
- Heazlewood J.L., Verboom R.E., Tonti-Filippini J., Small I., Millar A.H. (2007). SUBA: The Arabidopsis subcellular database. Nucleic Acids Res. 35(Database issue): D213–D218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P.K., Winship L.J. (2010). Calcium at the cell wall-cytoplast interface. J. Integr. Plant Biol. 52: 147–160 [DOI] [PubMed] [Google Scholar]
- Hirschi K.D. (1999). Expression of Arabidopsis CAX1 in tobacco: Altered calcium homeostasis and increased stress sensitivity. Plant Cell 11: 2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K.D. (2004). The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 136: 2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K.D., Zhen R.G., Cunningham K.W., Rea P.A., Fink G.R. (1996). CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc. Natl. Acad. Sci. USA 93: 8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong B.M., Ichida A., Wang Y.W., Gens J.S., Pickard B.G., Harper J.F. (1999). Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 119: 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.Q., Berkelman T., Franklin A.E., Hoffman N.E. (1993). Characterization of a gene encoding a Ca(2+)-ATPase-like protein in the plastid envelope. Proc. Natl. Acad. Sci. USA 90: 10066–10070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada N., Wildermuth M.C. (2005). Novel tissue preparation method and cell-specific marker for laser microdissection of Arabidopsis mature leaf. Planta 221: 9–16 [DOI] [PubMed] [Google Scholar]
- Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264 [DOI] [PubMed] [Google Scholar]
- Karley A.J., Leigh R.A., Sanders D. (2000a). Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends Plant Sci. 5: 465–470 [DOI] [PubMed] [Google Scholar]
- Karley A.J., Leigh R.A., Sanders D. (2000b). Differential ion accumulation and ion fluxes in the mesophyll and epidermis of barley. Plant Physiol. 122: 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenkov V., Hirschi K., Crutchfield J.D., Wagner G.J. (2007). Enhancing tonoplast Cd/H antiport activity increases Cd, Zn, and Mn tolerance, and impacts root/shoot Cd partitioning in Nicotiana tabacum L. Planta 226: 1379–1387 [DOI] [PubMed] [Google Scholar]
- Kralj J.G., Player A., Sedrick H., Munson M.S., Petersen D., Forry S.P., Meltzer P., Kawasaki E., Locascio L.E. (2009). T7-based linear amplification of low concentration mRNA samples using beads and microfluidics for global gene expression measurements. Lab Chip 9: 917–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., Kim H.S., Han H.J., Moon B.C., Kim C.Y., Harper J.F., Chung W.S. (2007). Identification of a calmodulin-regulated autoinhibited Ca2+-ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis. FEBS Lett. 581: 3943–3949 [DOI] [PubMed] [Google Scholar]
- Leegood R.C. (2008). Roles of the bundle sheath cells in leaves of C3 plants. J. Exp. Bot. 59: 1663–1673 [DOI] [PubMed] [Google Scholar]
- Leigh R.A. (1997). Solute composition of vacuoles. In Advances in Botanical Research Incorporating Advances in Plant Pathology, Vol. 25, Callow J.A., (London: Academic Press; ), pp. 171–194 [Google Scholar]
- Leigh R.A., Chater M., Storey R., Johnston A.E. (1986). Accumulation and subcellular-distribution of cations in relation to the growth of potassium-deficient barley. Plant Cell Environ. 9: 595–604 [Google Scholar]
- Leigh R.A., Storey R. (1993). Intercellular compartmentation of ions in barley leaves in relation to potassium nutrition and salinity. J. Exp. Bot. 44: 755–762 [Google Scholar]
- Lempe J., Balasubramanian S., Sureshkumar S., Singh A., Schmid M., Weigel D. (2005). Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet. 1: 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N., Kwak J.M., Robert N., Waner D., Leonhardt G., Schroeder J.I. (2004). Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman M., Mutaftschiev A., Jauneau B., Vian B., Catesson A.M., Goldberg R. (1999). Mungbean hypocotyl homogalacturonan: Localization, organisation and origin. Ann. Bot. (Lond.) 84: 225–233 [Google Scholar]
- Lionetti V., Raiola A., Camardella L., Giovane A., Obel N., Pauly M., Favaron F., Cervone F., Bellincampi D. (2007). Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 143: 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey F.J. (2003). Strategies for microarray analysis of limiting amounts of RNA. Brief. Funct. Genomics Proteomics 2: 31–36 [DOI] [PubMed] [Google Scholar]
- Lohaus G., Pennewiss K., Sattelmacher B., Hussmann M., Hermann Muehling K. (2001). Is the infiltration-centrifugation technique appropriate for the isolation of apoplastic fluid? A critical evaluation with different plant species. Physiol. Plant. 111: 457–465 [DOI] [PubMed] [Google Scholar]
- Lu Z., Neumann P.M. (1999). Low cell-wall extensibility can limit maximum leaf growth rates in rice. Crop Sci. 39: 126–130 [Google Scholar]
- Marín-Rodríguez M.C., Orchard J., Seymour G.B. (2002). Pectate lyases, cell wall degradation and fruit softening. J. Exp. Bot. 53: 2115–2119 [DOI] [PubMed] [Google Scholar]
- McAinsh M.R., Pittman J.K. (2009). Shaping the calcium signature. New Phytol. 181: 275–294 [DOI] [PubMed] [Google Scholar]
- McCartney L., Steele-King C.G., Jordan E., Knox J.P. (2003). Cell wall pectic (1—>4)-β-d-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 33: 447–454 [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S., Durachko D.M., Cosgrove D.J. (1992). Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4: 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. (2001). Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6: 414–419 [DOI] [PubMed] [Google Scholar]
- Moller I., Sørensen I., Bernal A.J., Blaukopf C., Lee K., Øbro J., Pettolino F., Roberts A., Mikkelsen J.D., Knox J.P., Bacic A., Willats W.G. (2007). High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 50: 1118–1128 [DOI] [PubMed] [Google Scholar]
- Morris J., Hawthorne K.M., Hotze T., Abrams S.A., Hirschi K.D. (2008). Nutritional impact of elevated calcium transport activity in carrots. Proc. Natl. Acad. Sci. USA 105: 1431–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Cheng N.H., Pittman J.K., Yoo K.S., Park J., Smith R.H., Hirschi K.D. (2005a). Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiol. 139: 1194–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kang T.S., Kim C.K., Han J.S., Kim S., Smith R.H., Pike L.M., Hirschi K.D. (2005b). Genetic manipulation for enhancing calcium content in potato tuber. J. Agric. Food Chem. 53: 5598–5603 [DOI] [PubMed] [Google Scholar]
- Pelloux J., Rustérucci C., Mellerowicz E.J. (2007). New insights into pectin methylesterase structure and function. Trends Plant Sci. 12: 267–277 [DOI] [PubMed] [Google Scholar]
- Pien S., Wyrzykowska J., McQueen-Mason S.J., Smart C., Fleming A. (2001). Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc. Natl. Acad. Sci. USA 98: 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman J.K., Shigaki T., Hirschi K.D. (2005). Evidence of differential pH regulation of the Arabidopsis vacuolar Ca2+/H+ antiporters CAX1 and CAX2. FEBS Lett. 579: 2648–2656 [DOI] [PubMed] [Google Scholar]
- Raser J.M., O’Shea E.K. (2004). Control of stochasticity in eukaryotic gene expression. Science 304: 1811–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A., Boscari A., Schreiber L., Kerstiens G., Jarvis M., Herzyk P., Fricke W. (2007). Cloning and expression analysis of candidate genes involved in wax deposition along the growing barley (Hordeum vulgare) leaf. Planta 226: 1459–1473 [DOI] [PubMed] [Google Scholar]
- Roth C., Menzel G., Petétot J.M., Rochat-Hacker S., Poirier Y. (2004). Characterization of a protein of the plastid inner envelope having homology to animal inorganic phosphate, chloride and organic-anion transporters. Planta 218: 406–416 [DOI] [PubMed] [Google Scholar]
- Roy S.J., et al. (2008). Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant Cell Environ. 31: 861–871 [DOI] [PubMed] [Google Scholar]
- Sattelmacher B. (2001). The apoplast and its significance for plant mineral nutrition. New Phytol. 149: 167–172 [DOI] [PubMed] [Google Scholar]
- Schaaf G., Catoni E., Fitz M., Schwacke R., Schneider A., von Wiren N., Frommer W.B. (2002). A putative role for the vacuolar calcium/manganese proton antiporter AtCAX2 in heavy metal detoxification. Plant Biol. 4: 612–618 [Google Scholar]
- Schneider J., Buness A., Huber W., Volz J., Kioschis P., Hafner M., Poustka A., Sültmann H. (2004). Systematic analysis of T7 RNA polymerase based in vitro linear RNA amplification for use in microarray experiments. BMC Genomics 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigaki T., Hirschi K.D. (2006). Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol (Stuttg) 8: 419–429 [DOI] [PubMed] [Google Scholar]
- Smyth G.K. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3: Article 3 [DOI] [PubMed] [Google Scholar]
- Storey R., Leigh R.A. (2004). Processes modulating calcium distribution in citrus leaves. An investigation using x-ray microanalysis with strontium as a tracer. Plant Physiol. 136: 3838–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain P.S., Elowitz M.B., Siggia E.D. (2002). Intrinsic and extrinsic contributions to stochasticity in gene expression. Proc. Natl. Acad. Sci. USA 99: 12795–12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.S. (2008). Space and time in the plant cell wall: Relationships between cell type, cell wall rheology and cell function. Ann. Bot. (Lond.) 101: 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomos A.D., Hinde P., Richardson P., Pritchard J., Fricke W. (1994). Microsampling and measurements of solutes in single cells. Plant Cell Biology: A Practical Approach, Harris K.O.N., (Oxford, UK: IRL Press; ), pp. 297–314 [Google Scholar]
- van Cutsem P., Messiaen J. (1996). Cell wall pectins: From immunochemical characterization to biological activity. Pectins and Pectinases,Vol. 17, Visser J., Voragen A.G.J., (Amsterdam, The Netherlands: Elsevier Science Publishers; ), pp. 135–149 [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren I., de Almeida Engler J., De Groodt R., Gheysen G. (2002). An Arabidopsis thaliana pectin acetylesterase gene is upregulated in nematode feeding sites induced by root-knot and cyst nematodes. Mol. Plant Microbe Interact. 15: 404–407 [DOI] [PubMed] [Google Scholar]
- Webb A.A.R., Larman M.G., Montgomery L.T., Taylor J.E., Hetherington A.M. (2001). The role of calcium in ABA-induced gene expression and stomatal movements. Plant J. 26: 351–362 [DOI] [PubMed] [Google Scholar]
- White P.J. (2001). The pathways of calcium movement to the xylem. J. Exp. Bot. 52: 891–899 [DOI] [PubMed] [Google Scholar]
- White P.J., Broadley M.R. (2003). Calcium in plants. Ann. Bot. (Lond.) 92: 487–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats W.G., McCartney L., Mackie W., Knox J.P. (2001). Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 47: 9–27 [PubMed] [Google Scholar]
- Yang Y., Costa A., Leonhardt N., Siegel R.S., Schroeder J.I. (2008). Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Shigaki T., Mei H., Guo Y.-Q., Cheng N.-H., Hirschi K.D. (2009). Interaction between Arabidopsis Ca2+/H+ exchangers CAX1 and CAX3. J. Biol. Chem. 284: 4605–4615 [DOI] [PubMed] [Google Scholar]
- Zhong J.F., Chen Y., Marcus J.S., Scherer A., Quake S.R., Taylor C.R., Weiner L.P. (2008). A microfluidic processor for gene expression profiling of single human embryonic stem cells. Lab Chip 8: 68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.